Abstract
Dementia is increasingly being recognized in cases of Parkinson's disease (PD); such cases are termed PD dementia (PDD). The spread of fibrillar α-synuclein (α-syn) pathology from the brainstem to limbic and neocortical structures seems to be the strongest neuropathological correlate of emerging dementia in PD. In addition, up to 50% of patients with PDD also develop sufficient amyloid-β plaques and tau-containing neurofibrillary tangles for a secondary diagnosis of Alzheimer's disease (AD), and these pathologies may act synergistically with α-syn pathology to confer a worse prognosis. Understanding the relationships between these three distinct pathologies and their resultant clinical phenotypes are crucial to the development of effective disease-modifying treatments for PD and PDD.
Introduction
Parkinson's disease (PD) is the most common neurodegenerative movement disorder associated with neuronal loss in the substantia nigra and inclusions containing the synaptic protein α-synuclein (α-syn) in the cell bodies and processes of surviving neurons known as Lewy bodies and Lewy neurites, respectively. However, PD also is now recognized to be a more complex clinicopathological entity that includes widespread α-syn pathology and cognitive impairments including dementia (PDD) in the majority of patients during the natural course of their illness (for a detailed review on the history of the major developments in PD research please see REF1).
Given this expanded view of PD as both a movement and cognitive disorder, here we examine the complex connections between the neuropathological aetiologies that underlie the variable expression of the cognitive deficits in PDD. By doing so, we aim to further the understanding of the pathobiology of PDD and to distinguish genetic, clinical and neuropathological subtypes of this condition, which will be important for developing and administering appropriate disease-modifying treatments.
Clinical features of PDD
Epidemiology and clinical impact of PDD
Cognitive impairment (i.e. cognitive dysfunction in the absence of a functional deficit sufficient for the diagnosis of dementia) is a common feature of PD and may be a natural consequence of end-stage disease2-4 as the overall prevalence of cognitive impairment in PD is roughly 30%3, 5 and approximately 80% of longitudinally followed patients with PD develop dementia over the course of their disease.2, 3, 6 It is estimated that the incidence rate of dementia in patients with PD is at least four times higher than it is in the general population.3, 5, 7, 8 Additionally, cognitive impairment not meeting the threshold for dementia can be present early in the disease course, with cognitive deficits seen in up to 24% of patients at initial diagnosis of PD.9 Furthermore, patients with PDD experience dementia for an average of 3–4 years during their disease course.4 Thus, including the period of cognitive impairment prior to progression to PDD, most patients with PD show cognitive deficits during the majority of their illness. This fact has implications for patient quality of life, public health and the cost of care.
Cognitive difficulties can exacerbate the disabilities caused by motor symptoms in PD. Indeed, the presence of cognitive impairment or dementia in PD patients is associated with a loss of independence, a lower quality of life and a reduction in survival time.10, 11 Furthermore, individuals with end-stage PD who develop dementia often exhibit a decline in function that follows a stereotypical pattern, indicating that dementia onset may herald impending residential care and mortality.4 Thus, the onset and progression of cognitive impairment and dementia in PD have an important influence on patient management and prognosis and understanding the pathophysiology underlying cognitive dysfunction in PD is critical for therapeutic development.
Cognitive features of mild cognitive impairment and dementia in PD
The specific cognitive symptoms associated with PD vary,12 most likely because of the heterogeneous nature of the underlying neuropathology. The most common cognitive impairments in PD are deficits in attention, executive functioning and visuo-spatial processing, although patients may also exhibit varying degrees of memory loss, which may be, in some cases, related more to a frontally mediated (i.e. executive functioning) retrieval deficit than to an intrinsic encoding problem(see REF.5 for a thorough review of cognitive studies in PDD). Similarly, PDD is also not characterized by an intrinsic core language deficit,5 but it can be associated with difficulties in sentence processing which are also related to executive dysfunction.13 Some researchers propose that executive difficulties in PD are an early phenomenon of altered dopaminergic tone in the frontal cortex, whereas deficits in visuospatial functioning and semantic memory correspond to the later neuropathological involvement of temporal and parietal cortices, and that these “posterior symptoms” confer an increased risk of PDD.8 The recognition of cognitive symptoms in PD has led to the recent development of clinical criteria for PDD5 and mild cognitive impairment (MCI) in PD (PD-MCI) (BOX 1).12 The formalized criteria for PD-MCI and PDD facilitate better detection and characterization of cognitive impairment earlier in PD and will allow for detailed comparative studies in the future.
Box 1. Clinical criteria for PD-MCI and PDD.
Recent clinical criteria have been proposed for better detection and characterization of PD dementia (PDD) and PD with mild cognitive impairment (PD-MCI). Both sets of criteria require the establishment of a diagnosis of PD according to the UK Brain Bank Criteria14 and a subsequent gradual decline in cognitive ability, with major distinctions of PDD from PD-MCI including the requirement of deficits in multiple cognitive domains and the subsequent emergence of functional impairments, while PD-MCI patients are, by definition, functionally independent.5, 12 The criteria for PD-MCI include two levels of assessment based on the availability of formal neuropsychological testing and provide subtyping for involvement of single (PD-MCI single domain) or multiple cognitive domains (PD-MCI multiple domain).12 The criteria for PDD includes two levels, probable and possible, differentiated by the presence of less typical PDD cognitive impairment features (i.e. language or pure amnestic symptoms) or factors that make the diagnosis of PDD uncertain (i.e. cerebrovascular co-morbidity or unknown motor-dementia interval) in possible PDD.
There is some similarity between the cognitive symptoms of PDD and Alzheimer's disease (AD), but patients with PDD may be differentiated from individuals with AD by the presence of hallucinations, cognitive fluctuations, depression and sleep disturbance.15 By contrast, the cognitive features of PDD are similar to, and often indistinguishable from, the clinical syndrome of dementia with Lewy bodies (DLB).5, 15, 16 The research criteria for DLB make a somewhat arbitrary distinction between these two entities, with a diagnosis of DLB being assigned to patients with an onset of dementia within one year after the onset of motor symptoms, and PDD being assigned to individuals when dementia occurs more than one year after PD onset.17 The underlying pathophysiology responsible for the different chronological patterns for the emergence of dementia in these clinical syndromes is a matter of intense research and continued debate.15
Patients with PD may also suffer from neuropsychiatric symptoms including depression, anxiety, apathy, hallucinations and delusions.5 Furthermore, such individuals may exhibit an impulse control disorder that is characterized by compulsive gambling, eating, purchasing and sexual behavior, and a dopamine dysregulation syndrome.18 The aetiology of impulse control disorders in PD is thought to be due to stimulation of hypersensitive ventral striatal-frontal connections by dopaminergic therapy rather than a direct consequence of the neurodegenerative process specific to PD and PDD.18, 19
Clinical risk factors for PDD
Identification of clinical features that may predict impending cognitive decline in PD is of interest for clinical practice and disease management. Age is the most prominent risk factor for PDD4, 7, 8, 20 The increased risk of dementia with age has been dissociated from the potential influence of age on PD onset;21 in other words, patients with PDD have a similar age of dementia onset regardless of when they first present with motor symptoms.4 Studies show that cognitive impairment in PD also correlates with the severity of motor disability22 and that the effects of ageing on cognition may be additive to the severity of the motor disturbance.7 Other factors and disease features linked to PDD include male gender,23 low education,23 visual hallucinations,3, 4, 16 and prominent axial rigidity and bradykinesia relative to tremor.3, 5, 24 The presence of MCI in PD,12 as revealed by poor performance on specific neuropsychological measures, including semantic fluency,8 constructional praxis,8 verbal memory25 and executive function25 has also been associated with increased risk of developing dementia.
The pathogenesis of PD
Role of Lewy bodies and Lewy neurites
α-syn is a 140 amino acid presynaptic protein that is involved in vesicular transport. This protein was implicated in the pathogenesis of PD when pathogenic mutations in the SNCA gene(which encodes α-syn)26, 27 and, later, multiplications of this gene28 were linked to hereditary forms of this disease. Some of these genetic abnormalities may increase the propensity of α-syn to fibrillize in vitro.29 Thus, molecular changes in the α-syn protein that increase protein misfolding and aggregation have a direct role in disease pathogenesis. The initial discovery of SNCA mutations was closely followed by evidence from post-mortem studies of PD and DLB brain that the characteristic Lewy bodies and Lewy neurites visualized through immunohistochemical methods using anti-α-syn antibodies were composed mainly of insoluble, fibrillar forms of α-syn.30 Several experimental models support a role for α-syn aggregation in neurodegeneration. For example, overexpression of the Ala53Thr mutation in transgenic animals31, 32 led to the formation of α-syn immune-reactive deposits that resembled Lewy body and neuritic pathology, as well as a cognitive and motor clinical phenotype and reduced survival time. Furthermore, suppression of this transgene resulted in decreases in neuropathology and clinical symptoms32 (see below for further discussion of the role of fibrillized α-syn in the progression of PD and REF33 for a comprehensive review of murine models of PD).
A detailed neuropathological examination of a large number of PD and control cases by Braak and colleagues34, 35 revealed a stereotypical caudal to rostral ascending progression of α-syn pathology from brainstem structures in early stages of the disease to limbic and neocortical areas in the later stages. Staging systems of α-syn pathology based on these observations have been proposed for PD34 and DLB.17 These hypothetical systems are supported by some independent studies;36-38 although not all studies demonstrate a similar topography of disease spread38, 39 and α-syn staging may be more applicable to younger-onset PD patients.6 Indeed, up to 30% of elderly patients with α-syn pathology at autopsy have no clinical signs of dementia or a movement disorder thereby prompting such cases to be termed incidental Lewy body disease (ILBD).35, 37, 40-43 Some infer from this that α-syn pathology may be protective rather than a cause of neuronal cell death.38, 44 Additionally, quantitative studies of Lewy bodies and Lewy neurites suggest a dissociation between α-syn pathology and neuron loss in PD.45, 46 The nature of this discrepancy is unclear, since individuals with ILBD could be in a prodromal state of PD and they would develop full blown disease if they survived longer,35 which is consistent with the locations of most incidental α-syn pathology in areas affected early in PD 35, 37 while not meeting a threshold of α-syn pathology required for to manifest clinical symptoms.47 In addition, α-syn pathology is also found in up to 50% of patients with AD with a distribution largely restricted to the amygdala in many cases.36, 37, 40, 48-50 The amygdala seems to be exceptionally vulnerable to other neurodegenerative inclusions, such as tau and TDP-43, and “amygdala-only”51 patterns of α-syn pathology in AD cases may represent a separate disease process compared to the pathogenic spread of these inclusions in PD and DLB, as has been proposed by Braak and colleagues36, 50 (for a detailed review of the Braak staging system and proposed revisions please see REFs44, 52).
Despite these exceptions to the staging systems proposed by Braak and colleagues, the stereotypical non-random progression of α-syn pathology in the majority of PD patients suggests that α-syn undergoes cell-to-cell transmission within individuals. In addition, α-syn pathology discovered in fetal dopaminergic neurons implanted in PD patients53-55 and recent in vitro cell56, 57 and in vivo animal58, 59 studies suggest that pathogenic α-syn can transfer between cells leading to neurodegeneration. These, and other transmission studies60-63 are reviewed in depth elsewhere,64, 65 but FIG. 1 summarizes the proposed mechanisms whereby the aggregation and spread of α-syn leads to deleterious consequences for the affected neurons.66 The evidence for α-syn transmission in synucleinopathies prompts speculations that PD and other neurodegenerative proteinopathies could spread between individuals similar to pathological prion proteins or PrPsc in human transmissible spongiform encephalopathies, but there is currently no definitive evidence of human-to-human transmission of clinical PD.67 BOX-2 summarizes evidence on cell-to-cell α-syn transmission in contrast to the infectivity of PrPsc in human spongiform encephalopathies.
Figure 1. Hypothetical model of α-syn toxicity and spread of pathology in PD and PDD.
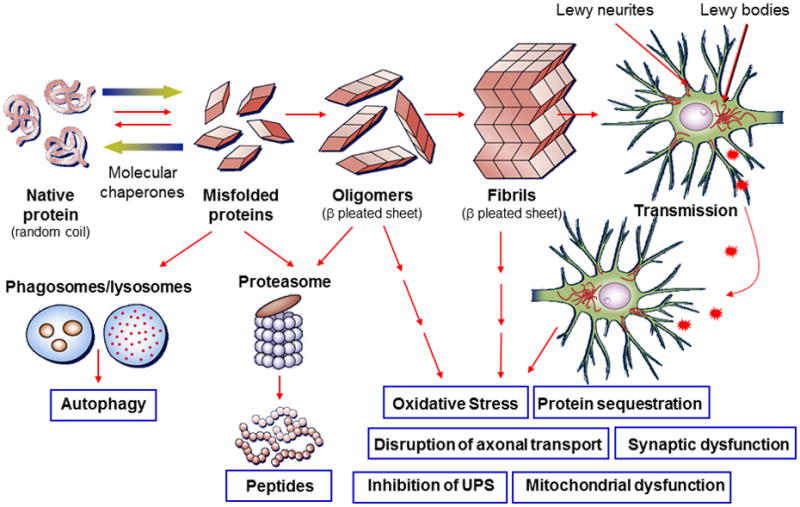
From left to right in the top row: native α-syn in normal conditions exists in a soluble random coil state and under pathological conditions undergoes misfolding into pathogenic species of α-syn (dimers, trimers, oligomers, etc.) that further aggregate into higher order structures (protofibrils, other intermediates and fibrils) which ultimately are the building blocks for pathological inclusions visualized under light microscopy at autopsy (i.e. Lewy bodies and Lewy Neurites).66 Genetic abnormalities and environmental factors may accelerate this process.27-29, 66, 119, 121 Normal quality-control systems (chaperones, ubiquitin proteosome and phagosome–lysosome systems) that prevent or reverse protein misfolding or eliminate misfolded proteins are overwhelmed (indicated by dashed lines).66 Remarkably, recent data suggest that the progression of PD and related disorders may be linked to the cell-to-cell spread of pathological species of α-syn,57-59, 64 as illustrated in the upper right of the figure. The inter-related toxic consequences of pathological α-syn transmission are listed in the lower right of the figure.66 It is unclear which species of pathogenic α-syn is directly toxic to neurons; however recent animal studies show that synthetic α-syn fibrils alone are sufficient to transmit disease, i.e. α-syn pathology, between neurons and cause clinical disease (indicated by the furthest right arrow).58, 59
Box 2. Contrasting evidence of transmission of α-syn and proteinaceous infectious particles (i.e. prions).
Evidence of neuron-to-neuron transmission of α-syn is similar to properties of the proteinaceous infectious particle, PrPsc,68 the causative agent of infectious spongiform encephalopathies.65 Iatrogenic forms of these disorders are caused by exposure by individuals to affected central nervous tissue from Prion disease patients containing PrPsc, causing templating and protein misfolding of native (PrPC) with spread throughout the brain resulting in neurodegeneration. Several lines of evidence draw similarities of pathogenic α-syn aggregation to prions. First, the non-random sequence of α-syn deposition into α-syn pathology in PD34 suggests that misfolded α-syn could spread between neurons within an individual. Further, indirect evidence for the transmission of pathological α-syn in humans comes from studies of PD patients wherein Lewy bodies were detected in grafted fetal mesencephalic cells in the striatum of a subset of patients who underwent experimental fetal graft treatment and survived for over 10 years.53-55 This raises the possibility that the Lewy bodies in the grafted neurons resulted from the transmission of pathological α-syn from the host tissue to the grafted neurons; however, the formation of Lewy bodies in the grafted neurons may be explained by other factors, such as the diseased local environment of the host striatum which could have “toxic” effects leading to the emergence of α-syn pathology in the grafted neurons.64, 69 Finally, several key animal and cell model studies have found experimental evidence of neuron-to-neuron transmission of pathogenic α-syn (review in REFs.64, 65). One major distinction between prions and non-prion neurodegenerative-disease proteins (e.g. those mediated by pathological and toxic α-syn, tau, Aβ species) is that there is no current evidence to suggest that PD or AD can be transmitted between individuals as seen in prion diseases. There is a large body of negative evidence from NIH transmission studies examining inoculation of human non-prion neurodegenerative disease CNS tissue into non-human primates and other animals, including 71 transmission experiments using tissue from 24 patients with PDD, that suggest these diseases are not clinically or pathologically transmissible between humans and non-human primates;70 although the pathological examination of these animals did not use modern immunohistochemical techniques to detect α-syn and other non-prion neurodegenerative disease proteins. A potential patient population that could provide evidence for human-to-human transmission of α-syn is individuals treated with cadaver-derived human growth hormone. These individuals were probably often exposed to pathological α-syn in the hypophysis of the donor tissue, but a systematic epidemiological evaluation of this patient cohort in the US67 found no evidence for the emergence of clinical AD or PD in any of the treated subjects for >20 years after this treatment program was terminated in 1985 when it was discovered that, PrPsc was transmitted between humans resulting in an outbreak of iatrogenic Creutzfeldt-Jacob disease through exposure to PrPsc in cadaveric donor tissue-derived human growth hormone preparations.71
The data reviewed here, including recent transmission studies showing that injections of synthetic α-syn pre-formed fibrils into dorsal striatum are followed first by Lewy body formation in the substantia nigra pars compacta and then later by neuron loss,58, 59 suggest that the direct effects of α-syn misfolding and aggregation within substantia nigra neurons result in neuron loss and the clinical syndrome of idiopathic PD. However, the nature of the pathogenic species of α-syn that are toxic and mediate cell-to-cell spread of pathological α-syn (i.e. dimers, oligomers, protofibrils, fibrils, ect.) is less clear, but α-syn pathologies are likely to be the proximal cause of neuron dysfunction and death in the cortex of patients with PDD, as discussed in more detail below.
Neuropathology underlying PDD
Clinicopathological correlations in PDD
The neuropathology underlying PDD is heterogeneous in nature (FIG. 2) and several factors have contributed to the difficulty of conducting accurate clinicopathological correlation studies in PDD. First, human post-mortem studies are limited to visualization of neuropathology at time of death from multiple potential aetiologies, some of which may precede end-stage disease and neuropathology. Although patients may be followed longitudinally, autopsy data are by definition cross-sectional in nature, which makes it difficult to directly correlate such data with antecedent clinical deficits that manifest years before death. Second, interpretations of the neuropathological substrates of cognitive status in PD are limited by the accuracy of clinical diagnosis and by the ability to perform adequate follow up in end stages of disease.2 There also may be several years between a patient's last clinic visit and autopsy because of the reduced mobility of patients with end stage PD renders office visits infeasible and, thus, large numbers of well-annotated cases are needed to make meaningful observations. Finally, criteria for neuropathological diagnoses have evolved over time and advanced immunochemical techniques have revealed higher burdens of cortical α-syn pathology in cases of PDD than were previously recognized.72, 73 Thus, for these reasons, there has been considerable variation in results from earlier clinicopathological studies as reviewed elsewhere.74
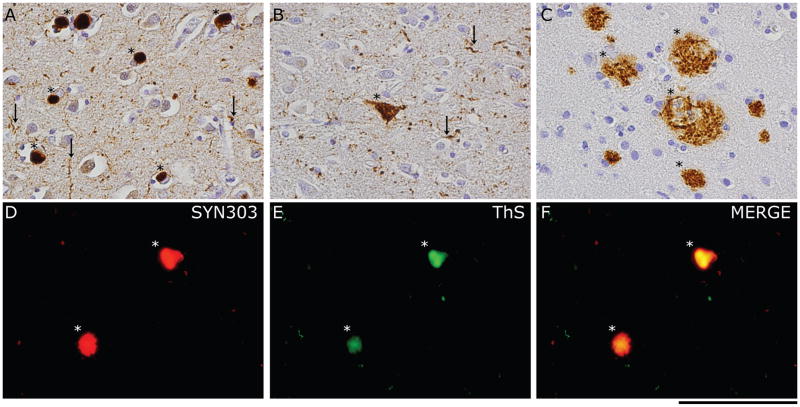
Figure 2. Neuropathology of PDD.
The main neuropathological features of PDD75 include: a) a severe burden of Lewy bodies (asterisks) and Lewy neurites (arrows) in the mid-frontal cortex (detected with SYN303 monoclonal-antibody (MAb)), b) moderate tau-reactive diffuse threads (arrows) and NFTs (asterisk) in the anterior cingulate gyrus (detected with PHF-1 MAb) and c) extensive diffuse Aβ-reactive plaque pathology in the superior temporal cortex (detected with the Nab228 MAb). d–f) The co-localization of pathological α-syn in LBs (asterisks; detected by SYN303) and the amyloid-binding dye Thioflavin-S reveal the fibrillar nature of the majority of α-syn pathology. Scale bar= 100 μm.
In general, cortical Lewy body/neuritic pathology is more extensive and severe in PDD than in PD without dementia and several lines of evidence from studies using α-syn immunohistochemistry implicate cortical α-syn pathology as the strongest correlate of dementia in PDD. First, most large studies have found that PDD cases are almost exclusively of the limbic/transitional or neocortical predominant stage17 of α-syn pathology3, 22, 74-77 and have higher levels of cortical α-syn pathology than do cases of PD.4, 72, 74-79 Many studies find that global cortical and limbic α-syn pathology can discriminate between PD and PDD,22, 74, 75 although some studies also implicate increased diagnostic accuracy of α-syn pathology severity in specific brain regions, including the parahippocampal gyrus79, 80 or anterior cingulate gyrus.80, 81 Second, strong correlations of advancing α-syn pathology stage or cortical α-syn pathology burden with decline on cognitive measures have been described.3, 22, 39, 80, 81 Finally, multivariate regression analyses that included multiple neuropatholoigcal and genetic variables implicated in PDD found that the burden of cortical and limbic Lewy bodies/neurites was the strongest correlate for dementia in a large well-annotated cohort of patients with PD or PDD.75
Despite these observations, some individuals with PD22, 72, 79 and some patients with ILBD38, 41 show a significant burden of cortical and limbic α-syn pathology. Conversely, a minority of patients with PDD were found to have minimal cortical α-syn pathology at autopsy.16, 22, 72, 75, 82 These findings suggest that cortical and limbic α-syn pathology is not exclusively required for development of dementia in PD, so the presence of cortical α-syn pathology at autopsy should be correlated with clinical information on the subject's cognitive status prior to death. These discrepancies might be explained by methodological difficulties in ascertainment of clinical symptoms across studies and/or by cognitive reserve, brain plasticity or other factors that could make some individuals more resistant to the detrimental effects of α-syn pathology, but further research is needed to clarify these issues.
Dementia in PD patients with limited cortical and limbic α-syn pathology could result from subcortical involvement or co-morbid neuropathologies. Cholinergic deficits occur in PDD,83, 84 with higher levels in those with a longer duration of Parkinsonism prior to dementia with lower cortical and limbic Lewy body/neuritic burden,83 and are ascribed to neuronal loss in basal forebrain cholinergic nuclei.84, 85 Furthermore, neuronal loss in these regions is associated with the transition of α-syn pathology into limbic and neocortical regions.22, 74 Moreover, a higher burden of α-syn pathology73, 77 and diffuse amyloid-β (Aβ) plaques (a feature of AD)86 have been found in the striatum in PDD than in PD without dementia and this striatal pathology has been considered to be a possible substrate for cognitive impairment. Indeed, one study of 92 patients with PDD found four cases with minimal cortical Lewy pathology and these cases all had significant cerebral vascular disease (CVD) or featured subcortical Lewy pathology, which could have explained the cognitive deficits.75
Despite the central role of α-syn pathology in PDD, some studies also find that Aβ plaques and tau neurofibrillary tangles (NFTs) — the hallmark pathologies of AD — correlate with cognitive status in PDD76, 78, 80, 87, 88 or a subset of patients with this condition.75, 81 FIG.2 illustrates the major neuropathologic correlates of PDD. In some studies, AD neuropathology seems to be a more specific correlate of dementia in PD than α-syn pathology, but the majority of PD patients with sufficient NFT and Aβ plaques for a diagnosis of co-morbid AD have clinical dementia and could be assigned a diagnosis of PDD+AD.75, 76 Indeed, a recent study found that a combination of measures of cortical α-syn, tau, and Aβ pathologies in the regression model was most predictive of dementia over any single marker alone.76 These results are similar to regression modeling in another large autopsy cohort study showing that inclusion of apolipoprotein epsilon genotype 4 (APOE ε4) status improves diagnostic accuracy for PDD above the model using severity of cortical α-syn pathology alone.75 Interestingly, the association of APOE ε4 genotype with PDD was independent of measures of AD neuropathology,75 which may be due, in part, to the measure of mature senile plaques and not diffuse Aβ deposits examined in this previous study.76 Overall, patients with PDD tend to have higher cortical Aβ plaque burden4, 22, 74-78, 89 and, to a lesser degree, a higher NFT4, 75-78, 87 burden than PD patients without dementia. The percentage of patients with PDD who have levels of NFTs and plaques that meet the threshold for a second diagnosis of another neurodegenerative dementia i.e. AD, varies between studies and depends on the criteria used; however, up to 50% of patients with PDD may have co-morbid AD, i.e. PDD+AD.40, 75, 81, 87, 90, 91 Thus, AD neuropathology, especially Aβ plaque pathology, appears to play an important role in the pathogenesis of PDD for a significant proportion of patients.
Interestingly, patients with PDD+AD seem to have higher levels of cortical and limbic α-syn pathology than patients with PDD without co-morbid AD75, 82 and increased severity of cortical SP and NFT burden correlates with increased cortical α-syn pathology density.75, 76, 79, 81, 87, 92 These data imply a potential synergy between α-syn pathology and AD-related pathology. Indeed, a potential interaction between these pathologies is also suggested by studies of transgenic mice overexpressing combinations of human wild type93 or Ala53Thr mutant94 α-syn with human mutant forms of tau94 and/or amyloid precursor protein (APP) and hence increased production of Aβ93, 94 which show enhanced neurodegenerative pathology and a more severe clinical phenotype. In addition, PD patients with the pathogenic Ala53Thr SNCA mutation exhibit marked tau pathology95 and in vitro experiments show that tau and α-syn can each cross-seed and enhance each other's polymerization into fibrils.96, 97
This putative additive effect of α-syn pathology, senile plaques and NFTs could potentially influence the clinical features of PDD. Indeed, patients with PDD+AD may have a shorter disease duration than those with PDD40, 75, 87, 88, 91 and PDD+AD is also associated with an older age at onset of motor symptoms.75, 88, 91 In addition, a higher Aβ plaque burden alone has been associated with shortened survival in patients with PD6, 76, 89, 98 and older age of PD-onset.6, 76 Thus, the presence of AD neuropathology in PD patients could lead to an older age of onset PD subtype with a more malignant course.6, 76 These prognostic findings for PDD+AD have important implications for patient care.
A few other potential contributing neuropathologies to PDD have been examined. One study found that argyrophilic grain disease was a common feature of PDD;99 however, it did not seem to correlate well with PDD in two large autopsy series.22, 75 TDP-43 inclusions, which are characteristic of frontotemporal lobar degeneration and amyotrophic lateral sclerosis, have also been found in limbic regions in a small number of PD and PDD patients,100 but they do not seem to influence the outcome of dementia in PD.75 Finally, CVD defined by varying methods has been described in a minority (roughly 10–15% or less) of individuals with PDD,2, 3, 75, 87, 90 although the presence of cerebrovascular lesions (including AD-associated amyloid angiopathy) was reported in 94% of patients with PDD compared to roughly 50% in PD in one cohort.78 Thus, systematic evaluation of CVD with validated neuropathological criteria is needed in PDD, but overall CVD does not seem to contribute significantly to dementia in the majority of patients with PDD.
In summary, these data implicate progression of Lewy body/neurite pathology from subcortical areas into limbic and cortical structures as the major driving force behind the development of dementia in most PDD patients, although there is notable heterogeneity in the underlying dementia-linked neuropathology of PDD. While, cortical α-syn pathology is often the sole pathological finding associated with dementia in PDD, it is not an exclusive finding, as there are a minority of patients with negligible burden of cortical/limbic α-syn pathology that develop dementia. Furthermore a significant number of PD patients with dementia have high cortical burdens of both α-syn and AD neuropathology (i.e.PDD+AD). Inter-individual variability of autopsy findings, such as PDD patients with dementia in the setting of relatively low levels of cortical and limbic α-syn pathology is perhaps due to the presence of co-morbidities (such as AD or CVD), while other individuals sustaining high levels of such pathology before exhibiting cognitive impairment may be because of cortical plasticity, cognitive reserve or other unknown factors.
Clinical subtypes of PD and DLB
In addition to the aforementioned clinical similarity between PDD and DLB, there is also neuropathological continuity between these syndromes, as the level and distribution of the underlying α-syn pathology in both of these patient groups are often indistinguishable;37, 50, 79, 83, 87, 101, 102 while some report differentiation of DLB and PDD based on increased striatal Aβ plaques in DLB.103, 104 The clinicopathological overlap with PD and AD helps contribute to the observed low diagnostic accuracy87, 105-107 for clinical DLB; co-morbid AD neuropathology can hinder the diagnosis of DLB,105, 106, 108 as clinical features of DLB such as hallucinations, fluctuations and executive impairments are less prominent in cases of this disorder with high levels of AD neuropathology.105, 106, 109 These findings suggest the clinical phenotype of disorders featuring Lewy body/neurite pathology could be masked in some cases by concomitant AD.15, 105 Thus, the aetiology of the differing pattern of emergence of cognitive deficits across PDD and DLB is unclear, but may be due in part to varying degrees of co-morbid AD neuropathology.
The effects of ageing102 also appear to play a role in the expression of dementia in PDD and DLB. The lack of correlation between α-syn pathology severity and disease duration39 and the variability of nigral neuronal cell loss in PD46 suggest that there is considerable heterogeneity in the rate of disease progression in PD. Indeed, early age of onset PD cases have been shown to have a longer course of disease on average6, 20, 110, 111 than older onset PD patients.6 Those older patients with a shorter interval (∼<5-10 y) of PD prior to dementia tend to have a higher degree of both cortical and limbic α-syn pathology and AD pathology.6, 75, 76, 83 In addition, age of onset of PD itself does not incur a greater risk of dementia21 but instead influences the interval to dementia,4 as early (which is associated with a long PD to dementia interval) and late-onset PD (which is associated with a short PD to dementia interval) patients have similar ages of dementia onset;4, 75, 83 which may be related in part to the presence of AD neuropathology.6, 75, 76, 92 Of note, the age of dementia onset in DLB and PDD is similar despite differences between the onset of motor features in these syndromes.2, 83
Patients with PD can also be clinically subdivided according to differences in motor phenotypes as well. Individuals with prominent postural instability and gait disorder symptoms or non-tremor dominant presentation seem to have a shorter survival time and are more likely to develop dementia than those with tremor-dominant presentation,3, 24, 88, 111 and a subset of rapidly progressive tremor-dominant patients with PD have been described who do not develop dementia.111 Furthermore, the emergence of the postural gait-instability phenotype in patients with an initial presentation of tremor-dominant PD was associated with an accelerated rate of cognitive decline.112 In addition, autopsy,111 CSF biomarker113-115 and in vivo Aβ neuroimaging116 data suggest that underlying AD Aβ pathology may be more common in non-tremor-dominant patients. Non-tremor-dominant phenotype patients have higher cortical and limbic Lewy body burden as well.111 Interestingly, the non-tremor dominant phenotype is reminiscent of the motor features of DLB107 and it is tempting to hypothesize that a clinical influence of AD Aβ pathology could potentially contribute to the varied clinical presentations between PDD and DLB. One caveat to this interpretation is the suspected accelerated progression of cortical and limbic α-syn pathology that causes early dementia in ‘pure’ DLB i.e. DLB without significant co-morbid AD neuropathology. This observation is in contrast to the slow progression of cortical and limbic α-syn pathology in PD patients with a long PD–dementia interval who, in most cases, are also thought to have a ‘pure’ synucleinopathy, i.e. they have negligible AD neuropathology.6 These findings suggest that a complex interaction of genetic and neuropathologic features resulting in the varying expression of clinical phenotypes across the Lewy body/neurite pathology spectrum (Box. 3); thus, we feel that PDD/DLB are most accurately viewed as continuous clinicopathological entities (i.e. synulceinopathies). For a detailed review on the relationships between clinical subtypes across the PDD/DLB spectrum and underlying neuropathology please see REF.117 Further detailed longitudinal clinical and biomarker studies with autopsy confirmation are needed to elucidate these correlations; however, the data described above suggest that deep clinical phenotyping with relation to emerging biomarkers of underlying neuropathology may yield important prognostic and perhaps diagnostic information for elucidating the underlying neuropathology of PDD and DLB.
Box 3. Relationship between neuropathology and timing of dementia across the PDD–DLB spectrum.
“Pure” synucleinopathies (i.e. DLB and PDD with minimal co-morbid AD neuropathology) and mixed pathology diagnoses (PDD+AD, DLB+AD) may have differing clinical and genetic phenotypes. PD without dementia is almost exclusively a pure synucleinopathy without co-morbid AD and α-syn pathology restricted largely to the brainstem.22, 75 Co-morbid AD neuropathology in PDD (PDD+AD) predicts a shorter time to dementia and shorter disease duration40, 75, 87, 88, 91 with higher cortical Lewy body/neurite burden than cases without AD (PDD).75, 82 PDD patients with a long PD-dementia interval are associated with lower cortical Lewy body/neurite burden.3, 24, 83, 88, 111 The postural/gait instability or non-tremor-dominant motor subtype are more associated with PDD than patients with a tremor-dominant phenotype.3, 24, 88, 111 In addition, the non-tremor dominant motor phenotype is associated with a higher cortical Lewy body/neurite burden burden111 and biomarkers of Aβ neuropathology.113-116 DLB cases also have less prominent tremor as compared with gait instability and rigidity symptoms.107 Typical DLB symptoms of hallucinations, fluctuations in cognition and prominent executive dysfunction are more common in “pure” PDD and DLB.105, 106, 109 APOE ε4 genotype is most commonly reported as being associated with increased risk of PDD and DLB in cases with co-morbid AD,125 while GBA mutations are more associated with “pure” PDD/DLB.120 Pathogenic mutations and duplication/triplication of SNCA are associated with PDD and DLB.27, 118 The MAPT H1/H1 haplotype confers risk of PD,130 but also PDD in some studies.8, 131
Genetic associations
Genetic factors may also play an important part in the expression of cognitive deficits in PDD and DLB. Indeed, some hereditary forms of PD have been associated with dementia, most notably those arising from a pathogenic mutation in or triplication of SCNA, or a mutation in the glucocerebrosidase gene (GBA). By contrast, mutations in leucine-rich repeat kinase 2 gene (LRRK2)and other less common genetic aetiologies of PD do not seem to be as strongly linked with PDD and DLB.27, 118 Homozygous mutations in GBA result in the lysosomal storage disorder Gaucher's disease, whereas heterozygous mutations in this gene are associated with increased risk of PD119 or DLB,120 with a higher rate of dementia and earlier age of dementia onset in PDD compared with PD/PDD non-carriers.121 Recently, an international multicenter study found that DLB patients were more than 8 times likely to harbor a GBA mutation associated with a more aggressive disease course compared to non-carrier DLB patients.122 Furthermore, autopsy studies show higher cortical and limbic α-syn pathology in GBA mutation carriers compared with non-carrier PD patients121 and an increased rate of carriers in ‘pure’ DLB (i.e. cases of DLB with minimal AD neuropathology) compared with DLB cases with a high degree of AD neuropathology (DLB+AD).120, 123 Given that GBA is functionally involved in lysosomal pathways, the findings described above suggest that alterations in GBA could contribute to increased α-syn deposition and influence cortical spread of α-syn pathology and resultant dementia.
Genetic changes other than those linked to hereditary forms of PD have also been implicated in PDD. Indeed, the APOE ε4 has been studied extensively as a risk factor for AD and may confer an increased risk of dementia in PD.75, 124, 125 However, no association was found between APOE ε4 carrier status and rate of cognitive decline in a population-based longitudinally followed PD cohort, and a meta-analysis of previous studies revealed a potential influence of publication bias and heterogeneity in odds-ratios as an alternative explanation for dementia in PDD.126 The APOE ε4 genotype may affect cognition in the late stage of PD,124 which could also explain the discrepancies mentioned above. Interestingly, APOE ε4 status is associated with both an increased number of Aβ plaques92, 127 and high cortical and limbic α-syn pathology burden in PD.43, 81, 125, 127 Furthermore, the association between APOE ε4 status and cognitive impairment was lost after adjusting for CSF Aβ1–42 levels in one study, suggesting that the observed influence of APOE ε4 on cognition in PD was mediated by associated AD neuropathological changes.128 By contrast, as aforementioned the APOE ε4 genotype was predictive of PDD, independent of AD-related pathology or α-syn pathology, in a multivariate analysis of a large PD/PDD autopsy cohort.75 Finally, the APOE ε4 genotype conferred an increased risk of ‘pure’ synucleinopathies (i.e. DLB, PDD) compared with controls, which was intermediate to that associated with DLB+AD and AD.125 Thus, the APOE ε4 genotype may contribute to neurodegeneration in PDD through pathways that are shared with and diverge from those involved in AD.
The tau gene MAPT contains two major haplotypes in humans: H1 and H2. The H1/H1 haplotype has been associated with increased risk of some tauopathies that clinically resemble PD but lack α-syn pathology (i.e. progressive supranuclear palsy).129 Interestingly, this variation in the tau gene has been associated with PD as well,130 providing another link between α-syn and tau in the pathologies in PD. The cognitive associations of H1/H1 haplotype in PD are less well studied; however, this haplotype has been associated with poor memory performance in PD in one study, without having an influence on cognitive decline,124 while others have found the H1/H1 haplotype in PD is an independent predictor of PDD.8, 131 Additionally the H1/H1 haplotype may have a synergistic effect on the risk of PDD with a polymorphism in SNCA.131 Furthermore, polymorphism in MAPT may influence the degree of co-morbid AD pathology in PD, as evidenced by altered CSF levels of tau in PDD risk allele carriers with low CSF Aβ1-42,132 further suggesting an influence of genetic factors on the variable expression of AD and α-syn pathology in PDD. In contrast, one large autopsy study found no association of the H1/H1 haplotype and PDD, as PD and PDD patients had nearly the same proportion of H1 carriers.75 These discrepancies may be due to differences in sample size or other poorly understood issues.
Further prospective studies of large cohorts of patients who meet the modern criteria for PDD with autopsy confirmation will help confirm these observations and clarify the impact of genetic factors on emergence of PDD. Therapeutic trial designs may benefit from taking account of genetic subgroups into their analyses.
Biomarker Studies
Biofluid biomarkers with strong predictive value for cognitive impairment in PD could be useful in clinical trials. Indeed, a recent biomarker exploratory study with an immune-based multiplex approach found that epidermal growth factor (EGF) plasma levels were lower in PDD than in PD and could predict progression from PD to PDD with 79% accuracy in a mixed PD–PDD cohort.133 Furthermore, most PD patients with low EGF levels converted to PDD in longitudinal follow-up.133 These findings were replicated in drug-naïve, newly diagnosed PD patients: here, EGF levels were inversely correlated with cognitive measures, including semantic fluency and executive function, at the 2 year follow-up.134 Thus, these results suggest that EGF could have utility as a biofluid biomarker for predicting cognitive decline in PD, although this possibility requires further validation.
CSF analytes are other biomarker candidates for PDD, as proteins characteristic of inclusions in neurodegenerative disease, such as tau and Aβ1–42, can be readily detected in CSF. Indeed, PDD was found to be associated with higher CSF levels of total tau (t-tau)135 and phosphorylated tau (p-tau)135, 136 than PD, although these levels were not as high as in AD.136 Patients with PDD have also been described to have lower CSF levels of Aβ1–42 than those found in individuals with PD, and intermediate to AD and healthy controls.135, 137 Furthermore, an “AD CSF biomarker signature” of (i.e. elevated t-tau and decreased Aβ1–42) was found in a higher percentage of PDD cases than PD cases.138 In addition, low Aβ1-42 levels in PDD correlated with poor performances on a memory task137 and executive tasks.135, 139 High t-tau levels in PDD also correlated with a poor performance on a memory task135 and, when combined in a ratio to Aβ1–42 levels, with poor performances in executive tasks.139 Finally, a prospective study found that low CSF Aβ1-42 levels diagnostic for AD predicts cognitive decline in PD across several cognitive domains.128 Thus, CSF measurements of tau and Aβ1-42 are associated with cognitive decline, have predictive value for PDD, and implicate AD neuropathology in cognitive decline in PD.
Immunoassays have been developed to detect forms of α-syn140, 141, 142 and proteins involved in inflammatory and oxidative stress pathways136, 143, 144 in CSF; However, the relationships between these potential biomarkers, PDD and cognitive status have not been systematically evaluated. Interestingly, early drug-naïve PD patients without dementia have lower levels of CSF t-tau than controls, which correlated to the lower CSF levels of α-syn.115 Longitudinal data on CSF tau in PD is lacking, but one possibility is that CSF tau may increase over the course of the disease,145 and could predict incipient dementia as indicated by the aforementioned associations of cognitive measures and elevated CSF tau in PD.
Neuroimaging studies provide a non-invasive method for determining risk of dementia in PD and, possibly, identifying underlying neuropathology. A volumetric MRI study using a specialized algorithm to score an individual's “AD-like pattern” of atrophy found that this spatial pattern of atrophy predicted cognitive decline in PD.146 Nuclear imaging using positron-emission scanning (PET) with a radioligand specific for Aβ deposition in the brain (i.e. Pittsburg compound B or PIB) provides in vivo evidence of AD neuropathology. Indeed, higher PIB retention, which reflects a higher burden of cortical Aβ plaques, corresponded to a higher likelihood that patients with PD would convert to a cognitively worse diagnosis (i.e., PD to PD-MCI or PD-MCI to PDD)147 and inversely correlated with measures of cognitive function.147, 148 Finally, functional PET imaging with 2-deoxy-2-[18F] fluro-D-glucose (18F-FDG PET) showed that patients with PD who eventually progressed to PDD showed hypometabolism in visual association and posterior cingulate cortex.149 Multi-modal analyses find that CSF measures of tau and Aβ are associated with structural changes on MRI in PD/PDD.145, 150 Note that in addition to these predictive studies, numerous comparative neuroimaging studies have been conducted in PD and PDD as reviewed in detail in REFs.5, 118
Prospective multi-modal studies of clinical, genetic, biochemical and neuroimaging biomarkers in initial drug naïve PD patients, such as the Parkinson's Progression Marker Initiative (PPMI),151 are ongoing and they will provide crucial insight into the dynamic changes that occur in these markers over time and their relationships with emerging dementia.
Therapeutic strategies and drug development
The current pharmacological strategy for treatment of PDD includes augmenting neurotransmitter deficits, similar to AD treatment. Acetylcholinesterase inhibitors have been shown to improve cognition and improve ability to perform activities of daily living in PDD,152 with the largest body of supportive data for rivastigmine.153 The glutaminergic agent memantine has inconsistent evidence for a benefit in PDD but further investigation is warranted.118, 153 Increasing cholinergic tone with acetylcholinesterase inhibitors has the potential to worsen motor symptoms and, conversely, dopamine agonists used to treat motor symptoms may worsen cognition, complicating therapeutic options in PDD.118 Furthermore, these agents do not target the underlying pathobiology of PDD and thus do not affect the progression of disease. The aforementioned studies on cell-to-cell transmission suggests that prevention of α-syn aggregation may help arrest motor and cognitive difficulties in PD. Concepts of drug development for prevention of α-syn mediated neurodegeneration are reviewed in detail elsewhere,154 but broadly include decreasing synthesis and aggregation of α-syn and promoting clearance of pathological inclusions. Furthermore, the role of tau and Aβ pathology in development of cognitive deficits in PD suggests that emerging disease-modifying therapies for AD may be beneficial in a subset of PDD cases. Immune-based therapies targeting α-syn are one potential approach for disease-modifying therapies in PD/PDD.155 Timing the initiation of potential disease-modifying therapies is also a critical issue, as it is unclear if halting the spread of α-syn aggregations in symptomatic PD patients would affect cognitive and motor symptoms or if preventative strategies through treatment of at risk or early PD patients would be more efficacious.155 Thus, biomarkers of incipient cognitive decline and pre-motor PD would be instrumental in the implementation and evaluation of α-syn directed therapies in a protective manner in highly-susceptible patients.
Conclusions
Dementia in PD is a common occurrence that causes a significant impact on patient well-being. While most evidence suggests a role for α-syn pathology in the development of clinical symptoms in PD, including the expression of cognitive impairment in PDD, notable heterogeneity exists between cases of PDD. This underlines the fact that complex interactions between multiple factors — including age, genetics and cognitive reserve, among others — contribute to the clinicopathological expression of symptoms in this condition. Furthermore, AD neuropathology appears to contribute to the emergence of dementia in PD and may indeed be the underlying basis for dementia in a subset of older PD patients by acting synergistically with α-syn pathology to promote the spread of these inclusions. These findings illustrate the need to consider PD, and all neurodegenerative diseases, as clinicopathological entities rather than purely clinical syndromes. Indeed, this view has emerged with new clinical and pathological criteria for AD156, 157 that incorporate biomarker evidence of Aβ plaque and NFT pathology to support clinical criteria. This approach has the benefit of providing refined prognostic information for different PD subtypes and could be useful in the selection of homogenous patient populations to more effectively study developing disease-modifying treatments. For example, patients with clinical phenotype and biomarker evidence of AD neuropathology may be predicted to have a shorter disease duration and a more rapid time to dementia, and such patients could be administered Aβ- or tau-directed therapies as they are developed. Thus, coordinated efforts between researchers from multiple backgrounds to further elucidate these relationships such as in the PPMI study will be crucial for the advancement of meaningful disease-modifying treatments that could help preserve patient independence and improve quality of life in PD.
Acknowledgments
We thank the patients and their families who have contributed to the research reviewed here, which has led to meaningful developments in our understanding of Parkinson's disease and related disorders. Funding for our research was provided by the National Institutes of Health grants P30 AG10124, AG17586, P50 NS53488 and T32-AG000255.
Glossary
- Mild cognitive impairment
Subjective cognitive complaints with objective findings of cognitive impairment in one or more cognitive domains that does not interfere with patient's ability to perform tasks of daily living. Mild cognitive impairment is thought to represent a prodromal state to AD and other dementias and, recently, clinical criteria has been defined for MCI in the setting of PD.
- Executive Function
Abilities in mental flexibility, planning, and working memory mediated by striatal-frontal networks.
- Semantic Memory
Memory for the meaning and context of objects and concepts mediated by the temporal lobe and its connections throughout the neocortex.
- Bradykinesia
Symptoms of slowed movement seen in PD and other disorders involving nigral-striatal dopaminergic pathways.
- Constructional praxis
The ability to draw or copy a figure (such as clock-drawing or drawing intersecting pentagons), which relies on attention, planning and organizing skills (executive function) and visuo-spatial perceptual abilities.
- Verbal memory
Episodic short-term memory mediated by language function (i.e. list-learning tasks).
- Amyloid fibril
An insoluble thread-like structure composed of polymerized protein monomers with notable β-sheet conformation and properties of amyloid.
- Fibrillar
Structure of an insoluble protein in amyloid inclusions of many neurodegenerative disease detected with amyloid-binding dyes (for example, Thioflavin-S)
- Transmission
The spread of a pathological protein in an altered conformation (for example, PrPsc) between neurons within an individual which does not necessarily imply the disease protein is infectious (i.e. can be spread between individuals).
- Cognitive reserve
This refers to the notion of relative resistance to clinical symptoms of neurodegeneration and other CNS insults that is thought to be mediated by neuroplasticity or an ability to recruit additional brain networks to compensate for the disease state, and this plasticity may be influenced by education or other environmental or genetic factors.
- Cerebrovascular disease
Damage to intracerebral blood vessels from atherosclerosis and lipohyalinosis, which are caused by systemic cardiovascular risk factors (for example, hypertension, diabetes and hyperlipidemia) and results in ischemic damage to the brain parenchyma (for example, lacunar infarcts).
- Amyloid angiopathy
A form of cerebral vasculopathy due to fibrillar Aβ deposition in blood vessel walls.
- Tauopathy
A family of neurodegenerative disease proteinopathies that are characterized by inclusions composed primarily of the microtubule-associated protein tau.
Footnotes
Financial Disclosures: The authors declare no competing financial interests.
References
- 1.Goedert M, Spillantini MG, Del Tredici K, Braak H. 100 years of Lewy pathology. Nat Rev Neurol. 2012;9:13–24. doi: 10.1038/nrneurol.2012.242. [DOI] [PubMed] [Google Scholar]
- 2.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23:837–44. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 3.Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60:387–92. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- 4.Kempster PA, O'Sullivan SS, Holton JL, Revesz T, Lees AJ. Relationships between age and late progression of Parkinson's disease: a clinico-pathological study. Brain. 2010;133:1755–62. doi: 10.1093/brain/awq059. [DOI] [PubMed] [Google Scholar]
- 5•.Emre M, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007;22:1689–707. doi: 10.1002/mds.21507. quiz 1837. The Movement Disorder Society Task Force criteria for PDD. [DOI] [PubMed] [Google Scholar]
- 6•.Halliday G, Hely M, Reid W, Morris J. The progression of pathology in longitudinally followed patients with Parkinson's disease. Acta Neuropathol. 2008;115:409–15. doi: 10.1007/s00401-008-0344-8. This is one of the largest prospectively followed PD autopsy series and finds that 80% of PD develop dementia after 20 years. [DOI] [PubMed] [Google Scholar]
- 7.Levy G, et al. Combined effect of age and severity on the risk of dementia in Parkinson's disease. Ann Neurol. 2002;51:722–9. doi: 10.1002/ana.10219. [DOI] [PubMed] [Google Scholar]
- 8.Williams-Gray CH, et al. The distinct cognitive syndromes of Parkinson's disease: 5 year follow-up of the CamPaIGN cohort. Brain. 2009;132:2958–69. doi: 10.1093/brain/awp245. [DOI] [PubMed] [Google Scholar]
- 9.Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65:1239–45. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- 10.Rosenthal E, et al. Association between cognition and function in patients with Parkinson disease with and without dementia. Mov Disord. 2010;25:1170–6. doi: 10.1002/mds.23073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo RY, et al. Clinical features in early Parkinson disease and survival. Arch Neurol. 2009;66:1353–8. doi: 10.1001/archneurol.2009.221. [DOI] [PubMed] [Google Scholar]
- 12•.Litvan I, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord. 2012;27:349–56. doi: 10.1002/mds.24893. The Movement Disorder Society Task Force criteria for PD-MCI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grossman M, et al. Difficulty processing temporary syntactic ambiguities in Lewy body spectrum disorder. Brain Lang. 2012;120:52–60. doi: 10.1016/j.bandl.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward CD, Gibb WR. Research diagnostic criteria for Parkinson's disease. Adv Neurol. 1990;53:245–9. [PubMed] [Google Scholar]
- 15•.Lippa CF, et al. DLB and PDD boundary issues: diagnosis, treatment, molecular pathology, and biomarkers. Neurology. 2007;68:812–9. doi: 10.1212/01.wnl.0000256715.13907.d3. In depth discussion of clinicopathological overlap of PDD/DLB. [DOI] [PubMed] [Google Scholar]
- 16.Galvin JE, Pollack J, Morris JC. Clinical phenotype of Parkinson disease dementia. Neurology. 2006;67:1605–11. doi: 10.1212/01.wnl.0000242630.52203.8f. [DOI] [PubMed] [Google Scholar]
- 17•.McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis. 2006;9:417–23. doi: 10.3233/jad-2006-9s347. DLB cosortium clinical and neuropathological criteria for DLB. [DOI] [PubMed] [Google Scholar]
- 18.Weintraub D. Dopamine and impulse control disorders in Parkinson's disease. Ann Neurol. 2008;64 Suppl 2:S93–100. doi: 10.1002/ana.21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weintraub D, Papay K, Siderowf A for the Parkinson's Progression Markers, I. Screening for impulse control symptoms in patients with de novo Parkinson disease: A case-control study. Neurology. 2013;80:176–180. doi: 10.1212/WNL.0b013e31827b915c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schrag A, Ben-Shlomo Y, Brown R, Marsden CD, Quinn N. Young-onset Parkinson's disease revisited--clinical features, natural history, and mortality. Mov Disord. 1998;13:885–94. doi: 10.1002/mds.870130605. [DOI] [PubMed] [Google Scholar]
- 21.Aarsland D, et al. The effect of age of onset of PD on risk of dementia. J Neurol. 2007;254:38–45. doi: 10.1007/s00415-006-0234-8. [DOI] [PubMed] [Google Scholar]
- 22.Braak H, Rub U, Jansen Steur EN, Del Tredici K, de Vos RA. Cognitive status correlates with neuropathologic stage in Parkinson disease. Neurology. 2005;64:1404–10. doi: 10.1212/01.WNL.0000158422.41380.82. [DOI] [PubMed] [Google Scholar]
- 23.Levy G, et al. Motor impairment in PD: relationship to incident dementia and age. Neurology. 2000;55:539–44. doi: 10.1212/wnl.55.4.539. [DOI] [PubMed] [Google Scholar]
- 24.Jankovic J, et al. Variable expression of Parkinson's disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology. 1990;40:1529–34. doi: 10.1212/wnl.40.10.1529. [DOI] [PubMed] [Google Scholar]
- 25.Levy G, et al. Memory and executive function impairment predict dementia in Parkinson's disease. Mov Disord. 2002;17:1221–6. doi: 10.1002/mds.10280. [DOI] [PubMed] [Google Scholar]
- 26•.Polymeropoulos MH, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–7. doi: 10.1126/science.276.5321.2045. Landmark discovery of SNCA mutations in PD. [DOI] [PubMed] [Google Scholar]
- 27.Poulopoulos M, Levy OA, Alcalay RN. The neuropathology of genetic Parkinson's disease. Mov Disord. 2012;27:831–42. doi: 10.1002/mds.24962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singleton AB, et al. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 29.Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4:1318–20. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 30•.Spillantini MG, et al. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–40. doi: 10.1038/42166. Landmark discovery of α-syn as the major component of Lewy pathology. [DOI] [PubMed] [Google Scholar]
- 31.Giasson BI, et al. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002;34:521–33. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 32.Lim Y, et al. alpha-Syn suppression reverses synaptic and memory defects in a mouse model of dementia with Lewy bodies. J Neurosci. 2011;31:10076–87. doi: 10.1523/JNEUROSCI.0618-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Magen I, Chesselet MF. Genetic mouse models of Parkinson's disease The state of the art. Prog Brain Res. 2010;184:53–87. doi: 10.1016/S0079-6123(10)84004-X. [DOI] [PubMed] [Google Scholar]
- 34.Braak H, et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 35.Del Tredici K, Rub U, De Vos RA, Bohl JR, Braak H. Where does parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol. 2002;61:413–26. doi: 10.1093/jnen/61.5.413. [DOI] [PubMed] [Google Scholar]
- 36.Dickson DW, Uchikado H, Fujishiro H, Tsuboi Y. Evidence in favor of Braak staging of Parkinson's disease. Mov Disord. 2010;25 Suppl 1:S78–82. doi: 10.1002/mds.22637. [DOI] [PubMed] [Google Scholar]
- 37.Jellinger KA. Lewy body-related alpha-synucleinopathy in the aged human brain. J Neural Transm. 2004;111:1219–35. doi: 10.1007/s00702-004-0138-7. [DOI] [PubMed] [Google Scholar]
- 38.Parkkinen L, Pirttila T, Alafuzoff I. Applicability of current staging/categorization of alpha-synuclein pathology and their clinical relevance. Acta Neuropathol. 2008;115:399–407. doi: 10.1007/s00401-008-0346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beach TG, et al. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117:613–34. doi: 10.1007/s00401-009-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mikolaenko I, et al. Alpha-synuclein lesions in normal aging, Parkinson disease, and Alzheimer disease: evidence from the Baltimore Longitudinal Study of Aging (BLSA) J Neuropathol Exp Neurol. 2005;64:156–62. doi: 10.1093/jnen/64.2.156. [DOI] [PubMed] [Google Scholar]
- 41.Parkkinen L, Kauppinen T, Pirttila T, Autere JM, Alafuzoff I. Alpha-synuclein pathology does not predict extrapyramidal symptoms or dementia. Ann Neurol. 2005;57:82–91. doi: 10.1002/ana.20321. [DOI] [PubMed] [Google Scholar]
- 42.Dickson DW, et al. Evidence that incidental Lewy body disease is pre-symptomatic Parkinson's disease. Acta Neuropathol. 2008;115:437–44. doi: 10.1007/s00401-008-0345-7. [DOI] [PubMed] [Google Scholar]
- 43.Saito Y, et al. Accumulation of phosphorylated alpha-synuclein in aging human brain. J Neuropathol Exp Neurol. 2003;62:644–54. doi: 10.1093/jnen/62.6.644. [DOI] [PubMed] [Google Scholar]
- 44.Wakabayashi K, et al. The Lewy body in Parkinson's disease and related neurodegenerative disorders. Mol Neurobiol. 2013;47:495–508. doi: 10.1007/s12035-012-8280-y. [DOI] [PubMed] [Google Scholar]
- 45.Milber JM, et al. Lewy pathology is not the first sign of degeneration in vulnerable neurons in Parkinson disease. Neurology. 2012;79:2307–2314. doi: 10.1212/WNL.0b013e318278fe32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parkkinen L, et al. Disentangling the relationship between Lewy bodies and nigral neuronal loss in Parkinson's disease. J Park Dis. 2011;1:277–286. doi: 10.3233/JPD-2011-11046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braak H, et al. Pathology associated with sporadic Parkinson's disease--where does it end? J Neural Transm Suppl. 2006:89–97. doi: 10.1007/978-3-211-45295-0_15. [DOI] [PubMed] [Google Scholar]
- 48.Hamilton RL. Lewy bodies in Alzheimer's disease: a neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol. 2000;10:378–84. doi: 10.1111/j.1750-3639.2000.tb00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lippa CF, et al. Lewy bodies contain altered alpha-synuclein in brains of many familial Alzheimer's disease patients with mutations in presenilin and amyloid precursor protein genes. Am J Pathol. 1998;153:1365–70. doi: 10.1016/s0002-9440(10)65722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iseki E. Dementia with Lewy bodies: reclassification of pathological subtypes and boundary with Parkinson's disease or Alzheimer's disease. Neuropathology. 2004;24:72–8. doi: 10.1111/j.1440-1789.2003.00530.x. [DOI] [PubMed] [Google Scholar]
- 51.Leverenz JB, et al. Empiric refinement of the pathologic assessment of Lewy-related pathology in the dementia patient. Brain Pathol. 2008;18:220–4. doi: 10.1111/j.1750-3639.2007.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jellinger KA. A critical reappraisal of current staging of Lewy-related pathology in human brain. Acta Neuropathol. 2008;116:1–16. doi: 10.1007/s00401-008-0406-y. [DOI] [PubMed] [Google Scholar]
- 53.Li JY, et al. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–3. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 54.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat Med. 2008;14:504–6. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 55.Kordower JH, Chu Y, Hauser RA, Olanow CW, Freeman TB. Transplanted dopaminergic neurons develop PD pathologic changes: a second case report. Mov Disord. 2008;23:2303–6. doi: 10.1002/mds.22369. [DOI] [PubMed] [Google Scholar]
- 56.Luk KC, et al. Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci U S A. 2009;106:20051–6. doi: 10.1073/pnas.0908005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Volpicelli-Daley LA, et al. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luk KC, et al. Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J Exp Med. 2012;209:975–86. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59•.Luk KC, et al. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in non-transgenic mice. Science. 2012;338(6109):949–953. doi: 10.1126/science.1227157. Landmark discovery of transmission of α-syn fibrils alone recapitulating human disease in wild type animals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee HJ, et al. Assembly-dependent endocytosis and clearance of extracellular alpha-synuclein. Int J Biochem Cell Biol. 2008;40:1835–49. doi: 10.1016/j.biocel.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 61.Desplats P, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106:13010–5. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mougenot AL, et al. Prion-like acceleration of a synucleinopathy in a transgenic mouse model. Neurobiol Aging. 2012;33:2225–8. doi: 10.1016/j.neurobiolaging.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 63.Masuda-Suzukake M, et al. Prion-like spreading of pathological alpha-synuclein in brain. Brain. 2013;136:1128–38. doi: 10.1093/brain/awt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steiner JA, Angot E, Brundin P. A deadly spread: cellular mechanisms of alpha-synuclein transfer. Cell Death Differ. 2011;18:1425–33. doi: 10.1038/cdd.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65•.Olanow CW, Brundin P. Parkinson's disease and alpha synuclein: is Parkinson's disease a prion-like disorder? Mov Disord. 2013;28:31–40. doi: 10.1002/mds.25373. Timely review of PD model transmission studies. [DOI] [PubMed] [Google Scholar]
- 66.Lee VM, Trojanowski JQ. Mechanisms of Parkinson's disease linked to pathological alpha-synuclein: new targets for drug discovery. Neuron. 2006;52:33–8. doi: 10.1016/j.neuron.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 67.Irwin DJ, et al. Evaluation of potential infectivity of Alzheimer's and Parkinson's disease proteins in recipients of cadaver-derived human growth hormone. JAMA Neurology. 2013;70(4):462–8. doi: 10.1001/jamaneurol.2013.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–44. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 69.Kordower JH, Brundin P. Propagation of host disease to grafted neurons: accumulating evidence. Exp Neurol. 2009;220:224–5. doi: 10.1016/j.expneurol.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 70.Brown P, et al. Human spongiform encephalopathy: the National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann Neurol. 1994;35:513–29. doi: 10.1002/ana.410350504. [DOI] [PubMed] [Google Scholar]
- 71.Brown P, Gajdusek DC, Gibbs CJ, Jr, Asher DM. Potential epidemic of Creutzfeldt-Jakob disease from human growth hormone therapy. The New England journal of medicine. 1985;313:728–731. doi: 10.1056/NEJM198509193131205. [DOI] [PubMed] [Google Scholar]
- 72.Hurtig HI, et al. Alpha-synuclein cortical Lewy bodies correlate with dementia in Parkinson's disease. Neurology. 2000;54:1916–21. doi: 10.1212/wnl.54.10.1916. [DOI] [PubMed] [Google Scholar]
- 73.Duda JE, Giasson BI, Mabon ME, Lee VM, Trojanowski JQ. Novel antibodies to synuclein show abundant striatal pathology in Lewy body diseases. Ann Neurol. 2002;52:205–10. doi: 10.1002/ana.10279. [DOI] [PubMed] [Google Scholar]
- 74.Apaydin H, Ahlskog JE, Parisi JE, Boeve BF, Dickson DW. Parkinson disease neuropathology: later-developing dementia and loss of the levodopa response. Arch Neurol. 2002;59:102–12. doi: 10.1001/archneur.59.1.102. [DOI] [PubMed] [Google Scholar]
- 75•.Irwin DJ, et al. Neuropathologic substrates of Parkinson disease dementia. Ann Neurol. 2012;72:587–98. doi: 10.1002/ana.23659. Large autopsy cohort with multivariate analysis of multiple clinical, genetic and neuropathological variables implicating Lewy body/neurite pathology as the most impactful in PDD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Compta Y, et al. Lewy- and Alzheimer-type pathologies in Parkinson's disease dementia: which is more important? Brain. 2011;134:1493–505. doi: 10.1093/brain/awr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsuboi Y, Uchikado H, Dickson DW. Neuropathology of Parkinson's disease dementia and dementia with Lewy bodies with reference to striatal pathology. Parkinsonism Relat Disord. 2007;13 Suppl 3:S221–4. doi: 10.1016/S1353-8020(08)70005-1. [DOI] [PubMed] [Google Scholar]
- 78.Jellinger KA, Attems J. Prevalence and impact of vascular and Alzheimer pathologies in Lewy body disease. Acta Neuropathol. 2008;115:427–36. doi: 10.1007/s00401-008-0347-5. [DOI] [PubMed] [Google Scholar]
- 79.Harding AJ, Halliday GM. Cortical Lewy body pathology in the diagnosis of dementia. Acta Neuropathol. 2001;102:355–63. doi: 10.1007/s004010100390. [DOI] [PubMed] [Google Scholar]
- 80.Kovari E, et al. Lewy body densities in the entorhinal and anterior cingulate cortex predict cognitive deficits in Parkinson's disease. Acta Neuropathol. 2003;106:83–8. doi: 10.1007/s00401-003-0705-2. [DOI] [PubMed] [Google Scholar]
- 81.Mattila PM, Rinne JO, Helenius H, Dickson DW, Roytta M. Alpha-synuclein-immunoreactive cortical Lewy bodies are associated with cognitive impairment in Parkinson's disease. Acta Neuropathol. 2000;100:285–90. doi: 10.1007/s004019900168. [DOI] [PubMed] [Google Scholar]
- 82.Pletnikova O, et al. Abeta deposition is associated with enhanced cortical alpha-synuclein lesions in Lewy body diseases. Neurobiol Aging. 2005;26:1183–92. doi: 10.1016/j.neurobiolaging.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 83.Ballard C, et al. Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology. 2006;67:1931–4. doi: 10.1212/01.wnl.0000249130.63615.cc. [DOI] [PubMed] [Google Scholar]
- 84.Perry EK, et al. Cholinergic correlates of cognitive impairment in Parkinson's disease: comparisons with Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1985;48:413–21. doi: 10.1136/jnnp.48.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Whitehouse PJ, Hedreen JC, White CL, 3rd, Price DL. Basal forebrain neurons in the dementia of Parkinson disease. Ann Neurol. 1983;13:243–8. doi: 10.1002/ana.410130304. [DOI] [PubMed] [Google Scholar]
- 86.Kalaitzakis ME, Graeber MB, Gentleman SM, Pearce RK. Striatal beta-amyloid deposition in Parkinson disease with dementia. J Neuropathol Exp Neurol. 2008;67:155–61. doi: 10.1097/NEN.0b013e31816362aa. [DOI] [PubMed] [Google Scholar]
- 87.Jellinger KA. Morphological substrates of parkinsonism with and without dementia: a retrospective clinico-pathological study. J Neural Transm Suppl. 2007:91–104. doi: 10.1007/978-3-211-73574-9_12. [DOI] [PubMed] [Google Scholar]
- 88.Jellinger KA, Seppi K, Wenning GK, Poewe W. Impact of coexistent Alzheimer pathology on the natural history of Parkinson's disease. J Neural Transm. 2002;109:329–39. doi: 10.1007/s007020200027. [DOI] [PubMed] [Google Scholar]
- 89.Kotzbauer PT, et al. Pathologic accumulation of alpha-synuclein and Abeta in Parkinson disease patients with dementia. Arch Neurol. 2012;69:1326–31. doi: 10.1001/archneurol.2012.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hughes AJ, Daniel SE, Blankson S, Lees AJ. A clinicopathologic study of 100 cases of Parkinson's disease. Arch Neurol. 1993;50:140–8. doi: 10.1001/archneur.1993.00540020018011. [DOI] [PubMed] [Google Scholar]
- 91.Sabbagh MN, et al. Parkinson disease with dementia: comparing patients with and without Alzheimer pathology. Alzheimer Dis Assoc Disord. 2009;23:295–7. doi: 10.1097/WAD.0b013e31819c5ef4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lashley T, et al. Cortical alpha-synuclein load is associated with amyloid-beta plaque burden in a subset of Parkinson's disease patients. Acta Neuropathol. 2008;115:417–25. doi: 10.1007/s00401-007-0336-0. [DOI] [PubMed] [Google Scholar]
- 93.Masliah E, et al. beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer's disease and Parkinson's disease. Proc Natl Acad Sci U S A. 2001;98:12245–50. doi: 10.1073/pnas.211412398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ, LaFerla FM. Synergistic Interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. J Neurosci. 2010;30:7281–9. doi: 10.1523/JNEUROSCI.0490-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Duda JE, et al. Concurrence of alpha-synuclein and tau brain pathology in the Contursi kindred. Acta Neuropathol. 2002;104:7–11. doi: 10.1007/s00401-002-0563-3. [DOI] [PubMed] [Google Scholar]
- 96.Lee VM, Giasson BI, Trojanowski JQ. More than just two peas in a pod: common amyloidogenic properties of tau and alpha-synuclein in neurodegenerative diseases. Trends Neurosci. 2004;27:129–34. doi: 10.1016/j.tins.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 97.Giasson BI, et al. Initiation and synergistic fibrillization of tau and alpha-synuclein. Science. 2003;300:636–40. doi: 10.1126/science.1082324. [DOI] [PubMed] [Google Scholar]
- 98.Jellinger KA, Wenning GK, Seppi K. Predictors of survival in dementia with lewy bodies and Parkinson dementia. Neurodegener Dis. 2007;4:428–30. doi: 10.1159/000107703. [DOI] [PubMed] [Google Scholar]
- 99.Sabbagh MN, et al. Correlation of clinical features with argyrophilic grains at autopsy. Alzheimer Dis Assoc Disord. 2009;23:229–33. doi: 10.1097/WAD.0b013e318199d833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nakashima-Yasuda H, et al. Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol. 2007;114:221–9. doi: 10.1007/s00401-007-0261-2. [DOI] [PubMed] [Google Scholar]
- 101.Colosimo C, Hughes AJ, Kilford L, Lees AJ. Lewy body cortical involvement may not always predict dementia in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2003;74:852–6. doi: 10.1136/jnnp.74.7.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Richard IH, Papka M, Rubio A, Kurlan R. Parkinson's disease and dementia with Lewy bodies: one disease or two? Mov Disord. 2002;17:1161–5. doi: 10.1002/mds.10274. [DOI] [PubMed] [Google Scholar]
- 103.Jellinger KA, Attems J. Does striatal pathology distinguish Parkinson disease with dementia and dementia with Lewy bodies? Acta Neuropathol. 2006;112:253–60. doi: 10.1007/s00401-006-0088-2. [DOI] [PubMed] [Google Scholar]
- 104.Halliday GM, Song YJ, Harding AJ. Striatal beta-amyloid in dementia with Lewy bodies but not Parkinson's disease. J Neural Transm. 2011;118:713–9. doi: 10.1007/s00702-011-0641-6. [DOI] [PubMed] [Google Scholar]
- 105.Merdes AR, et al. Influence of Alzheimer pathology on clinical diagnostic accuracy in dementia with Lewy bodies. Neurology. 2003;60:1586–90. doi: 10.1212/01.wnl.0000065889.42856.f2. [DOI] [PubMed] [Google Scholar]
- 106.Verghese J, Crystal HA, Dickson DW, Lipton RB. Validity of clinical criteria for the diagnosis of dementia with Lewy bodies. Neurology. 1999;53:1974–82. doi: 10.1212/wnl.53.9.1974. [DOI] [PubMed] [Google Scholar]
- 107.Litvan I, et al. Accuracy of the clinical diagnoses of Lewy body disease, Parkinson disease, and dementia with Lewy bodies: a clinicopathologic study. Arch Neurol. 1998;55:969–78. doi: 10.1001/archneur.55.7.969. [DOI] [PubMed] [Google Scholar]
- 108.Nelson PT, et al. Low sensitivity in clinical diagnoses of dementia with Lewy bodies. J Neurol. 2010;257:359–66. doi: 10.1007/s00415-009-5324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kraybill ML, et al. Cognitive differences in dementia patients with autopsy-verified AD, Lewy body pathology, or both. Neurology. 2005;64:2069–73. doi: 10.1212/01.WNL.0000165987.89198.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.van Rooden SM, et al. The identification of Parkinson's disease subtypes using cluster analysis: a systematic review. Mov Disord. 2010;25:969–78. doi: 10.1002/mds.23116. [DOI] [PubMed] [Google Scholar]
- 111.Selikhova M, et al. A clinico-pathological study of subtypes in Parkinson's disease. Brain. 2009;132:2947–57. doi: 10.1093/brain/awp234. [DOI] [PubMed] [Google Scholar]
- 112.Alves G, Larsen JP, Emre M, Wentzel-Larsen T, Aarsland D. Changes in motor subtype and risk for incident dementia in Parkinson's disease. Mov Disord. 2006;21:1123–30. doi: 10.1002/mds.20897. [DOI] [PubMed] [Google Scholar]
- 113.Prikrylova Vranova H, et al. CSF markers of neurodegeneration in Parkinson's disease. J Neural Transm. 2010;117:1177–81. doi: 10.1007/s00702-010-0462-z. [DOI] [PubMed] [Google Scholar]
- 114.Alves G, et al. Cerebrospinal fluid amyloid-beta and phenotypic heterogeneity in de novo Parkinson's disease. J Neurol Neurosurg Psychiatry. 2012;85(5):537–43. doi: 10.1136/jnnp-2012-303808. [DOI] [PubMed] [Google Scholar]
- 115.Kang JH, et al. Association of cerebrospinal fluid Aβ1-42, t-tau, p-tau181 and α-synuclein levels with clinical 1 features of early drug naïve Parkinson's disease patients. JAMA Neurology. 2013 doi: 10.1001/jamaneurol.2013.3861. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Muller ML, et al. beta-amyloid and postural instability and gait difficulty in Parkinson's disease at risk for dementia. Mov Disord. 2012;28(3):296–01. doi: 10.1002/mds.25213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Halliday GM, Holton JL, Revesz T, Dickson DW. Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathol. 2011;122:187–204. doi: 10.1007/s00401-011-0852-9. [DOI] [PubMed] [Google Scholar]
- 118.Svenningsson P, Westman E, Ballard C, Aarsland D. Cognitive impairment in patients with Parkinson's disease: diagnosis, biomarkers, and treatment. Lancet Neurol. 2012;11:697–707. doi: 10.1016/S1474-4422(12)70152-7. [DOI] [PubMed] [Google Scholar]
- 119.Sidransky E, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N Engl J Med. 2009;361:1651–61. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tsuang D, et al. GBA mutations increase risk for Lewy body disease with and without Alzheimer disease pathology. Neurology. 2012;79:1944–50. doi: 10.1212/WNL.0b013e3182735e9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Neumann J, et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson's disease. Brain. 2009;132:1783–94. doi: 10.1093/brain/awp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nalls MA, et al. A Multicenter Study of Glucocerebrosidase Mutations in Dementia With Lewy Bodies. JAMA Neurol. 2013:1–9. doi: 10.1001/jamaneurol.2013.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Clark LN, et al. Association of glucocerebrosidase mutations with dementia with lewy bodies. Arch Neurol. 2009;66:578–83. doi: 10.1001/archneurol.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Morley JF, et al. Genetic influences on cognitive decline in Parkinson's disease. Mov Disord. 2012;27:512–8. doi: 10.1002/mds.24946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tsuang D, Leverenz JB, Lopez O, et al. APOE ε4 Increases Risk for Dementia in Pure Synucleinopathies. JAMA Neurology. 2013;70(2):223–8. doi: 10.1001/jamaneurol.2013.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Williams-Gray CH, et al. Apolipoprotein E genotype as a risk factor for susceptibility to and dementia in Parkinson's disease. J Neurol. 2009;256:493–8. doi: 10.1007/s00415-009-0119-8. [DOI] [PubMed] [Google Scholar]
- 127.Wakabayashi K, et al. Apolipoprotein E epsilon4 allele and progression of cortical Lewy body pathology in Parkinson's disease. Acta Neuropathol. 1998;95:450–4. doi: 10.1007/s004010050824. [DOI] [PubMed] [Google Scholar]
- 128.Siderowf A, et al. CSF amyloid {beta} 1-42 predicts cognitive decline in Parkinson disease. Neurology. 2010;75:1055–61. doi: 10.1212/WNL.0b013e3181f39a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Baker M, et al. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum Mol Genet. 1999;8:711–5. doi: 10.1093/hmg/8.4.711. [DOI] [PubMed] [Google Scholar]
- 130.Zabetian CP, et al. Association analysis of MAPT H1 haplotype and subhaplotypes in Parkinson's disease. Ann Neurol. 2007;62:137–44. doi: 10.1002/ana.21157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Goris A, et al. Tau and alpha-synuclein in susceptibility to, and dementia in, Parkinson's disease. Ann Neurol. 2007;62:145–53. doi: 10.1002/ana.21192. [DOI] [PubMed] [Google Scholar]
- 132.Compta Y, et al. High cerebrospinal tau levels are associated with the rs242557 tau gene variant and low cerebrospinal beta-amyloid in Parkinson disease. Neurosci Lett. 2011;487:169–73. doi: 10.1016/j.neulet.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 133.Chen-Plotkin AS, et al. Plasma epidermal growth factor levels predict cognitive decline in Parkinson disease. Ann Neurol. 2011;69:655–63. doi: 10.1002/ana.22271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pellecchia MT, et al. Serum epidermal growth factor predicts cognitive functions in early, drug-naive Parkinson's disease patients. J Neurol. 2012;260(2):438–44. doi: 10.1007/s00415-012-6648-6. [DOI] [PubMed] [Google Scholar]
- 135.Compta Y, et al. Cerebrospinal tau, phospho-tau, and beta-amyloid and neuropsychological functions in Parkinson's disease. Mov Disord. 2009;24:2203–10. doi: 10.1002/mds.22594. [DOI] [PubMed] [Google Scholar]
- 136.Hall S, et al. Accuracy of a Panel of 5 Cerebrospinal Fluid Biomarkers in the Differential Diagnosis of Patients With Dementia and/or Parkinsonian Disorders. Arch Neurol. 2012:1–8. doi: 10.1001/archneurol.2012.1654. [DOI] [PubMed] [Google Scholar]
- 137.Alves G, et al. CSF amyloid-beta and tau proteins, and cognitive performance, in early and untreated Parkinson's disease: the Norwegian ParkWest study. J Neurol Neurosurg Psychiatry. 2010;81:1080–6. doi: 10.1136/jnnp.2009.199950. [DOI] [PubMed] [Google Scholar]
- 138.Montine TJ, et al. CSF Abeta(42) and tau in Parkinson's disease with cognitive impairment. Mov Disord. 2010;25:2682–5. doi: 10.1002/mds.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Leverenz JB, et al. Cerebrospinal fluid biomarkers and cognitive performance in non-demented patients with Parkinson's disease. Parkinsonism Relat Disord. 2011;17:61–4. doi: 10.1016/j.parkreldis.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mollenhauer B, et al. Direct quantification of CSF alpha-synuclein by ELISA and first cross-sectional study in patients with neurodegeneration. Exp Neurol. 2008;213:315–25. doi: 10.1016/j.expneurol.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 141.Wang Y, et al. Phosphorylated alpha-synuclein in Parkinson's disease. Sci Transl Med. 2012;4:121ra20. doi: 10.1126/scitranslmed.3002566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tokuda T, et al. Detection of elevated levels of alpha-synuclein oligomers in CSF from patients with Parkinson disease. Neurology. 2010;75:1766–72. doi: 10.1212/WNL.0b013e3181fd613b. [DOI] [PubMed] [Google Scholar]
- 143.Hong Z, et al. DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson's disease. Brain. 2010;133:713–26. doi: 10.1093/brain/awq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shi M, et al. Cerebrospinal fluid biomarkers for Parkinson disease diagnosis and progression. Ann Neurol. 2011;69:570–80. doi: 10.1002/ana.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Compta Y, et al. Grey matter volume correlates of cerebrospinal markers of Alzheimer-pathology in Parkinson's disease and related dementia. Parkinsonism Relat Disord. 2012;18:941–7. doi: 10.1016/j.parkreldis.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 146.Weintraub D, et al. Alzheimer's disease pattern of brain atrophy predicts cognitive decline in Parkinson's disease. Brain. 2011;135(Pt 1):170–80. doi: 10.1093/brain/awr277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gomperts SN, et al. Amyloid is linked to cognitive decline in patients with Parkinson disease without dementia. Neurology. 2013;80:85–91. doi: 10.1212/WNL.0b013e31827b1a07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Petrou M, et al. Abeta-amyloid deposition in patients with Parkinson disease at risk for development of dementia. Neurology. 2012;79:1161–7. doi: 10.1212/WNL.0b013e3182698d4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bohnen NI, et al. Cerebral glucose metabolic features of Parkinson disease and incident dementia: longitudinal study. J Nucl Med. 2011;52:848–55. doi: 10.2967/jnumed.111.089946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Beyer MK, et al. Cerebrospinal fluid Abeta levels correlate with structural brain changes in Parkinson's disease. Mov Disord. 2013;28:302–10. doi: 10.1002/mds.25282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Marek K, et al. The Parkinson Progression Marker Initiative (PPMI) Prog Neurobiol. 2011;95:629–35. doi: 10.1016/j.pneurobio.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rolinski M, Fox C, Maidment I, McShane R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson's disease dementia and cognitive impairment in Parkinson's disease. Cochrane Database Syst Rev. 2012;3:CD006504. doi: 10.1002/14651858.CD006504.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Seppi K, et al. The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the non-motor symptoms of Parkinson's disease. Mov Disord. 2011;26 Suppl 3:S42–80. doi: 10.1002/mds.23884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154•.Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of alpha-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci. 2013;14:38–48. doi: 10.1038/nrn3406. Recent review highlighting the pathophysiology of synuclein toxicity in PD and potential therapeutic startegies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Valera E, Masliah E. Immunotherapy for neurodegenerative diseases: Focus on alpha-synucleinopathies. Pharmacol Ther. 2013;138:311–22. doi: 10.1016/j.pharmthera.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sperling RA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Montine TJ, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]