A short history of how we got here
The adaptive immune system endows vertebrates with the ability to identify and target pathogens for elimination during primary infections and to form memory cells that provide accelerated responses and/or protection upon subsequent encounters with that pathogen. One of the pillars of modern immunology is the clonal selection theory first put forward by Burnet (1), which posits that there exists in the body a vast array of lymphocytes expressing distinct receptors that are present before an infection, and those rare lymphocytes that can specifically interact with a pathogen are recruited from this ‘pre-immune’ repertoire to participate in the anti-pathogen response. An attractive feature of this theory was (and is) that it provides a Darwinistic explanation for the adaptive immune system; it allows lymphocytes to randomly generate receptors with the potential to recognize and distinguish between an unknown universe of foreign pathogens, and relies on selection processes to identify and expand those that have the appropriate specificities. However, it is implicit in this model that an immune repertoire with sufficient diversity to anticipate an infinite assortment of foreign antigens would also generate receptors that could react with the immense collection of self-antigens derived from the organism that the immune system occupies. Based on theoretical grounds, then, it was proposed that one or more mechanisms must exist to modify this pre-immune repertoire in such a way as to prevent self-reactivity and achieve a state of immunological self-tolerance. The most prominent of these potential mechanisms included the possibilities that lymphocytes bearing autoreactive receptors could be physically eliminated during development (generally termed deletion), that they might be inactivated because of a failure to receive appropriate secondary signals (generally termed anergy induction), or that they might be subjected to active regulation either through the formation of interactive networks or via the production of specialized ‘suppressor’ cells. For many years, experimental analyses of these proposed mechanisms were made difficult by the sheer complexity of the immune system. However, in the 1970s and 1980s, a number of technologies became available that allowed the processes underlying clonal selection to be understood in more detail. Although there were many important developments, principal among the new technologies was the advent of molecular biology, which allowed the discovery of the mechanisms by which gene rearrangement gives rise to the vast diversity of T-cell receptors (TCRs) and immunoglobulins that are used by T and B cells to recognize antigens (2). The development of flow cytometry allowed the quantitative analysis and purification of large numbers of lymphocytes; coupled with the ability to introduce (or eliminate) genes from the mouse genome (including genes that encode TCRs or immunoglobulins), it became possible to demonstrate that lymphocytes that could react with self-antigens could indeed be either eliminated during their development (deletion) or forced into an inactive state (anergy) (3).
The idea that suppressor cells might play a role in regulating autoreactive lymphocytes lost acceptance during this same time period. A wide variety of studies had resulted in descriptions of phenomena that were consistent with the existence of suppressor cells, which had been studied in large part because of the possibility that such cells, if they could be isolated, might be used to manipulate the immune system under adverse conditions such as autoimmunity or allergy. However, molecular biology failed to provide evidence to support the existence of these cells, and during the 1980s, it was discovered that CD4+ T cells could differentiate into distinct phenotypes [e.g. T-helper 1 (Th1) versus Th2 cells], and that these differentiated CD4+ T-cell subsets could crossregulate one another (4). This discovery provided a convincing alternative explanation for many of the phenomena that had previously been interpreted as reflecting immune suppression. For example, in studies in which investigators used delayed type hypersensitivity (which is now known to be a classical Th1-type response) to measure an immune response, simultaneous manipulations to induce a Th2-type response could suppress the development of the original Th1 response. These observations gave rise the idea of immune deviation, and these processes of counter-regulation by different T-cell subsets are likely very important in regulating both the magnitude and the quality of the immune responses that develop in a variety of settings. However, this concept of immune deviation and counter-regulation is distinct from the theory that specialized cell types and processes may exist by which the immune system actively regulates lymphocytes expressing receptors with the potential to react with the host’s own cells and tissues.
The current renaissance of regulatory T cells owes much to three sets of studies that occurred during the period when a general consensus existed in the immunological community that suppression was perhaps largely an experimental phenomenon. Thus, Sakaguchi’s group (5) showed that a day 3 thymectomy led to the development of multi-organ autoimmune disease that could be prevented by transfer of a population of CD4+ T cells that expressed CD25 and constituted around 5 to 10% of the CD4+ T cells in unmanipulated mice. In another approach, LeDourain and colleagues (6) used cell engraftment techniques in avian and then mouse chimeras to show that thymic epithelium induces the formation of cells that exert dominant tolerance. Lastly, Mason’s group (7) demonstrated that peripheral CD4+ T cells and CD4 single positive (CD4SP) thymocytes could be fractionated into subsets that were either autoagressive or regulatory based on their levels of expression of CD45RB, which led Mason to propose that the formation of regulatory T (Treg) cells is the ‘third function of the thymus’ (along with mediating positive selection and deletion of developing thymocytes). These seminal studies were followed by the discovery that a severe wasting disease that had previously been identified in ‘scurfy’ mice, was due to mutation of the transcription factor Forkhead box protein 3 (Foxp3) (8). Foxp3 was further shown to be expressed selectively by CD4+CD25+ T cells and to play a crucial role in establishing the ability of Treg cells to prevent the development of an autoaggressive lymphoproliferative disease that develops spontaneously when these cells are absent (9).
Where we are now, and where we are going next
The firm identification of CD4+CD25+Foxp3+ Treg cells as a specialized subset that is required to prevent the immune system from becoming autoaggressive has led to an explosion of research over the past dozen or so years. The reviews found in this volume aim to summarize three broad areas of regulatory cell biology that have been the subject of intense research and which are summarized schematically in Fig. 1. It seems reasonable to introduce the reviews in this volume as they pertain to these areas, although several of the reviews reach across these topics and are accordingly are introduced more than once.
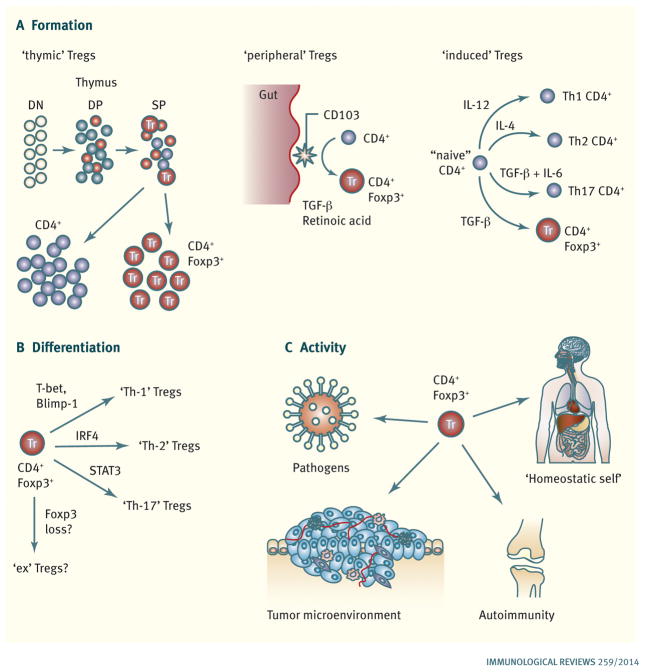
Fig. 1. Major themes in regulatory T cell biology.
(A). Pathways leading to Treg cell formation. CD4+Foxp3+ ‘thymic’ Treg cells are a major source and are generated during T-cell development in the thymus. Conventional CD4+ T cells can undergo conversion to become CD4+Foxp3+ ‘peripheral’ Treg cells in the periphery, most notably in response to CD103+ dendritic cells presenting microbial and food antigens encountered via the gut (by a TGF-β- and retinoic acid-dependent mechanism). Conventional CD4+ T cells can also become ‘induced’ Foxp+ Treg cells in response to TCR stimulation in the presence of TGF-β in vitro by a process that is analogous to the cytokine-driven differentiation of conventional CD4+ effector T cells into different effector subsets (Th-1, Th-2, and Th-17 cells). (B). Treg cell differentiation. Following activation, Foxp3+ Treg cells can use the same transcription factors required for the acquisition of effector phenotypes by conventional CD4+ T cells to adopt novel characteristics that allow them to suppress CD4+ T cells with the corresponding phenotype. There is also evidence that Treg cells can also lose Foxp3 expression and become ‘ex-Tregs’ that could potentially possess autoaggressive properties. (C). In vivo Treg cell activity. Foxp3+ Treg cells are required to prevent the development of an autoagressive lymphoproliferative disease and maintain quiescence in the steady state immune system (‘homeostatic self’). Foxp3+ Treg cells have also been implicated in and/or shown to be able to modulate ongoing immune responses in settings such and infection, autoimmunity, and cancer.
Regulatory T-cell formation
Three major mechanisms have been identified for the formation of Foxp3+ Treg cells (Fig. 1A). As introduced above, early studies establishing the existence of Treg cells implicated the thymus as an important source of these cells, and Weissler and Caton (10) describe studies carried out using transgenic mice that established that ‘thymic’ Treg cell formation can be driven by the specificity of a thymocyte TCR for a self-peptide. These studies further show that differentiation into a Foxp3+ Treg cell is an alternative fate to deletion for autoreactive thymocytes, although the relationship between these processes and the factors that promote these distinct outcomes are not yet understood. Attridge and Walker (11) also focus on the use of TCR transgenic mice to study Foxp3+ Treg cell formation, and in this case, their review concentrates on the role that ongoing interactions with self-peptides in the periphery play in the maintenance and expansion of thymic Treg cells. They also introduce the idea that additional signals, such as those from interleukin-2 (IL-2) and the costimulatory molecule CD28, play a role in maintaining Treg cell homeostasis. The review by Campbell’s group (12) further discusses the role of TCR signaling, IL-2, and costimulation in the maintenance of immune homeostasis and additionally considers how expression of specific chemokine receptors can affect Treg cell homeostasis and localization, for example by providing Treg cells with access to IL-2 in secondary lymphoid tissues.
Convincing evidence that Treg cells can also form outside of the thymus has come from studies showing that conventional CD4+ T cells can differentiate into Foxp3+ Treg cells upon encountering cognate antigen presented by a subset of dendritic cells in the gut (13, 14). As is described in the review by Hsieh’s group (15), these ‘peripheral’ Treg cells likely play an important role in establishing immune tolerance to food antigens and commensal gut-residing bacteria, although under other circumstances, it must still be possible to generate an effector immune response in the gut that can combat invading pathogenic organisms. The review by Belkaid’s group (16) focuses on the role that specialized antigen-presenting cell populations in the gut play in establishing tolerance to orally acquired antigens in the steady state, and in regulating acute inflammation during infection.
A third pathway to Foxp3+ Treg cell formation is the activation of naive conventional CD4+ cells in vitro in the presence of cytokines such as transforming growth factor-β (TGF-β). Shevach and Thornton (17) describe studies in which they have been able to generate such ‘induced’ Treg cells and compare their abilities to modulate immune responses in vivo with those of Foxp3+ Treg cells that have been isolated from naive mice. Notably, these studies have provided evidence that induced Treg cells can suppress immune responses in vivo by different mechanism(s) than are used by Treg cells that form naturally in mice. This review also recounts studies aimed at identifying molecules that can distinguish between thymic and peripheral Treg cells, with the collective goal of understanding more about how different Treg cells achieve suppression, so that one might eventually be able to be accurately manipulate Treg cell function in therapeutic settings.
Regulatory T-cell differentiation
A second major area covered in these reviews is the ability of Treg cells to undergo further differentiation following their initial activation. One of the more exciting recent findings in Treg cell biology has been the discovery that Foxp3+ Treg cells use the same transcription factors required for CD4+ conventional T-cell acquisition of effector phenotypes to adopt novel characteristics that allow them to suppress CD4+ T cells with the corresponding phenotype (18). For example, T-bet is required for conventional CD4+ Th1 cell differentiation but also confers on Foxp3+ Treg cells the ability to traffic to areas of Th1-mediated inflammation and secrete the anti-inflammatory cytokine IL-10. Additional studies in which the transcription factors GATA-3 and interferon regulatory factor-4 (IRF-4) were selectively eliminated from Foxp3+ cells have shown that they are necessary for Treg cell-mediated suppression of Th2 and Th17-mediated pathology, respectively (18). The review from Malek’s group (19) highlights the significant diversity of phenotypes that Foxp3+ Treg cells can display, along with studies that aim to determine the relationships between these phenotypes and the extent to which they arise in an ordered progression to generate activated subset(s) that are necessary to maintain immune homeostasis.
This question of differentiation and phenotypic diversity among Treg cells extends to Foxp3 itself; it is well established that Foxp3 can be transiently expressed (at least in human cells) following activation without necessarily establishing commitment to a Treg cell lineage. Conversely, some studies have provided evidence that Foxp3+ Treg cells can lose Foxp3 expression (‘ex-Tregs’) and acquire effector phenotypes, although this area remains quite controversial. The reviews by Barbi, Pardoll and Pan (20), by Picirrillo’s group (21), and by Hori (22) all focus on the question of Foxp3+ Treg cell plasticity and stability, and address some of the controversial and conflicting findings that have been made in this highly dynamic area. The idea that Foxp3+ Treg cells can lose Foxp3 expression and become ex-Tregs has attracted much attention not least because of the evidence that Foxp3+ Treg cells are generated based on specificity for self-antigens, and the extent to which loss of Foxp3 may cause such cells to acquire pathologic anti-self effector functions and/or novel regulatory phenotypes is of much interest. The reviews by Sawant and Vignali (23) and by Morikawa and Sakaguchi (24) also examine this theme and place considerable emphasis on the role that epigenetic changes play in coordinating the phenotypic variations that can arise among Treg cells, including loss of Foxp3 expression. Indeed, Morikawa and Sakaguchi (24) present the case that such epigenetic changes can be more important than Foxp3 expression itself in establishing the regulatory identity of Treg cells. The many reviews that are presented in this area highlight both the nascent nature of this field and the importance of understanding the phenotypic and functional diversity and stability of Treg cells in different settings that their role in both disease states and therapeutic settings can be understood.
In vivo Treg cell activity
The early studies identifying Treg cells and the role of Foxp3 stemmed from interest in the crucial role these cells play in maintaining immune system homeostasis, as evidenced by the wasting disease and autoaggressive lymphoproliferative syndrome that develops in their absence. There is also clear evidence that Treg cells can modulate immune responses in a variety of inflammatory conditions, as assessed in most cases by analyzing the effects of their depletion in different circumstances, such as autoimmunity, cancer, and during immune responses to pathogens. However, the exact targets that are recognized by Treg cells in these diverse settings are poorly understood, as are the mechanisms by which they can modulate immune responses in vivo.
Several of the reviews that have already been introduced include studies designed to assess the in vivo function of Treg cells, especially the mechanisms by which Treg cells maintain self-tolerance in the steady state (‘homeostatic self’). The concept that Treg cells control immune responses to pathogens in the gut was introduced in the reviews by Hsieh (15) and Belkaid’s groups (16). The review by Finlay, Walsh and Mills (25) describes the regulation of immune responses to and by helminths, including studies that have been carried out in humans and can include induction of Foxp3 expression in conventional CD4+ T cells. This review additionally reports on the occurrence of counter-regulatory effects exerted by different T-cell subsets (e.g. Th2 versus Th17 cells) and by different antigen-presenting cell types.
Despite studies showing that Treg cells can be formed based on their reactivity with self-peptides, how the specificity of the TCR directs their activity in different in vivo settings is not well understood. Weissler and Caton (10) describe studies showing that thymic Foxp3+ Treg cells can modify immune responses to influenza virus infection by recognizing a viral antigen and differentiating into ‘Th1-Tregs’. They also recount studies in a mouse model of autoimmune arthritis in which polyclonal Treg cells, but not a monoclonal population of Treg cells capable of recognizing a target autoantigen, could suppress arthritis development. Interestingly, the review by Shevach and Thornton (17) describes similar studies in which polyclonal Treg cells can suppress autoimmune disease in mouse models (including a model with a well-characterized autoantigen), and it remains unclear exactly how TCR recognition of self-peptides contributes to the ability of Treg cells to prevent autoimmune disease. Determining the role of TCR specificity in Treg cell function is even more challenging in human studies, and the review by Kleinewietfeld and Hafler (26) describes work analyzing how Foxp3+ Treg cells and IL-10-secreting Tr1 cells participate during autoimmune neuroinflammation in human patients. Treg cells can also be active in the tumor microenvironment, and the review by Savage, Leventhal, and Malchow (27) includes elegant studies in murine models in which Foxp3+ Treg cells have been shown to directly recognize tumor antigens and to differentiate based on specificity for tumor antigen expressed intrathymically (under control of the AIRE transcription factor). Thus, in different settings, TCR specificity appears to play distinct roles in directing Treg cell function, and the successful application of technologies such as chimeric antigen receptors to direct Treg cell function will clearly require a more thorough understanding of this issue.
The final review in this volume comes from Candando, Lykken, and Tedder (28) and describes studies analyzing the role of B10 cells in regulating immune responses. B10 cells have also been called ‘Bregs’ and have been shown to be capable of modulating immune responses in a variety of settings, principally through their production of IL-10. However, as is described in the review, it is not clear that B10 cells belong to a specialized subset, as is the case for CD4+Foxp3+ T cells. As such, they might represent an example of a cell type with counter-regulatory properties that certainly modifies immune responses and may prove to be useful in the treatment of aberrant immune responses. They are included in this volume as an example of the expanding world of regulatory cells that have re-emerged in the renaissance driven by the identification of Foxp3+ T cells.
Concluding remarks
The articles compiled in this issue of Immunological Reviews describe what is currently known regarding the formation, differentiation, and in vivo activity of regulatory cells, and the factors that can guide these events. They highlight the important role that these cells play in the delicate balance that the immune system must maintain between the ability to generate an effective response against unknown foreign antigens, and the need to limit self-reactivity and prevent autoimmune pathology. Treg cells are absolutely required to prevent wasting disease and death stemming from uncontrolled lymphoproliferation and autoaggression, and these cells can form in the thymus following recognition of self-antigen, in the gut due to interactions with specialized antigen-presenting cell subsets, or in vitro in response to cytokines like TGF-β. The various ways in which Treg cells with distinct origins are similar and/or different remain unclear, and it will be important to understand how each subgroup functions and the circumstances in which each is required before Treg cells can be used as a therapeutic intervention. Once formed, Treg cells can undergo further differentiation, and while much has been learned in the relatively short time since this observation was first made, a lot of questions remain regarding the signals driving differentiation and the situations in which it may be necessary. The answers to these questions could potentially allow one to manipulate Treg cell activity either to promote suppression of autoimmune disease driven by known helper T-cell subsets, or to limit Treg cell differentiation, and therefore trafficking and activity, in contexts such as cancer where they may limit a beneficial immune response. The precise signals leading to activation of Treg cell suppressive function in vivo also remain a matter of some debate, and why autoimmune disease can continue to occur in human patients despite the presence of Treg cells is still unknown. It will be interesting to learn whether the polyclonal populations of Treg cells that have been shown to prevent autoimmune disease development in mouse models work because they include Treg cells with necessary TCR specificities that are not present in the host or through a more generalized suppressive mechanism. In the contexts of infection and cancer, further information on how Treg cells come to participate in and impact effector cell function during the immune response could be useful in designing more effective treatments and vaccines. We look forward to seeing many of these questions answered and advances made in the manipulation of Treg cells for therapeutic purposes in the coming years.
Acknowledgments
Our work in this area is supported by NIH grants AI24541 and AI59916, by NCI core grant P30 CA10815, by the Commonwealth of Pennsylvania, and by Sibley Memorial Hospital.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Burnet FM. The Clonal Selection Theory of Acquired Immunity. New York: Cambridge University Press; 1959. [Google Scholar]
- 2.Tonegawa S. Somatic generation of antibody diversity. Nature. 1983;302:575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- 3.Nossal GJV. Cellular mechanisms of immunologic tolerance. Annu Rev Immunol. 1983;1:33–62. doi: 10.1146/annurev.iy.01.040183.000341. [DOI] [PubMed] [Google Scholar]
- 4.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 5.Sakaguchi S. Regulatory T cells: Key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 6.Le Douarin N, et al. Evidence for a thymus-dependent form of tolerance that is not based on elimination or anergy of reactive T cells. Immunol Rev. 1996;149:35–53. doi: 10.1111/j.1600-065x.1996.tb00898.x. [DOI] [PubMed] [Google Scholar]
- 7.Seddon B, Mason D. The third function of the thymus. Immunol Today. 2000;21:95–99. doi: 10.1016/s0167-5699(99)01559-5. [DOI] [PubMed] [Google Scholar]
- 8.Bennett CL, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 9.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 10.Weissler KA, Caton AJ. The role of T-cell receptor recognition of peptide:MHC complexes in the formation and activity of Foxp3+ regulator T cells. Immunol Rev. 2014;259 doi: 10.1111/imr.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Attridge K, Walker LSK. Homeostasis and function of regulatory T cells (Tregs) in vivo: lessons from TCR-transgenic Tregs. Immunol Rev. 2014;259 doi: 10.1111/imr.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smigiel KS, Srivastava S, Stolley JM, Campbell DJ. Regulatory T-cell homeostasis: steady-state maintenance and modulation during inflammation. Immunol Rev. 2014;259 doi: 10.1111/imr.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ai TL, Solomon BA, Hsieh C-S. T-cell selection and intestinal homeostasis. Immunol Rev. 2014;259 doi: 10.1111/imr.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grainger JR, Askenase MH, Guimont-Desrochers F, Morais da Fonseca D, Belkaid Y. Contextual functions of antigen-presenting cells in the gastrointestinal tract. Immunol Rev. 2014;259 doi: 10.1111/imr.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shevach EM, Thornton AM. tTregs, pTregs, and iTregs: similarities and differences. Immunol Rev. 2014;259 doi: 10.1111/imr.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11:119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan X, Cheng G, Malek TR. The importance of regulator T-cell heterogeneity in maintaining self-tolerance. Immunol Rev. 2014;259 doi: 10.1111/imr.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbi J, Pardoll DM, Pan F. Treg functional stability and its responsiveness to the microenvironment. Immunol Rev. 2014;259 doi: 10.1111/imr.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bin Dhuban K, Kornete M, Mason E, Piccirollo CA. Functional dynamics of Foxp3+ regulatory T cells in mice and humans. Immunol Rev. 2014;259 doi: 10.1111/imr.12168. [DOI] [PubMed] [Google Scholar]
- 22.Hori S. Linage stability and phenotypic plasticity of Foxp3+ regulatory T cells. Immunol Rev. 2014;259 doi: 10.1111/imr.12175. [DOI] [PubMed] [Google Scholar]
- 23.Sawant DV, Vignali DAA. Once a Treg, always a Treg? Immunol Rev. 2014;259 doi: 10.1111/imr.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morikawa H, Sakaguchi S. Genetic and epigenetic basis of Treg cell development and function: from a FoxP3-centered view to an epigenome-defined view of natural Treg cells. Immunol Rev. 2014;259 doi: 10.1111/imr.12174. [DOI] [PubMed] [Google Scholar]
- 25.Finlay CM, Walsh KP, Mills KHG. Induction of regulatory cells by helminth parasites: exploitation for the treatment of inflammatory diseases. Immunol Rev. 2014;259 doi: 10.1111/imr.12164. [DOI] [PubMed] [Google Scholar]
- 26.Kleinewietfeld M, Hafler DA. Regulatory T cells in autoimmune neuroinflammation. Immunol Rev. 2014;259 doi: 10.1111/imr.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savage PA, Leventhal DS, Malchow S. Shaping the repertoire of tumor-infiltrating effector and regulatory T cells. Immunol Rev. 2014;259 doi: 10.1111/imr.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Candando KM, Lykken JM, Tedder TF. B10 cell regulation of health and disease. Immunol Rev. 2014;259 doi: 10.1111/imr.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]