Abstract
Objectives
Anorexia nervosa (AN) is characterized by low weight, aberrant eating attitudes, body image distortion and hypogonadism. Anxiety is a common co-morbid condition. Estrogen replacement reduces anxiety in animal models, and reported variations in food intake across the menstrual cycle may be related to gonadal steroid levels. The impact of estrogen replacement on anxiety, eating attitudes, and body image has not been reported in AN. We hypothesized that physiologic estrogen replacement would ameliorate anxiety and improve eating attitudes, without affecting body image in AN.
Methods
Girls with AN (DSM-IV) 13–18 yo were randomized to transdermal estradiol (100-mcg twice weekly) with cyclic progesterone (AN-E+) or placebo patches and pills (AN-E−) for 18-months, between 2002 and 2010. The Spielberger’s State-Trait Anxiety Inventory for Children (STAIC), the Eating Disorders Inventory II (EDI II), and the Body Shape Questionnaire (BSQ-34) were administered. 72 girls completed these at baseline (38 AN-E+ and 34 AN-E−), and 37 at 18-months (20 AN-E+ and 17 AN-E−). The primary outcome measure was the change in these scores over 18-months.
Results
Estrogen replacement caused a decrease in STAIC-trait scores (−3.05±1.22 vs. 2.07±1.73, p=0.02), without impacting STAI-state scores (−1.11±2.17 vs. 0.20±1.42, p=0.64). There was no effect of estrogen replacement on EDI II or BSQ-34 scores. BMI changes did not differ between groups, and effects of estrogen replacement on STAIC-trait scores persisted after controlling for BMI changes (p=0.03). There was an inverse association between serum estradiol changes and changes in STAIC-trait scores (r=−0.66, p=0.001).
Conclusions
Estrogen replacement improved trait anxiety (the tendency to experience anxiety), but did not impact eating attitudes or body shape perception.
Keywords: Estrogen, anxiety, eating behavior, body shape, anorexia nervosa, adolescents
Introduction
Anorexia nervosa (AN) is reported in 0.2–1% of all adolescent girls, and is characterized by low weight, aberrant eating attitudes and behavior (restriction, binge/purge behaviors), body image disturbance and hypothalamic amenorrhea. Anxiety is a common co-morbid condition 1–3 and is reported in as many as two thirds of women presenting with AN 3; when present, it precedes the diagnosis of the eating disorder in 69% 4. Levels of gonadal steroids, both estrogen and testosterone, are low in AN 5, 6, and contribute to pathology associated with AN, such as low bone density. We have shown that physiologic estrogen replacement is effective in increasing bone density and maintaining bone density Z-scores in adolescent girls with AN 7. Studies indicate that estrogen status may also impact cognitive function 8, mood and anxiety 9, 10, body shape perception, and eating behavior 11, 12. However, the effect of physiologic estrogen replacement on these endpoints has not been assessed in AN.
Estrogen, acting though estrogen receptor-β, is anxiolytic in animals 9, and levels of anxiety change across the estrus cycle 10. Ovariectomized, hypogonadal rats treated with estrogen perform better than controls who do not receive estrogen during a forced swim test and the open field test 13, 14. Additionally, women are more likely to develop anxiety disorders than men 15. Of note, adolescents and adults with Turner Syndrome or premature ovarian failure (hypogonadal conditions) have higher anxiety levels than controls 16–18, although this does not improve with estrogen administration17. Estrogen may also be involved with perception of body shape, and may account for greater body shape concerns in females than males. Additionally, caloric intake is lower during the follicular phase, when estrogen levels are low, than in the luteal phase, suggesting that variations in food intake across the menstrual cycle may be related to levels of gonadal hormones 11, 12.
Few studies have assessed the impact of estrogen status on cognitive and psychiatric outcomes in adolescents or adults with AN. One study reported an improvement in certain cognitive measures in adult women with a history of AN who spontaneously resumed menses or were treated with estrogen-progesterone combination pills, whereas this was not observed in women who recovered weight alone without resuming menses. These data suggest that gonadal steroid status, rather than weight recovery alone, is important for improving cognitive function in AN 8. Another study reported an improvement in anxiety trait scores with weight recovery in AN 2. However, the impact of estrogen replacement on anxiety, body image, eating attitudes and behavior has not been assessed in patients with AN, and specifically adolescents, and merits investigation.
We assessed changes in anxiety scores, perception of body shape, and eating attitudes and behavior in adolescent girls with AN randomized to physiologic estrogen replacement or placebo. We hypothesized that physiologic estrogen replacement in adolescents with AN would ameliorate anxiety and improve eating behavior, without affecting body image.
Subjects and Methods
Subject Selection and Protocol
Girls with AN 13–18 years old and a bone age of at least 15 years were randomized for 18 months to physiologic estrogen replacement or placebo by the Massachusetts General Hospital (MGH) Research Pharmacy based on a pre-determined computer generated randomization sequence. We have previously published data regarding effects of estrogen replacement on bone density in adolescent girls with AN in the larger study cohort, but not related to psychiatric outcome 7. Inclusion criteria included a diagnosis of AN based on DSM-IV criteria, and a chronological age between 13–18 years. Exclusion criteria included (i) active suicidality, psychosis or substance abuse, and (ii) hematocrit <30 %, potassium <3.0 mmol/L, or glucose <50 mg/dl. Because the larger randomized controlled trial examined effects of physiologic estrogen replacement on bone density, additional exclusion criteria included use of prescription medications within three months of study participation known to affect bone metabolism, and other diseases known to affect bone metabolism.
All subjects with AN from this larger cohort (n=110) who had a bone age of at least 15 years and completed psychiatric questionnaires at baseline were included in the current analysis. Seventy-two girls with AN completed the questionnaires at baseline (38 randomized to estrogen and 34 to placebo), and 37 at 18 months follow-up (20 randomized to estrogen and 17 to placebo). The remainder of the girls were either lost to follow-up or did not complete the questionnaires at 18 months. The completers and non-completers did not differ at baseline for BMI and questionnaire scores for anxiety symptoms, eating attitudes and behavior and body image. Subjects were recruited at MGH, Boston, and the Hospital for Sick Children (Sick-Kids), Toronto. Recruitment strategies, and inclusion and exclusion criteria for the study have been previously reported 7. Use of psychiatric medications was not an exclusion criterion for study participation. All girls with AN had multidisciplinary treatment teams in place. The study team did not assume clinical care of these subjects. The study was approved by the Partners HealthCare Institutional Review Board (for MGH) and the Research Ethics Board (for Sick-Kids), and informed consent and assent were obtained from parents and subjects.
The diagnosis of AN was confirmed by the study psychiatrist or psychologist. Girls with AN received either (i) transdermal 17β-estradiol (100 mcg twice weekly; Novartis Pharmaceuticals, Inc.) with cyclic progesterone (2.5 mg of medroxyprogesterone acetate) (AN-E+), or (ii) placebo patches and cyclic placebo pills (AN-E−) for the study duration. Progesterone or placebo pills were taken during the first 10 days of each month to prevent unopposed estrogen stimulation of the uterus. We assessed compliance with study medications every two months using verbal questionnaires and calendars given to subjects to record any missed study medication doses. All used and unused medications were collected. Groups did not differ for compliance with study medications.
In order to investigate the effects of physiologic estrogen on anxiety symptoms, eating attitudes and behavior and body image, the Spielberger’s State-Trait Anxiety Inventory for Children (STAIC) 19, the Eating Disorders Inventory II questionnaire (EDI II) 20, and the Body Shape Questionnaire (BSQ-34) 21 were administered at baseline and 18 months. On the STAIC 19, the State-Anxiety scale includes 20 statements that measure transitory anxiety states, or subjective, consciously perceived feelings of apprehension, tension, and worry that vary in intensity and fluctuate over time 22. The Trait-Anxiety scale includes 20 statements that measure relatively stable individual differences in anxiety proneness, or differences between children in the tendency to experience anxiety states. The statements are assessed on a three-point rating scale. The EDI II is a measure of eating disordered attitudes and behaviors used to diagnose eating disorders and evaluate treatment success in intervention studies 20. It includes 91 questions, divided into 11 subscales. Each question is on a 6 point scale (ranging from ‘always’ to ‘never’), rated 0–3. The score for each sub-scale is then summated. The EDI-II subscales include Drive for Thinness, Bulimia, Body Dissatisfaction, Ineffectiveness, Perfectionism, Interpersonal Distrust, Interoceptive awareness, Maturity fears, Asceticism, Impulse Regulation and Social Insecurity. The BSQ-34 asks 34 questions related to body shape perception that range from 1 to 6. Higher scores on these scales indicate greater psychopathology.
Subjects were weighed in a hospital gown at the Clinical Research Center at MGH or the Clinical Investigation Unit at Sick-Kids on a single wall-mounted stadiometer (average of three measurements to the nearest 0.1 cm) and using an electronic scale (to the nearest 0.1 kg). Blood samples were drawn for estradiol, testosterone and sex hormone binding globulin (SHBG) levels at these visits. We assessed compliance with study medications every two months using verbal questionnaires and by collecting calendars provided to subjects to record missed study medications. We also collected used and unused patches and pills. Groups did not differ for compliance with study medications.
Biochemical Analysis
We used a radioimmunoassay (RIA) to measure estradiol (Diagnostic Systems Laboratories, Inc., Webster, TX; limit of detection 2.2 pg/ml, intra-assay CV 6.5–8.9%). The Access chemiluminescent immunoassay (Beckman Coulter, Fullerton, CA) was used to measure testosterone (limit of detection 10 ng/dl, intra-assay CV 1.67–3.93%) and SHBG (limit of detection 0.33 nmol/L, intra-assay CV 4.5–4.8%). Free androgen index (FAI) was calculated with the following formula: [total testosterone*3.47]/SHBG. Data at 18 months were available for 27 subjects for gonadal steroids. Samples were stored at −80 C until analysis, and run in duplicate.
Statistical Methods
Data are reported as means ± SE. A p value of <0.05 on a two-tailed test was used to indicate significance. Our primary endpoint was the prospective change in questionnaire scores in AN E+ versus AN E− over 18 months. Baseline characteristics of AN E+ versus AN E−, and the difference in questionnaire scores in the two groups from baseline to 18 months (18-month – baseline) was compared using the Student t-test. In comparing AN E+ versus AN E−, we controlled for weight and BMI changes over 18 months using analysis of covariance, given that these are known determinants of prospective changes in certain questionnaire measures over time 2. We also used correlation analysis to determine associations of changes in weight, BMI and estradiol levels with changes in questionnaire scores over 18 months, and performed multivariate analysis to control for potential confounders of these associations. We use Spearman (non-parametric) correlations when determining associations of changes in questionnaire scores with changes in estradiol levels, as the latter were not normally distributed, and Pearson (parametric) correlations for all other associations. Baseline data are provided for all subjects who completed the questionnaires at baseline, and follow-up data are provided for those who also completed the questionnaires at 18-month follow-up.
Results
Baseline Characteristics
Girls with AN randomized to physiologic estrogen replacement did not differ from those randomized to placebo for age, bone age, height, weight, BMI, baseline STAIC-state and trait scores, EDI II and BSQ-34 scores, and estradiol and testosterone levels (Table 1). 52.6% of girls in the AN-E+ group and 50.0% in the AN-E− group were on psychotropic medication/s at baseline (p not significant). Additionally, AN-E+ and AN-E− groups did not differ for use of SSRIs/SSNRIs (44.7 vs. 44.1%, p=1.00), atypical antipsychotics (21.1 vs. 14.7%; p=0.55), or benzodiazepines (13.2 vs. 5.9%, p=0.43). Girls who did or did not complete the questionnaires at follow-up or were lost to follow-up did not differ for baseline characteristics including use of psychotropic medication/s.
Table 1.
Baseline characteristics of girls with anorexia nervosa randomized to physiologic estrogen replacement (AN-E+) or placebo (AN-E−)
| AN-E+ (n=38) | AN-E− (n=34) | p | |
|---|---|---|---|
| Age (years) | 16.9 ± 0.2 | 16.6 ± 0.2 | 0.39 |
| Bone age (years) | 16.7 ± 0.1 | 16.4 ± 0.2 | 0.10 |
| Body mass index (kg/m2) | 17.2 ± 0.2 | 17.5 ± 0.2 | 0.34 |
| Percent ideal body weight (for height) | 79.1 ± 1.6 | 82.1 ± 2.0 | 0.25 |
| Percent ideal body weight (based on height and 50th percentile of BMI for age) | 83.0 ± 1.1 | 84.8 ± 1.1 | 0.25 |
| Percent body fat | 18.6 ± 0.8 | 17.2 ± 0.9 | 0.27 |
| Age at menarche (years) | 12.3 ± 0.2 | 12.2 ± 0.2 | 0.79 |
| Duration since diagnosis (months) | 14.5 ± 2.8 | 13.6 ± 2.6 | 0.82 |
| EDI II scores | |||
| Drive for Thinness | 11.7 ± 1.2 | 13.2 ± 1.2 | 0.39 |
| Bulimia | 1.7 ± 0.5 | 0.9 ± 0.2 | 0.23 |
| Body Dissatisfaction | 15.2 ± 1.6 | 15.9 ± 1.5 | 0.77 |
| Ineffectiveness | 9.4 ± 1.2 | 8.7 ± 1.3 | 0.69 |
| Perfectionism | 7.7 ± 0.9 | 7.3 ± 0.8 | 0.75 |
| Interpersonal Distrust | 6.3 ± 0.8 | 5.4 ± 0.8 | 0.42 |
| Interoceptive Awareness | 7.1 ± 1.0 | 7.4 ± 1.1 | 0.83 |
| Maturity Fears | 7.3 ± 0.5 | 7.6 ± 0.7 | 0.69 |
| Asceticism | 7.8 ± 0.9 | 8.6 ± 1.1 | 0.58 |
| Impulse Regulation | 3.7 ± 0.7 | 3.0 ± 0.8 | 0.56 |
| Social Insecurity | 7.4 ± 0.8 | 7.7 ± 0.9 | 0.82 |
| BSQ-34 scores | 119.8 ± 7.3 | 129.8 ± 7.8 | 0.35 |
| STAIC-State scores | 36.2 ± 1.1 | 36.7 ± 1.2 | 0.77 |
| STAIC-Trait scores | 43.0 ± 1.5 | 41.6 ± 1.4 | 0.49 |
| Estradiol (pg/ml) | 43.2 ± 7.6 | 32.3 ± 6.3 | 0.28 |
| Testosterone (ng/dl) | 43.0 ± 6.4 | 36.8 ± 5.7 | 0.47 |
| Free androgen index | 3.1 ± 0.5 | 2.6 ± 0.5 | 0.57 |
Means ± SE
Changes in STAIC, EDI II and BSQ-34 Scores
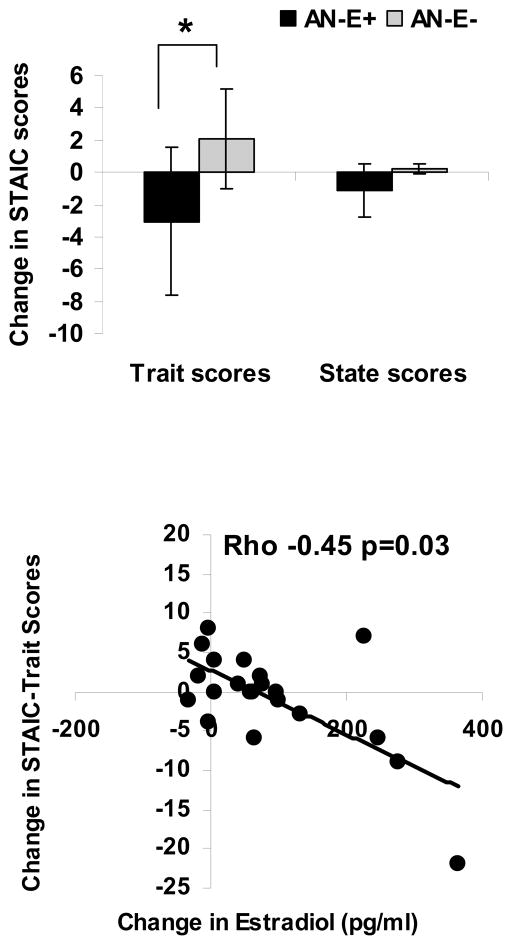
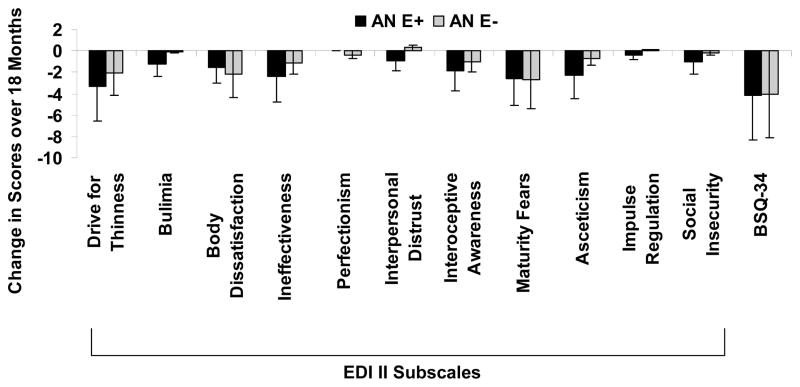
Estrogen replacement in girls with AN led to a significant decrease in the STAIC-trait scores (−3.05±1.22 vs. 2.07±1.73, p=0.02), without impacting STAIC-state scores (−1.11±2.17 vs. 0.20±1.42, p=0.64) (Figure 1). AN-E+ and AN-E− groups did not differ for changes in EDI II subscale scores or BSQ-34 scores over 18 months (Figure 2).
Figure 1.
Upper Panel: Change in STAIC trait (left) and state (right) scores in girls with anorexia nervosa randomized to physiological estrogen with cyclic progesterone (AN-E+) or placebo patches and cyclic placebo pills (AN-E−). Physiologic estrogen replacement led to a significant decrease in anxiety trait (but not state) scores. * p<0.05. Means±SE. Lower Panel: Association of change in estradiol levels with change in STAIC-Trait scores. An increase in estradiol levels was associated with a decrease in STAIC-Trait scores (Spearman Rho −0.45, p=0.03)
Figure 2.
Changes in EDI II subscale scores and BSQ-34 scores in girls with anorexia nervosa randomized to physiological estrogen with cyclic progesterone (AN-E+) or placebo patches and cyclic placebo pills (AN-E−). Physiologic estrogen replacement had no significant impact on these scores. Means±SE
The groups did not differ for changes in weight, BMI or percent body fat over 18 months (Table 2). Estradiol levels, as expected, increased significantly in the AN-E+ group compared with the AN-E− group; however, changes in total testosterone levels and the free androgen index did not differ between groups. Differences between AN-E+ and AN-E− for change in STAIC-trait scores remained significant after controlling for changes in weight or BMI over the 18 months (p=0.02 and 0.03 respectively).
Table 2.
Changes in body composition parameters and hormones over 18 months in girls with anorexia nervosa randomized to physiologic estrogen replacement (AN-E+) or placebo (AN-E−)
| AN-E+ (n=20) | AN-E− (n=17) | p | |
|---|---|---|---|
| Change in weight (18-0 m) (kg) | 3.8 ± 1.1 | 3.3 ± 1.1 | 0.73 |
| Change in BMI (18-0 m) (kg/m2) | 1.36 ± 0.45 | 1.19 ± 0.42 | 0.79 |
| Change in percent body fat (18-0 m) | 2.8 ± 1.5 | 4.1 ± 1.0 | 0.48 |
| Change in estradiol (18-0 m) (pg/ml) | 117.0 ± 28.0 | 22.4 ± 18.8 | 0.01 |
| Change in testosterone (18-0 m) (ng/dl) | −15.2 ± 9.4 | −16.6 ± 11.8 | 0.93 |
| Change in free androgen index (18-0 m) | −0.6 ± 0.5 | 1.2 ± 1.8 | 0.28 |
Means ± SE
There was an inverse association between change in estradiol and change in STAIC-trait scores over the study duration for the group as a whole (Spearman Rho= −0.45, p=0.03), and this association was more marked in the AN-E+ group (Spearman Rho = −0.60, p=0.03). The association persisted after controlling for change in BMI for the group as a whole (p=0.002) and within AN-E+ (p=0.0007). There were no associations of changes in testosterone or the free androgen index with questionnaire scores. Differences between the groups for STAIC-trait scores persisted after controlling for baseline estradiol and baseline STAIC-trait scores (p=0.03).
Only eight subjects were assessed at follow-up on days during which they were on progesterone or placebo. Differences between the groups remained significant after excluding these subjects on a subgroup analysis (p=0.02). None of the girls in the AN-E− group resumed regular menses (although about 50% had irregular cycles or spotting), and we were thus unable to assess the impact of spontaneous menstrual recovery on questionnaire scores.
Other Determinants of Changes in EDI II, BSQ-34 and STAIC Scores
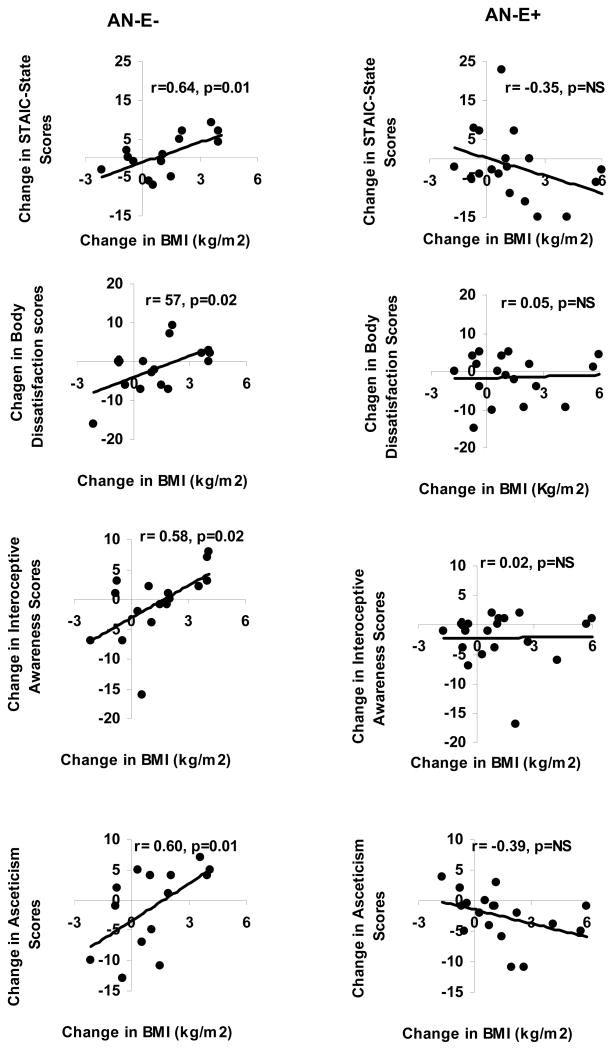
In AN-E−, changes in weight or in BMI were associated positively with changes in the STAIC-state scores (r=0.64; p=0.01 for both), and the following EDI II subscale scores: body dissatisfaction (r=0.59 and 0.57; p=0.01 and 0.02), interoceptive awareness (r=0.58 for both; p=0.01 and 0.02) and asceticism (r=0.58 and 0.60; p=0.01). However, these associations were not observed in the AN-E+ group. In contrast, there was an inverse association between change in BMI and change in ineffectiveness (EDI II subscale) in the AN-E+ group (r= −0.46, p=0.048).
Discussion
We demonstrate a reduction in trait anxiety (the tendency to experience anxiety) with estrogen replacement in adolescent girls with AN independent of weight changes in a randomized placebo-controlled study. However, estrogen replacement did not directly impact eating attitudes and behaviors, body shape perception, or state anxiety.
Our findings are consistent with data from animal experiments that report a decrease in depressive behavior and anxiety in animal models of hypogonadism following estrogen replacement 13, 14, 23. Ovariectomy leads to hypoestrogenism and has been shown to be anxiogenic in rats 13, 14. However, ovariectomized rats treated with estrogen perform better than those treated with vehicle during a forced swim test by swimming more and struggling less 13, 23. Following estrogen administration, when exposed to a circular open field, ovariectomized rats tend to be less immobile, venture more often into the centre of the field and spend more time there 14, 23. Estrogen replacement also results in less anxiety like behavior on the mirror maze test and with light-dark transition tasks 23. In our study, use of physiological replacement doses of the 17β-estradiol transdermal patch was associated with a reduction in trait anxiety scores, and this effect persisted after controlling for weight changes. We also found correlations between increases in estradiol and decreases in trait anxiety scores over 18 months, and this association remained significant after controlling for weight changes. In addition, the impact on trait anxiety scores was not related to progesterone intake as differences between groups persisted after excluding subjects who were assessed during the days they were on progesterone/placebo pills. The fact that ‘trait’ rather than ‘state’ anxiety was impacted by estrogen replacement is likely related to ‘trait’ reflecting a pattern of responding or proneness to respond or to feel a certain way, whereas ‘state’ reflects feelings at any given moment. Our data suggest that estrogen replacement improves chronic patterns of anxiety, rather than feelings at any given moment, which may be more likely to be impacted by coincident triggers and stimuli. Of note, although the constructs of state and trait anxiety are the same across the age spectrum, the language in the child measure (used in this study) is simplified. Future studies should include use of the STAI rather than the STAIC in this adolescent age group. We did not assess depressive symptoms in our subjects in this study, and this will be important to evaluate in future studies.
Estrogen replacement was not associated with a decrease in scores on the EDI II subscales or on the BSQ-34, suggesting that estrogen replacement does not improve eating attitudes and behavior or body shape perception in AN. However, girls with AN randomized to placebo had a positive association between changes in weight or BMI and (i) changes in various subscale scores of the EDI II including body dissatisfaction, interoceptive awareness and asceticism, and (ii) changes in STAIC state scores. These data suggest that when estrogen levels are low, an increase in weight or BMI alone is associated with an increase in eating disorder psychopathology and state anxiety. Importantly, this association was not evident in girls with AN randomized to transdermal estradiol, indicating that in an estrogen replete state, an increase in weight or BMI is reassuringly not associated with an increase in psychopathology or state anxiety. In fact, in girls with AN randomized to transdermal estradiol, an increase in weight/BMI was associated with a decrease in feelings of ineffectiveness on correlation analysis. This has implications for better chances at sustaining recovery in an estrogen replete than an estrogen deficient state.
Estrogen administration has been associated with improved cognitive performance in ovariectomized rats 23. In addition, in women with AN, spontaneous resumption of menses and/or use of oral estrogen is associated with improvement in certain cognitive measures 8. We did not assess cognitive function following transdermal estradiol use in our study, and this will be important to evaluate.
A limitation of the study is our reliance on self-report questionnaires. Although study participants were aware that the questionnaires were for research purposes only, there may be a bias towards under-reporting psychopathology. However, this bias would be expected to be distributed equally across the randomization groups. Also, these are validated questionnaires for assessing anxiety, eating attitudes and behavior, and body shape perception 19, 20. An additional limitation is that multiple factors, such as weight changes over time, may mediate longitudinal changes in anxiety, self body image and eating attitudes and behavior. Controlling for such covariates in multivariate models is one strategy to assess whether effects of estrogen replacement on prospective changes in questionnaires scores are independent of other confounders. Indeed, differences between treatment groups persisted for STAIC trait scores even after controlling for changes in weight or BMI over the study duration. Additionally, the groups did not differ for changes in weight or BMI over time. Another study limitation is that treatment status and current emotional and physical state of the subject may impact performance on questionnaires, as may comorbid conditions such as anxiety and depression. Although we had complete information about use of psychotropic drugs, our study subjects were not always clear about specific indications for these drugs, limiting our ability to determine effects of specific comorbidities. Again, in a randomized trial, these modifiers would be evenly distributed across groups. Finally, a higher retention rate would have been desirable over the study duration. Our attrition rate was comparable to other reports in AN 24, although a recent study in adolescents reported significant success with retention 25. This may relate to perceived benefits from study participation, with studies involving treatment of the state of AN being perceived as more beneficial than those involving treatment of associated morbidities such as low bone density. Importantly, girls who did or did not complete the study did not differ for baseline characteristics including use of psychotropic medications.
We thus demonstrate an improvement in trait anxiety in girls with AN randomized to physiologic replacement doses of transdermal estradiol, even after controlling for weight changes. We also demonstrate that associations of increases in weight with increase in eating disorder psychopathology and state anxiety (observed in estrogen deficient girls with AN) are not evident in those who receive estrogen replacement. Further studies are necessary to confirm these findings and to assess the impact of physiologic estrogen replacement on long-term cognitive outcomes and depressive symptoms in AN, and to assess the effect of other forms of estrogen administration (such as estrogen-progesterone combination pills), not considered physiologic replacement, on anxiety, cognitive outcomes and depressive symptoms.
Figure 3.
Associations of changes in BMI over 18 months with changes in STAIC-State scores, and with changes in Body Dissatisfaction, Interoceptive Awareness and Asceticism scores on the EDI-II subscales in girls with anorexia nervosa randomized to physiological estrogen with cyclic progesterone (AN-E+) or placebo patches and cyclic placebo pills (AN-E−). Positive associations of changes in these scores over 18 months were noted with changes in BMI in AN-E−, but not in AN-E+.
Clinical Points.
Physiologic estrogen replacement as the transdermal estrogen patch improves trait anxiety (the tendency to experience anxiety), but not state anxiety, eating attitudes or body shape perception.
Further studies are necessary to understand the impact of other forms and doses of estrogen administration
Acknowledgments
Grant Support: This work was supported by National Institutes of Health Grants R01 DK 062249, M01-RR-01066 and 1 UL1 RR025758-03, USA
We thank the skilled nursing and bionutrition staff of the Clinical Research Center of Massachusetts General Hospital, Boston, MA, USA, and Clinical Investigation Center of the Hospital for Sick Children, Toronto, ON, USA, for their help with carrying out this study. Finally, we thank our subjects, without whom this study would not have been possible.
Footnotes
The authors report no financial or other relationship relevant to the subject of this article.
Clinical Trials Registration: NCT00088153
References
- 1.Hambrook D, Brown G, Tchanturia K. Emotional intelligence in anorexia nervosa: Is anxiety a missing piece of the puzzle? Psychiatry Res. 2012 Nov 30;200(1):12–19. doi: 10.1016/j.psychres.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 2.Sala L, Mirabel-Sarron C, Gorwood P, Pham-Scottez A, Blanchet A, Rouillon F. The level of associated depression and anxiety traits improves during weight regain in eating disorder patients. Eat Weight Disord. 2011 Dec;16(4):e280–284. doi: 10.1007/BF03327473. [DOI] [PubMed] [Google Scholar]
- 3.Kaye WH, Bulik CM, Thornton L, Barbarich N, Masters K. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am J Psychiatry. 2004 Dec;161(12):2215–2221. doi: 10.1176/appi.ajp.161.12.2215. [DOI] [PubMed] [Google Scholar]
- 4.Swinbourne J, Hunt C, Abbott M, Russell J, St Clare T, Touyz S. The comorbidity between eating disorders and anxiety disorders: prevalence in an eating disorder sample and anxiety disorder sample. Aust N Z J Psychiatry. 2012 Feb;46(2):118–131. doi: 10.1177/0004867411432071. [DOI] [PubMed] [Google Scholar]
- 5.Misra M, Aggarwal A, Miller KK, et al. Effects of anorexia nervosa on clinical, hematologic, biochemical, and bone density parameters in community-dwelling adolescent girls. Pediatrics. 2004 Dec;114(6):1574–1583. doi: 10.1542/peds.2004-0540. [DOI] [PubMed] [Google Scholar]
- 6.Soyka L, Misra M, Frenchman A, et al. Abnormal bone mineral accrual in adolescent girls with anroexia nervosa. J Clin Endocrinol Metab. 2002;87:4177–4185. doi: 10.1210/jc.2001-011889. [DOI] [PubMed] [Google Scholar]
- 7.Misra M, Katzman D, Miller KK, et al. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J Bone Miner Res. 2011 Oct;26(10):2430–2438. doi: 10.1002/jbmr.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chui HT, Christensen BK, Zipursky RB, et al. Cognitive function and brain structure in females with a history of adolescent-onset anorexia nervosa. Pediatrics. 2008 Aug;122(2):e426–437. doi: 10.1542/peds.2008-0170. [DOI] [PubMed] [Google Scholar]
- 9.Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology. 2005 Feb;146(2):797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- 10.Mora S, Dussaubat N, Diaz-Veliz G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology. 1996 Oct;21(7):609–620. doi: 10.1016/s0306-4530(96)00015-7. [DOI] [PubMed] [Google Scholar]
- 11.Gong EJ, Garrel D, Calloway DH. Menstrual cycle and voluntary food intake. Am J Clin Nutr. 1989 Feb;49(2):252–258. doi: 10.1093/ajcn/49.2.252. [DOI] [PubMed] [Google Scholar]
- 12.Li ET, Tsang LB, Lui SS. Menstrual cycle and voluntary food intake in young Chinese women. Appetite. 1999 Aug;33(1):109–118. doi: 10.1006/appe.1999.0235. [DOI] [PubMed] [Google Scholar]
- 13.Rachman IM, Unnerstall JR, Pfaff DW, Cohen RS. Estrogen alters behavior and forebrain c-fos expression in ovariectomized rats subjected to the forced swim test. Proc Natl Acad Sci U S A. 1998 Nov 10;95(23):13941–13946. doi: 10.1073/pnas.95.23.13941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diz-Chaves Y, Kwiatkowska-Naqvi A, Von Hulst H, Pernia O, Carrero P, Garcia-Segura LM. Behavioral effects of estradiol therapy in ovariectomized rats depend on the age when the treatment is initiated. Exp Gerontol. 2012 Jan;47(1):93–99. doi: 10.1016/j.exger.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Seeman MV. Psychopathology in women and men: focus on female hormones. Am J Psychiatry. 1997 Dec;154(12):1641–1647. doi: 10.1176/ajp.154.12.1641. [DOI] [PubMed] [Google Scholar]
- 16.Lasaite L, Lasiene D, Lasas L. Cognition, emotions and quality of life in Lithuanian girls with Turner syndrome after growth hormone therapy discontinuation. J Pediatr Endocrinol Metab. 2010 May;23(5):443–450. [PubMed] [Google Scholar]
- 17.Cardoso G, Daly R, Haq NA, et al. Current and lifetime psychiatric illness in women with Turner syndrome. Gynecol Endocrinol. 2004 Dec;19(6):313–319. [PubMed] [Google Scholar]
- 18.Schmidt PJ, Cardoso GM, Ross JL, Haq N, Rubinow DR, Bondy CA. Shyness, social anxiety, and impaired self-esteem in Turner syndrome and premature ovarian failure. JAMA. 2006 Mar 22;295(12):1374–1376. doi: 10.1001/jama.295.12.1374. [DOI] [PubMed] [Google Scholar]
- 19.Spielberger CD. State-Trait Anxiety Inventory for Children. Palo Alto, Calif: Consulting Psychologists Press; 1973 pp. [Google Scholar]
- 20.Garner DM. Eating Disorder Inventory-II: Professional manual. Odessa, FL: Psychological Assessment Resources; 1991. [Google Scholar]
- 21.Cooper PJ, Taylor MJ, Cooper Z, Fairburn CG. The development and validation of the Body Shape Questionnaire. Int J Eat Disord. 1986;6:485–494. [Google Scholar]
- 22.http://www.mindgarden.com/products/staisch.htm
- 23.Walf AA, Paris JJ, Frye CA. Chronic estradiol replacement to aged female rats reduces anxiety-like and depression-like behavior and enhances cognitive performance. Psychoneuroendocrinology. 2009 Jul;34(6):909–916. doi: 10.1016/j.psyneuen.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halmi KA, Agras WS, Crow S, et al. Predictors of treatment acceptance and completion in anorexia nervosa: implications for future study designs. Arch Gen Psychiatry. 2005 Jul;62(7):776–781. doi: 10.1001/archpsyc.62.7.776. [DOI] [PubMed] [Google Scholar]
- 25.Brownstone L, Anderson K, Beenhakker J, Lock J, Le Grange D. Recruitment and retention in an adolescent anorexia nervosa treatment trial. Int J Eat Disord. 2012 Sep;45(6):812–815. doi: 10.1002/eat.22010. [DOI] [PMC free article] [PubMed] [Google Scholar]