Abstract
OBJECTIVE
Pulmonary disease represents an important extra-articular manifestation of rheumatoid arthritis (RA). While the association of RA and interstitial lung disease is widely acknowledged, obstructive lung disease (OLD) in RA is less well understood. We therefore aimed to assess incidence, risk factors and mortality of OLD in patients with RA.
METHODS
We examined a population-based incident cohort of patients with RA and a comparison cohort of individuals without RA. OLD was defined using a strict composite criterion. Cox-proportional hazards models were used to compare OLD incidence between the RA and comparator cohort, to investigate risk factors and to explore the impact of OLD on patient survival.
RESULTS
594 patients with RA and 596 subjects without RA were followed for a mean of 16.3 and 19.4 years, respectively. The lifetime risk of developing OLD was 9.6% for RA patients and 6.2% for subjects without RA; hazard ratio (HR) 1.54 (95% CI 1.01 to 2.34). The risk of developing OLD was higher among male patients, current or former smokers and for individuals with more severe RA. Survival of RA patients diagnosed with OLD was worse compared to those without OLD (HR 2.09, 95% CI 1.47 to 2.97).
CONCLUSION
Patients with RA are at higher risk of developing OLD, which is significantly associated with premature mortality. Effective diagnostic and therapeutic strategies to detect and manage OLD in patients with RA may help to improve survivorship in these patients.
Keywords: obstructive lung disease, rheumatoid arthritis, incidence, risk factors, mortality
INTRODUCTION
While the association of interstitial lung disease (ILD) and rheumatoid arthritis (RA) is widely acknowledged, the risk and impact of obstructive lung disease (OLD) in patients with RA is less clear. Studies exploring this potential association have so far relied on the prevalence of pulmonary obstruction in consecutive samples, focusing on differences in pulmonary function testing and CT morphology between patients with RA and comparator subjects. To date, no study has been performed evaluating the incidence of OLD in a population-based cohort.
A wide variety of pulmonary disorders can result in airway obstruction, with chronic obstructive pulmonary disease (COPD) and asthma as the two most common forms of OLD in the general population. Importantly, clinical symptoms of airway obstruction as well as an obstructive pattern on pulmonary function testing can also occur with bronchiolar involvement in ILD (constrictive or follicular bronchiolitis) and large airway disease (bronchiectasis). Case series have suggested that bronchiectasis and constrictive bronchiolitis may represent distinct extraarticular manifestations of RA (1). However, estimates based on patients with RA who are seen in academic centers carry the inherent risk of over - estimating various clinical endpoints, since extra-articular manifestations of RA are more frequently found in patients with more severe disease (2). In light of the significant mortality risk associated with extra-articular disease in RA (3), a clear definition of the individual subtypes of RA-associated co-morbidities would be an essential step towards improving patient care. We therefore aimed to investigate the incidence, risk factors and mortality of OLD in patients with RA in a population-based setting.
PATIENTS AND METHODS
The population of Rochester, Minnesota is well suited for investigation of long-term outcomes of patients with RA. A medical records linkage system, the Rochester Epidemiology Project, allows ready access to the complete records from all health care providers for the local population. The potential of this data system for population-based research has been previously described (4). This system ensures virtually complete clinical and vital status information on all clinically recognized cases of RA among Rochester residents.
Cohort of patients with RA
A population-based incidence cohort of all cases of RA, first diagnosed between January 1, 1955 and January 1, 1994, among Rochester, Minnesota residents ≥ 18 years of age was assembled as previously described (5–7). All cases fulfilled the 1987 American College of Rheumatology (ACR) classification criteria for RA (8). Incidence date was defined as the first date of fulfilment of four out of the seven ACR criteria. This RA incidence cohort consists of 603 subjects.
Comparison cohort of patients without RA
For each of the 603 subjects with RA, an individual without RA with similar birth year (± 3 years) and sex was randomly selected from the same source population. Each subject in this cohort was assigned an index date corresponding to the RA incidence date of the corresponding patient with RA.
Data collection
The data abstraction process has been described in detail (5–7). Briefly, all subjects were followed up longitudinally through their complete medical records beginning at age 18 years and continuing until death, migration from Rochester, or January 1, 2006. Comorbidities were abstracted as previously described (9)
Classification of obstructive lung disease
The criteria used for classification of OLD were based on consensus forming discussions between two rheumatologists (TB, ELM) and two pulmonologists (JHR, RV). OLD was defined as the presence of airflow obstruction based on at least one spirometry result with a FEV1/FVC ratio <0.7, consistent with ATS/ERS diagnostic criteria(10). In addition, patients classified as having OLD needed at least one documented physician diagnosis of airway or parenchymal lung disease such as COPD (incl. emphysema and/or chronic bronchitis), asthma, ILD, bronchiolitis or bronchiectasis. The medical records of all 1206 subjects were reviewed and pulmonary diagnoses, spirometry results and chest radiographic data were abstracted into number/identifier codes. Of note, final classification of a patient as having OLD was made after completion of the chart review: we applied a computer-based algorithm to the pulmonary function testing results and pulmonary diagnoses abstracted for every study subject: if a FEV1/FVC ratio <0.7 and a physician diagnosis of airway/parenchymal pulmonary disease were present, the study subject was classified as having OLD. For every subject with OLD, we applied additional criteria for sub-classification (table 1).
Table 1.
Obstructive lung disease (FEV1/FVC<0.7): definition of subtypes
| OLD subtype | Criterion |
|---|---|
| COPD | Physician’s diagnosis of COPD (chronic bronchitis or emphysema) OR chest radiograph or CT: diagnosis of emphysema OR airflow obstruction with <20% improvement of FEV1 with bronchodilator and no alternative explanation for the patient’s respiratory symptoms |
| Asthma | Physician’s diagnosis of asthma AND ≥20% improvement of FEV1 with bronchodilator |
| Bronchiectasis | Diagnosis of bronchiectasis by a pulmonologist based on clinical and radiologic data OR chest radiograph or CT: diagnosis of bronchiectasis according to radiologist |
| Obstructive bronchiolar disorders | Diagnosis of bronchiolitis obliterans (or obliterative/constrictive bronchiolitis) by pulmonologist OR CT diagnosis of bronchiolitis obliterans (or obliterative/constrictive bronchiolitis) by radiologist |
| ILD associated airflow obstruction | Physician’s diagnosis of ILD PLUS CT/chest radiograph consistent with ILD and/or 1 of the following criteria TLC≤80% of predicted Bronchoscopic or surgical lung biopsy results consistent with ILD |
OLD=obstructive lung disease; FEV1=forced expiratory volume; FVC=forced vital capacity; COPD = chronic obstructive pulmonary disease; CT= computed tomography; ILD=intersitial lung disease; TLC=total lung capacity
Cause of death: For all patients diagnosed with OLD who died during the period of follow-up, we determined the cause of death using death certificate data and hospitalization records. In the case of discrepancy between the death certificate diagnosis and physician notes/hospitalization records, the cause of death was determined by one of the physician reviewers (TB) based on chart review.
Statistical analysis
Descriptive statistics were used to summarize the data. Demographics were compared using two-sample t-tests and chi-square tests. Cumulative incidence of OLD was estimated adjusting for the competing risks of death. Cox proportional hazards models were used to compare the incidence of OLD in patients with RA versus individuals without RA, and to investigate possible associations of demographic and clinical variables with OLD. Time-dependent covariates were used to represent risk factors that could develop over time. Kaplan Meier methods were used to estimate survival. Cox proportional hazards models were used to compare the mortality in patients with RA and OLD to patients with OLD who did not have RA, and to compare survival of patients with RA and OLD to patients with RA who did not develop OLD. In this case, OLD was modeled using a time-dependent covariate to account for the development of OLD during follow-up. Cox-proportional hazard models were also used to evaluate the impact of risk factors on mortality following OLD in RA patients. Finally, we estimated the risk of mortality attributable to OLD in the RA cohort. Attributable risk is commonly calculated according to the equation AR = (P[D] − P[D, no F])/P(D), where AR is the attributable risk, P(D) is the probability of disease (i.e., death), and P(D, no F) is the conditional probability of disease among individuals without the risk factor. The cumulative incidence of death was used to estimate the probability of death, and these estimates were obtained from Cox models to allow for adjustment for age and sex. The conditional probability of death for those without OLD was estimated from the same Cox models, but with a target cohort that matched the observed cohort except for the fact that the subjects did not have OLD. The analyses were performed using SAS Version 9 (SAS Institute Inc., Cary, NC) and Splus (Insightful Corp., Seattle, WA) software.
RESULTS
Study subjects
The population-based cohort of patients with RA and the comparison cohort comprised 603 patients each. After exclusion of 9 patients with RA and 7 without RA who developed OLD prior to RA incidence/index diagnosed, 594 patients with incident RA and 596 comparison subjects were used for analysis. The mean age at RA incidence/index date was 58 years. The mean duration of follow-up was 16.3 years for RA patients and 19.4 years for subjects without RA. 73% of patients were female. Smoking was more common among RA patients: 28.5% of RA patients were current smokers and 23.6% were former smokers compared to 23.8% current and 19.6% former smokers in the non-RA comparator group (p<0.01). The number of individuals who did undergo pulmonary function testing or chest imaging at any point during follow-up was similar between RA and non-RA subjects. There were also no statistically significant differences among patients with and without RA regarding alcohol consumption, diabetes, obesity and coronary heart disease (table 2).
Table 2.
Demographic and clinical characteristics
| Patients with RA (N=594) |
Subjects without RA (N=596) |
P value | |
|---|---|---|---|
| Age at RA diagnosis/index date, mean ± SD | 57.8 ± 15.2 | 58.2 ± 15.3 | 0.71 |
| Years of follow up, mean ± SD | 16.3 ± 10.5 | 19.4 ± 11.1 | -- |
| Female (n, %) | 435 (73.2%) | 438 (73.5%) | 0.92 |
| Smoking status | 0.01* | ||
| Current smoker (n,%) | 169 (28.5%) | 142 (23.8%) | |
| Former smoker (n,%) | 140 (23.6%) | 117 (19.6%) | |
| Pulmonary function available (n,%)* | 114 (19.2%) | 97 (16.3%) | 0.18 |
| Chest x-ray available (n,%)* | 585 (98.5%) | 582 (97.7%) | 0.40 |
| Comorbidities (n,%) | |||
| Alcoholism | |||
| baseline | 12 (2.0%) | 17 (3.0%) | 0.28 |
| ever | 41 (6.9%) | 26 (4.6%) | -- |
| Diabetes | |||
| baseline | 43 (7.2%) | 40 (6.7%) | 0.72 |
| ever | 111 (18.7%) | 143 (24.0%) | -- |
| Obesity | |||
| baseline | 71 (12.8%) | 65 (12.2%) | 0.75 |
| ever | 136 (22.9%) | 140 (23.5%) | -- |
| Heart disease† | |||
| baseline | 76 (12.8%) | 72 (12.1%) | 0.71 |
| ever | 281 (47.3%) | 233 (47.3%) | -- |
multigroup comparison never vers. current vers. former smoker;
ever preformed until last date of follow up;
angina pectoris, coronary artery disease, coronary insufficiency, ischemic heart disease, myocardial infarction, heart failure, pulmonary edema and coronary revascularization procedures. SD: standard deviation; N: number of subjects; %: percentage
Incidence of OLD in patients with RA
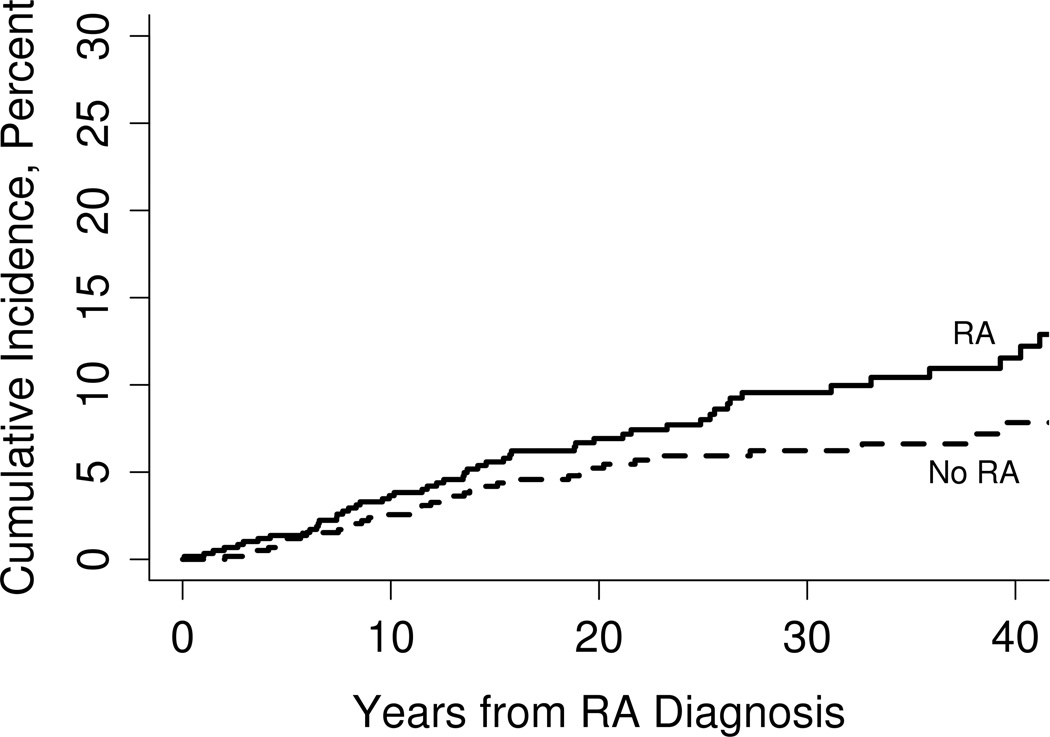
During follow-up, 52 RA patients and 40 subjects without RA met the OLD classification criterion. The 10-, 20- and 30-year cumulative incidence rates for OLD (adjusted for the competing risk of death) were 3.7%, 6.9% and 9.6%, respectively. Among comparison subjects, the 10-, 20- and 30-year cumulative incidence rates were 2.6%, 5.2% and 6.2%, respectively (figure 1). The risk of developing OLD in patients with RA was significantly higher than in subjects without RA (hazard ratio (HR): 1.54; 95% confidence interval (CI): 1.01 to 2.34 after adjusting for age, sex, smoking and alcoholism. The risk of developing OLD in patients with RA who never smoked was higher than in subjects without RA who never smoked, but this difference did not reach statistical significance (HR: 1.98; 95% CI: 0.73 to 5.40 after adjusting for age and sex.
Figure 1.
Incidence of obstructive lung disease in patients with rheumatoid arthritis (RA; continued line) and subjects without rheumatoid arthritis (dotted line)
Subtypes of OLD in patients with and without RA
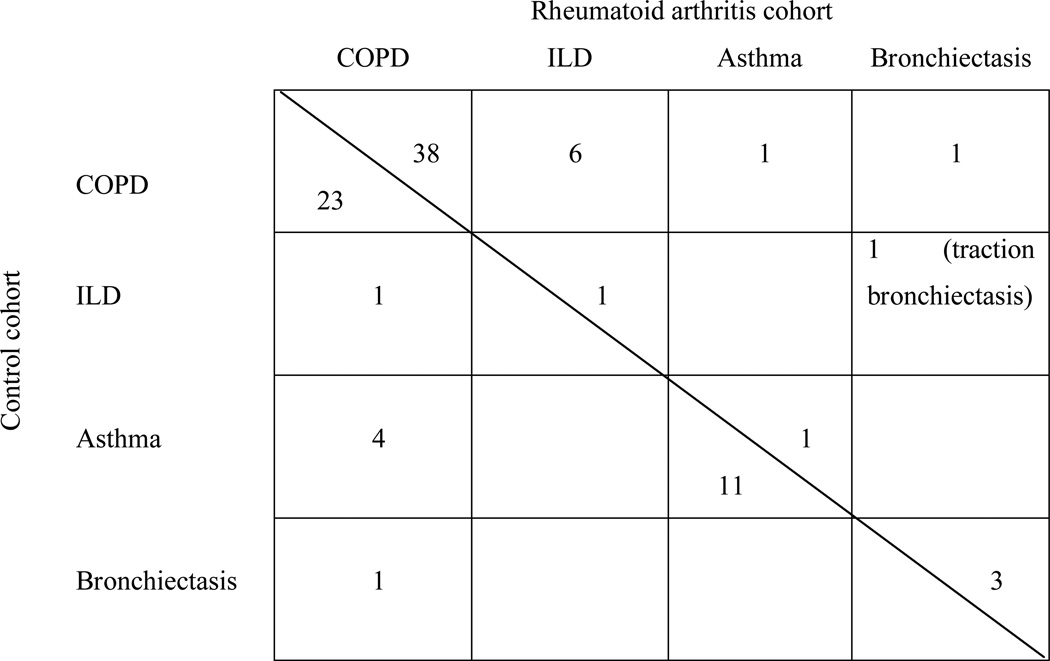
COPD was the most common subtype in subjects with OLD. COPD and ILD were much more common in patients with RA compared to subjects in the non-RA cohort. Conversely, asthma was diagnosed more frequently among subjects without RA than in patients with RA. A detailed depiction of OLD subtypes is given in table 3. Although the low number of patients with pulmonary disease who never smoked precluded any meaningful statistical analysis, we stratified OLD subtypes by “never”- versus “ever” smoking status: among the 9 RA patients with OLD who never smoked, 5 were diagnosed with COPD, 3 with bronchiectasis and 1 subject was affected by asthma. Conversely, the most common diagnosis among the 7 non-RA subjects who never smoked was asthma (5 individuals), followed by asthma COPD overlap and bronchiectasis in one patient each.
Table 3.
Types of lung disease (including overlap of two different pathologies) underlying airway obstruction in rheumatoid arthritis and non-rheumatoid arthritis patients. Patients with Rheumatoid arthritis are displayed in the upper right, control patients in the lower left part of the table. By comparing row and column label, the reader can identify the various forms of OLD overlap in both cohorts. (Example: 6 patients with RA were affected by ILD and COPD overlap; 11 patients in the non-RA cohort did meet criteria for a diagnosis of asthma without any overlap with other types of OLD)
 |
Demographic and clinical characteristics of subjects with OLD
Patients with RA were younger at the time of OLD diagnosis compared with subjects without RA. The characteristics of OLD in RA and non-RA patients are presented in table 4. Abnormalities detected with chest imaging were more frequent in the RA group compared with non-RA patients. The minimum FEV1 had a lower mean value among the patients with RA (mean ±SD: 1.30 ± 0.73) compared to subjects without RA (mean ±SD: 1.45 ± 0.56), indicating more severe obstruction in patients with RA. However, this difference was not statistically significant.
Table 4.
Characteristics of obstructive lung disease in patients with RA and non-RA patients
| RA (52 patients) | Non-RA (40 patients) | |
|---|---|---|
| Mean ± SD age at RA diagnosis, years | 54.0 ± 13.2 | 57.5 ± 14.1 |
| Mean ± SD age at OLD diagnosis, years | 69.2 ± 12.9 | 74.4 ± 10.4 |
| Men (n, %) | 23 (44.2) | 16 (40.0) |
| Current smoker (n, %) | 31 (59.6) | 21 (52.5) |
| Former smoker (n, %) | 12 (23.1) | 12 (30.0) |
| X-ray or CT abnormal (at any time) | ||
| Emphysema (n,%) | 27 (51.9) | 14 (35.0) |
| Bronchiectasis (n,%) | 5 (9.6) | 1 (2.5.) |
| Interstitial lung disease (n, %) | 7 (13.5) | 1 (2.5.) |
| Minimum FEV1, L (at any time) | ||
| Mean ± SD | 1.30 ± 0.73 | 1.45 ± 0.56 |
| 0.37 to <0.84 (n, %) | 17 (32.7%) | 6 (15.0%) |
| 0.84 to <1.29 (n, %) | 13 (25.0%) | 10 (25.0%) |
| 1.29 to <1.85 (n, %) | 11 (21.2%) | 12 (30.0%) |
| 1.85 to <3.41 (n, %) | 11 (21.2%) | 12 (30.0%) |
SD=standard deviation; RA=rheumatoid arthritis; OLD=obstructive lung disease; CT=computer tomography; FEV1=forced expiratory volume; FVC=forced vital capacity
Risk factors for OLD in patients with RA
The incidence of RA-associated OLD was higher in men (HR 2.43; 95% CI: 1.40, 4.21) and among RA patients who were active or past smokers (HR 4.38; 95% CI: 2.14, 8.99), table 5. Other risk factors which had a statistically significant association with OLD were related to disease severity including rheumatoid factor, elevated erythrocyte sedimentation rate (ESR) levels, corticosteroid and disease-modifying antirheumatic drug (DMARD) use. Other features of disease severity (extraarticular disease, large joint swelling, and decreased functional status) all had HRs above 1.5, albeit statistically not significant (table 5).
Table 5.
Risk factors for obstructive lung disease in patients with rheumatoid arthritis (RA)*
| Risk Factor | RA patients without OLD (N=542) |
RA patients with OLD (N=52) |
Hazard Ratio† |
95% CI† |
|---|---|---|---|---|
| Age at RA (per 10 year increase) | 58.2 ± 15.4 | 54.0 ± 13.2 | 1.20 | 0.96, 1.48 |
| Sex (male) | 136 (25.1) | 23 (44.2) | 2.43 | 1.40, 4.21 |
| Smoking (ever) | 266 (49.1) | 43 (82.7) | 4.38 | 2.14, 8.99 |
| Extraarticular disease (ever)** | 84 (15.5) | 8 (15.4) | 1.84 | 0.86, 3.95 |
| Rheumatoid factor (ever) | 341 (66.3) | 42 (82.4) | 2.93 | 1.41, 6.10 |
| Joint erosions on radiographs (ever) | 85 (17.7) | 6 (12.2) | 0.76 | 0.32, 1.81 |
| Erosions or destructive changes (ever) | 273 (57.0) | 26 (53.1) | 0.95 | 0.53, 1.70 |
| ESR - 3 values ≥ 60 (ever) | 157 (29.0) | 15 (28.8) | 1.86 | 1.01, 3.43 |
| Large joint swelling (ever) | 450 (83.0) | 46 (88.5) | 2.13 | 0.90, 5.07 |
| Rheumatoid nodules (ever) | 169 (31.2) | 17 (32.7) | 1.36 | 0.75, 2.45 |
| DMARD use (ever) | 303 (55.9) | 34 (65.4) | 2.01 | 1.10, 3.64 |
| Methotrexate use (ever) | 121 (22.3) | 10 (19.2) | 1.18 | 0.58, 2.41 |
| Steroid use (ever) | 281 (51.8) | 31 (59.6) | 2.50 | 1.43, 4.40 |
| Functional capacity†† = 1+2 | 431 (80.1) | 37 (74.0) | 1.71 (3,4 vers. 1,2) | 0.90, 3.24 |
| Functional capacity†† = 3+4 | 107 (19.9) | 13 (26.0) |
Except where indicated otherwise, values are the number (%) of patients.
Felty’s syndrome, pleuritis, pericarditis, rheumatoid myocarditis, rheumatoid vasculitis.
Models in rows 4–15 are adjusted for age at RA, sex, and ever smoker.
According to Steinbrocker index; OLD = obstructive lung disease; RA = rheumatoid arthritis; CI = confidence interval; ESR = erythrocyte sedimentation rate; DMARD = disease-modifying antirheumatic drug
Mortality associated with OLD in patients with RA
Kaplan-Meier estimates of survival following diagnosis of OLD were similar for RA patients and non-RA subjects. The 5-year survival rate was 59% among RA patients and 64% among non RA patients. The difference in mortality risk between patients with RA and OLD and subjects without RA and OLD was not statistically significant after adjustment for age, sex, smoking status and alcoholism (HR 1.81; 95% CI 1.00 to 3.28), p-value 0.052.
We also compared the survival experience of RA patients with OLD to RA patients who did not develop OLD. The development of OLD among patients with RA was significantly associated with worse survival compared to RA patients without OLD after adjustment for age, sex, smoking status and alcoholism (HR 2.09; 95% CI 1.47 to 2.97).
Among RA patients with OLD, a higher mortality risk was significantly associated with age at OLD onset (per 10 year increase in age, increase in HR 1.94; 95% CI: 1.32, 2.84) and a low functional capacity (level 3–4 versus level 1–2 adjusted for age, sex, and smoking status: HR 3.16; 95% CI: 1.43, 6.99).
Cause of death
Among the 52 patients with RA and OLD, 21 (48%) died of pulmonary causes. “Respiratory failure secondary to COPD” was the most common death diagnosis in 12 of these patients, followed by “respiratory infection (pneumonia)” in 7 individuals, and cor pulmonale and pneumothorax in 1 patient each.
Conversely, only 5 subjects (12.5%) among the 40 subjects in the control cohort died of pulmonary causes. “Respiratory failure secondary to COPD” was the death diagnosis in 3 subjects, while 2 patients succumbed to metastatic lung cancer.
Excess mortality of patients with RA attributable to OLD
The cumulative incidence of death was 71.8% in patients with RA at 30 years after RA incidence date, compared to 60.2% in patients without RA. Based on this, the RA cohort has an excess mortality of 12.6% at 30 years after RA incidence.
When reducing the rate of OLD in RA patients to the rate of OLD in the non-RA cohort, the cumulative mortality of RA patients dropped by 0.8%. Thus, the excess deaths in RA would be reduced by 6.3% (0.8% / 12.6%), if the risk of OLD in RA was the same as in non-RA subjects.
DISCUSSION
The results of our population-based study indicate an increased risk for OLD in patients with RA. Furthermore, we provide evidence that airway obstruction is associated with the higher mortality of patients with RA.
Our population-based approach does offer the advantage of a comprehensive assessment of all patients diagnosed with RA in a defined geographic area, thereby minimizing the risk of referral bias. We utilized an acknowledged, objective criterion for pulmonary obstruction based on pulmonary function testing, in order to avoid misclassification which can occur when relying on ICD-codes or claims data (11).
Although we were unable to identify previously published data on the incidence of OLD in RA, several studies have explored the prevalence of OLD among RA patients in consecutive samples or claims-based databases. In a 1979 study (12), the prevalence of “airways obstruction” in patients with RA was 24% versus 3% in control subjects. This number is more than twice as high as the 30-year cumulative incidence in our study. In that study, the definition of airway obstruction differed significantly from our classification criterion: a FEV1/FVC ratio <84% of predicted was used as a criterion for presence of disease. An even higher prevalence of OLD in patients with RA was reported by other authors who found that 18 (41%) of 43 patients with RA had a FEV1/FVC<0.7 (13). Several distinctive features of this cohort have to be taken into account: 77% of subjects were smokers, patients had very severe RA requiring hospitalization (mean “total joint count” 34, mean ESR 40mm/1h) and an unusually high proportion of male study subjects (50%). Such a cohort is unlikely to be representative of the full spectrum of RA in the population.
In a more recent study, 100 patients with RA were investigated for OLD (14). The diagnosis of respiratory disorders was based on clinical, radiological and spirometry findings. OLD affected 11% of patients with RA. Although the estimate is much closer to our 30-year cumulative incidence, the complete absence of any OLD in the control cohort of this trial appears unusual, given that approximately 10% of the US population is affected by COPD (15).
In general, the differing estimates of OLD in patients with RA among studies have to be interpreted in the context of variable disease definitions, different study population and the varying extent of pulmonary function and imaging based testing applied.
What could be the explanation for the observed association of RA and OLD? Considering the role of smoking as an important risk factor for both rheumatoid arthritis and COPD, it appears possible that the observed association between RA and OLD is non-causal and merely the result of confounding. This could even be the case when adjusting for crude categories such as never smoker, current smoker and previous smoker, since this categorization would not take into account the individual “dose” of smoking. Because smoking is a risk factor for RA (16), it is possible that cohorts of RA patients could include heavier smokers than persons of similar age and gender without RA, thereby also increasing the likelihood of COPD in these patients. Adjusting for broad categories of smoking behavior would not adjust for this difference. However, when limiting our analysis to non-smokers, pulmonary obstruction was still twice as likely among RA patients when compared to non-RA subjects (although the low number of non-smokers did not allow a statistically meaningful analysis), arguing in favor of a true casual association.
So far as is known to date, the risk genes identified for RA and COPD do not appear to overlap. While many of the risk genes for COPD are positioned in pathways of oxidative stress response (17), RA risk genes are pointing towards pathways of T-cell activation and antigen presentation (18). Thus, there is currently no evidence that the observed association would be due to common risk genes that are shared between both diseases. It has been speculated that pulmonary injury may predispose patients to development of RA (19, 20). However, a recent population-based study did not reveal a higher risk of RA in patients with established COPD (21).
The three subtypes of OLD that were more frequently observed in patients with RA as compared to non-RA subjects were COPD, bronchiectasis and ILD-associated obstruction. For the idiopathic forms of those 3 pulmonary diseases (which may have a different underlying pathophysiology than their RA-associated counterparts), autoimmunity and activation of various inflammatory pathways are thought to play an important role. Although still controversial, it has been suggested that COPD represents an autoimmune disease (22). T-cells generated under cigarette smoke exposure promote the development of an emphysema-like phenotype in mice and transfer of these T-cells into Rag-2-deficient mice could induce this phenotype independent of smoke exposure (23).
Similarly, autoantibody and T-cell responses to several auto-antigens expressed in pulmonary tissue have been described in patients with ILD (24, 25). In patients with bronchiectasis, recent findings support a predisposing role for innate immune mechanisms (26) and the association with certain HLA class II haplotypes does implicate involvement of the adaptive immune response (27).
Considering the similarities between pathogenic mechanisms in these various pulmonary diseases and the pathophysiology of RA, it can be hypothesized that RA-related immune dysfunction may also predispose to abnormal inflammatory responses in extra-articular tissues.
There are some shortcomings to our retrospective study approach. Because of the inherent limitations of a chart review based diagnosis, it is likely that our estimates do not reflect the full extent of OLD in this population. Tests for the detection of lung disease (including radiographs, CT, pulmonary function testing) were not systematically used for each patient, and the lack of some information due to time trend effects (for example, CT became available in the 1970’s), may lead to an underestimation of the true incidence.
Additionally, patients with RA have more frequent physician visits than the average patient in the population, and physicians may be more attuned to the possibility of lung disease in RA patients than healthy people, leading to an overestimation of lung involvement in subjects with RA compared to those without RA (surveillance bias). However, the overall number of chest X-rays and pulmonary function tests that were performed during the follow-up period was balanced between RA and non-RA subjects.
In summary, we have shown that OLD is more common in patients with RA compared to subjects without RA. Similar to what has been observed with other extra-articular manifestations of RA (3), OLD increases the mortality risk of affected patients. When considering recent data on the incidence and mortality of RA-associated ILD (28), it becomes apparent that pulmonary disease does represent a major contributor to the overall burden of disease and the risk of premature death in patients with RA. Of note, we cannot exclude that the association of OLD with premature mortality in patients with RA is affected by confounders such as high RA disease activity, use of more potent DMARDs and associated adverse events or additional extraarticular diseases, which were found to be more common among patients with RA and OLD. However, the high proportion of pulmonary death diagnoses among RA patients with OLD (48%) is pointing towards a causal relationship between obstructive pulmonary disease and premature death in RA patients. Whether patients with RA should be screened for pulmonary disease in a preclinical stage and if detection of subclinical pulmonary pathologies should have any impact on the choice of RA treatment is yet unclear. Future studies will have to clarify how and when OLD in patients with RA should be addressed, and how early effective therapies directed towards joint disease may modify the subsequent risk of developing pulmonary manifestations.
SIGNIFICANCE AND INNOVATION.
Obstructive lung disease is more common in patients with rheumatoid arthritis (RA) compared to individuals without RA
Obstructive lung disease does contribute to the excess mortality in patients with rheumatoid arthritis
Acknowledgments
Grant support: NIH grant UL1-RR-024150, R01-AR-30582 and R01-AR-46849
References
- 1.Tsuchiya Y, Takayanagi N, Sugiura H, Miyahara Y, Tokunaga D, Kawabata Y, et al. Lung diseases directly associated with rheumatoid arthritis and their relationship to outcome. Eur Respir J. 2011;37(6):1411–1417. doi: 10.1183/09031936.00019210. [DOI] [PubMed] [Google Scholar]
- 2.Turesson C, O'Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Extra-articular disease manifestations in rheumatoid arthritis: incidence trends and risk factors over 46 years. Annals of the rheumatic diseases. 2003;62(8):722–727. doi: 10.1136/ard.62.8.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turesson C, O'Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Occurrence of extraarticular disease manifestations is associated with excess mortality in a community based cohort of patients with rheumatoid arthritis. The Journal of rheumatology. 2002;29(1):62–67. [PubMed] [Google Scholar]
- 4.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clinic proceedings. 1996;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 5.Doran MF, Pond GR, Crowson CS, O'Fallon WM, Gabriel SE. Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty-year period. Arthritis and rheumatism. 2002;46(3):625–631. doi: 10.1002/art.509. [DOI] [PubMed] [Google Scholar]
- 6.Gabriel SE, Crowson CS, Kremers HM, Doran MF, Turesson C, O'Fallon WM, et al. Survival in rheumatoid arthritis: a population-based analysis of trends over 40 years. Arthritis and rheumatism. 2003;48(1):54–58. doi: 10.1002/art.10705. [DOI] [PubMed] [Google Scholar]
- 7.Gabriel SE, Crowson CS, O'Fallon WM. The epidemiology of rheumatoid arthritis in Rochester, Minnesota, 1955–1985. Arthritis and rheumatism. 1999;42(3):415–420. doi: 10.1002/1529-0131(199904)42:3<415::AID-ANR4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 8.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis and rheumatism. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 9.Myasoedova E, Crowson CS, Nicola PJ, Maradit-Kremers H, Davis JM, 3rd, Roger VL, et al. The influence of rheumatoid arthritis disease characteristics on heart failure. The Journal of rheumatology. 2011;38(8):1601–1606. doi: 10.3899/jrheum.100979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 11.Cooke CR, Joo MJ, Anderson SM, Lee TA, Udris EM, Johnson E, et al. The validity of using ICD-9 codes and pharmacy records to identify patients with chronic obstructive pulmonary disease. BMC Health Serv Res. 2011;11:37. doi: 10.1186/1472-6963-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geddes DM, Webley M, Emerson PA. Airways obstruction in rheumatoid arthritis. Annals of the rheumatic diseases. 1979;38(3):222–225. doi: 10.1136/ard.38.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins RL, Turner RA, Johnson AM, Whitley NO, McLean RL. Obstructive pulmonary disease in rheumatoid arthritis. Arthritis and rheumatism. 1976;19(3):623–628. doi: 10.1002/art.1780190316. [DOI] [PubMed] [Google Scholar]
- 14.Vergnenegre A, Pugnere N, Antonini MT, Arnaud M, Melloni B, Treves R, et al. Airway obstruction and rheumatoid arthritis. Eur Respir J. 1997;10(5):1072–1078. doi: 10.1183/09031936.97.10051072. [DOI] [PubMed] [Google Scholar]
- 15.Halbert RJ, Isonaka S, George D, Iqbal A. Interpreting COPD prevalence estimates: what is the true burden of disease? Chest. 2003;123(5):1684–1692. doi: 10.1378/chest.123.5.1684. [DOI] [PubMed] [Google Scholar]
- 16.Albano SA, Santana-Sahagun E, Weisman MH. Cigarette smoking and rheumatoid arthritis. Semin Arthritis Rheum. 2001;31(3):146–159. doi: 10.1053/sarh.2001.27719. [DOI] [PubMed] [Google Scholar]
- 17.Hancock DB, Eijgelsheim M, Wilk JB, Gharib SA, Loehr LR, Marciante KD, et al. Meta-analyses of genome-wide association studies identify multiple loci associated with pulmonary function. Nat Genet. 2010;42(1):45–52. doi: 10.1038/ng.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stahl EA, Raychaudhuri S, Remmers EF, Xie G, Eyre S, Thomson BP, et al. Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat Genet. 2010;42(6):508–514. doi: 10.1038/ng.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demoruelle MK, Weisman MH, Simonian PL, Lynch DA, Sachs PB, Pedraza IF, et al. Brief report: airways abnormalities and rheumatoid arthritis-related autoantibodies in subjects without arthritis: early injury or initiating site of autoimmunity? Arthritis and rheumatism. 2012;64(6):1756–1761. doi: 10.1002/art.34344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klareskog L, Stolt P, Lundberg K, Kallberg H, Bengtsson C, Grunewald J, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis and rheumatism. 2006;54(1):38–46. doi: 10.1002/art.21575. [DOI] [PubMed] [Google Scholar]
- 21.Bergstrom U, Jacobsson LT, Nilsson JA, Berglund G, Turesson C. Pulmonary dysfunction, smoking, socioeconomic status and the risk of developing rheumatoid arthritis. Rheumatology (Oxford) 2011;50(11):2005–2013. doi: 10.1093/rheumatology/ker258. [DOI] [PubMed] [Google Scholar]
- 22.Agusti A, MacNee W, Donaldson K, Cosio M. Hypothesis: does COPD have an autoimmune component? Thorax. 2003;58(10):832–834. doi: 10.1136/thorax.58.10.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motz GT, Eppert BL, Wesselkamper SC, Flury JL, Borchers MT. Chronic cigarette smoke exposure generates pathogenic T cells capable of driving COPD-like disease in Rag2−/− mice. Am J Respir Crit Care Med. 2010;181(11):1223–1233. doi: 10.1164/rccm.200910-1485OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurosu K, Takiguchi Y, Okada O, Yumoto N, Sakao S, Tada Y, et al. Identification of annexin 1 as a novel autoantigen in acute exacerbation of idiopathic pulmonary fibrosis. J Immunol. 2008;181(1):756–767. doi: 10.4049/jimmunol.181.1.756. [DOI] [PubMed] [Google Scholar]
- 25.Yang Y, Fujita J, Bandoh S, Ohtsuki Y, Yamadori I, Yoshinouchi T, et al. Detection of antivimentin antibody in sera of patients with idiopathic pulmonary fibrosis and non-specific interstitial pneumonia. Clin Exp Immunol. 2002;128(1):169–174. doi: 10.1046/j.1365-2249.2002.01811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyton RJ. Regulation of immunity in bronchiectasis. Med Mycol. 2009;47(Suppl 1):S175–S182. doi: 10.1080/13693780802163370. [DOI] [PubMed] [Google Scholar]
- 27.Boyton RJ, Smith J, Jones M, Reynolds C, Ozerovitch L, Chaudhry A, et al. Human leucocyte antigen class II association in idiopathic bronchiectasis, a disease of chronic lung infection, implicates a role for adaptive immunity. Clin Exp Immunol. 2008;152(1):95–101. doi: 10.1111/j.1365-2249.2008.03596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bongartz T, Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2010;62(6):1583–1591. doi: 10.1002/art.27405. [DOI] [PMC free article] [PubMed] [Google Scholar]