Abstract
Lenalidomide is the approved treatment for patients with red blood cell (RBC) transfusion-dependent lower-risk myelodysplastic syndromes (MDS) and chromosome 5q deletion (del(5q)). We report the long-term outcomes (median follow-up 3.2 years) in patients treated with lenalidomide in the MDS-003 trial. RBC transfusion independence (TI) ⩾8 weeks was achieved in 97 of 148 treated patients (65.5%), with a median response duration of 2.2 years. Partial or complete cytogenetic response was achieved by 63 of 88 evaluable patients (71.6%). Median overall survival (OS) was longer in patients achieving RBC-TI ⩾8 weeks (4.3 vs 2.0 years in non-responders; P<0.0001) or cytogenetic response (4.9 vs 3.1 years in non-responders; P=0.010). Time to acute myeloid leukemia (AML) progression was longer in patients achieving RBC-TI ⩾8 weeks or any cytogenetic response versus non-responders (P=0.001 and P=0.0002, respectively). In a landmark multivariate analysis, RBC-TI ⩾8 weeks was associated with prolonged OS (P<0.001) and a trend toward reduced relative risk of AML progression (P=0.080). Among these lower-risk MDS patients with del(5q), lenalidomide was associated with prolonged RBC-TI and cytogenetic responses, which were linked to improved OS and reduced risk of AML progression.
Keywords: acute myeloid leukemia, del(5q), lenalidomide, myelodysplastic syndromes, transfusion independence
Introduction
Myelodysplastic syndromes (MDS) with chromosome 5q deletion (del(5q)) is clinically and pathologically distinct from other MDS subtypes. It is characterized by hypo-proliferative anemia arising from impaired ribosome assembly, accompanied by dysplastic megakaryocytes.1, 2, 3 Most patients become red blood cell (RBC)-transfusion dependent within the first year of diagnosis and have less durable responses to recombinant erythropoiesis-stimulating agents compared with other MDS subtypes.4, 5, 6 The 5q− syndrome was first described in 1974 as a constellation of features, including isolated del(5q), hypoplastic anemia, mild leukopenia, normal or elevated platelet count with atypical megakaryocytes and an indolent clinical course.7 Subsequent reports confirmed that patients with del(5q) MDS have a favorable prognosis compared with most other MDS subtypes, but that bone marrow blast counts >5%, greater cytogenetic complexity, RBC transfusion-dependence and significant non-erythroid cytopenias were all associated with reduced overall survival (OS) and a greater risk of acute myeloid leukemia (AML) progression.4, 8, 9
Lenalidomide is approved in the United States for treatment of transfusion-dependent anemia in patients with International Prognostic Scoring System (IPSS) defined Low- or Intermediate (Int)-1-risk MDS, associated with del(5q), with or without additional cytogenetic abnormalities. In the multicenter, phase II registration trial of lenalidomide, MDS-003, 67% of patients achieved RBC-transfusion independence (TI), which was sustained for a median duration of 2.2 years.10, 11 Unlike with erythropoiesis-stimulating agents, lenalidomide was effective in heavily transfusion-dependent patients, and it was associated with suppression of the del(5q) clone, with all cytogenetic responders achieving RBC-TI.10 The efficacy of lenalidomide was later confirmed in a phase III randomized, placebo-controlled trial (MDS-004).12 Prolonged suppression of the primary MDS clone in patients with adverse cytogenetic features may favorably impact the risk for disease progression and possibly extend survival in patients with del(5q). To examine the potential benefit of prolonged suppression of the del(5q) clone with lenalidomide, we analyzed the long-term outcomes of OS and AML progression in patients enrolled in the MDS-003 trial.
Patients and methods
Patients and study design
In the MDS-003 study, RBC transfusion-dependent patients with IPSS-defined Low- or Int-1-risk MDS and del(5q), with or without additional chromosomal abnormalities, were treated with lenalidomide 10 mg for 21 days of each 28-day cycle or daily. Full details of patient eligibility criteria and study design have been described previously.10
A non-interventional extension study was conducted between October 2009 and 8 October 2010 to collect long-term follow-up data in accordance with good clinical practice and regulatory requirements, including the Declaration of Helsinki, and was approved by institutional review boards at the participating centers. As of June 2009, 76 of 148 patients enrolled in MDS-003 had died; therefore, 72 patients were eligible for follow-up. Of these, 18 patients were excluded because the study site did not participate (16 patients) or patient consent was unavailable (2 patients). Informed consent (or, for deceased patients, legal consent from next of kin or a legal representative) was obtained for 33 patients, and information was obtained for the remaining 21 patients from other sources, including the United States Social Security Death Index, public records, hospital records and verbal reports from healthcare professionals, next of kin or legal representatives. For patients with AML progression, clinical and laboratory documentation was collected to confirm the diagnosis. For deceased patients with confirmed AML, the primary cause of death was determined based on available documentation, including autopsy reports, hospitalization, emergency department or out-patient medical records, death certificates, verbal reports, United States Social Security Database or public records. All analyses were performed at Celgene Corporation, Summit, NJ, USA. All authors had access to the data, were involved in analyzing and interpreting data and approved the final version of the manuscript.
Clinical end points
Clinical end points included erythroid and cytogenetic response, duration of RBC-TI, OS and time to AML progression. Erythroid and cytogenetic responses were evaluated according to modified International Working Group criteria.13 RBC-TI was defined as a transfusion-free period of ⩾8 consecutive weeks accompanied by an increase in hemoglobin of ⩾1 g/dl. RBC-TI for ⩾26 weeks was also assessed. Bone marrow assessments for pathologic and cytogenetic responses were performed at weeks 24 and 53 at treatment termination and when clinically indicated. Cytogenetic response was determined in patients who had ⩾20 bone marrow cells in metaphase analyzed at baseline and during active treatment. Cytogenetic progression was defined as any of the following: (a) cytogenetic relapse, reappearance of the clonal abnormality present at baseline after a complete cytogenetic response or an increase in number of abnormal metaphases after a cytogenetic partial response, so that the criteria for partial response were no longer met; (b) clonal evolution, development of secondary abnormalities within the del(5q) clone or (c) development of independent clones distinct from the del(5q) clone. Disease progression was defined as progression to a more advanced French-American-British (FAB) subtype or AML (according to FAB criteria). Duration of OS was defined as time from first study dose of lenalidomide to death. Time to AML progression was defined as time from first study dose to diagnosis of AML. One patient diagnosed with AML at baseline was excluded from the analysis.
Statistical analysis
Analyses were based on combined data from the original MDS-003 study and the follow-up collection phase. The data reported here may differ from those in the original report due to a difference in the data cutoff used: 15 July 2005 in the original report10 and 8 October 2010 in the current analysis. Median OS, duration of RBC-TI and AML progression were estimated using the Kaplan–Meier method. Patients without a reported death date were censored at the last known date they were alive. Patients with unknown status or missing date of AML progression (including 18 patients who did not participate in follow-up) were censored at date of last contact they were known to be AML-free. Time to AML progression was also analyzed using Gray's test, with death as a competing risk.
Univariate comparisons were performed using Fisher's exact test or t-test. All reported P values are two-sided, with a P value<0.05 considered statistically significant. Multivariate analysis of covariates associated with OS and AML progression was performed using a Cox proportional hazards model. A backward elimination variable-selection approach was applied to remove variables from the model until all remaining variables had P<0.15. The following variables were used: age (years); sex (male vs female); IPSS risk (Int-1-, Int-2- or High-risk vs Low-risk); FAB classification (refractory anemia with excess blasts (RAEB) or chronic myelomonocytic leukemia (CMML) vs refractory anemia (RA) or RA with ringed sideroblasts (RARS)); time since MDS diagnosis (years); transfusion burden (units/8 weeks); bone marrow blast count (⩾5 vs <5%); number of cytopenias (2 or 3 vs 1); platelet count (109/l); neutrophil count (109/l); minimum hemoglobin concentration during the 8-week baseline period (g/dl); cytogenetic complexity (del(5q) plus ⩾1 abnormality vs isolated del(5q)); World Health Organization prognostic scoring system risk group (High or Very High vs Intermediate or Low); and RBC-TI ⩾8 weeks as a time-dependent variable.
To reduce potential bias, a 6-month landmark analysis was used to assess OS (excluding patients who died within the first 6 months) by RBC-TI ⩾8 weeks and cytogenetic response.
Results
Patient characteristics
Baseline characteristics are summarized in Table 1. The median age was 71.0 years and 65.5% of patients were female. Median time since MDS diagnosis was 2.5 years and 72.3% of patients had a transfusion burden of ⩾4 RBC units per 8 weeks. Most patients had RA or RARS (52.7% and 10.8%, respectively). Overall, 74.3% of patients had isolated del(5q) (including 27.0% with 5q− syndrome) and 25.0% had del(5q) plus ⩾1 additional abnormality. Median follow-up was 3.2 years (range: 0.03–6.8). Baseline characteristics of patients who were included in the follow-up phase were comparable with those who were not included in the follow-up phase, with the exception of age (P=0.001), karyotype (P=0.042), karyotype IPSS risk score (P=0.044) and absolute neutrophil count (P=0.029) (Table 1).
Table 1. Baseline characteristics of patients in the MDS-003 study.
| Characteristic | All patients (N=148) | Patients in follow-up phase (n=54) | Patients not in follow-up phase (n=94) |
|---|---|---|---|
| Age, years | |||
| Median | 71.0 | 65.5 | 72.1 |
| Range | 37.0–95.0 | 37.0–91.0 | 45.0–95.0 |
| Sex, n (%) | |||
| Female | 97 (65.5) | 39 (72.2) | 58 (61.7) |
| Male | 51 (34.5) | 15 (27.8) | 36 (38.3) |
| FAB classification (central review), n (%) | |||
| Refractory anemia | 78 (52.7) | 33 (61.1) | 45 (47.9) |
| Refractory anemia with ringed sideroblasts | 16 (10.8) | 4 (7.4) | 12 (12.8) |
| Refractory anemia with excess blasts | 30 (20.3) | 7 (13.0) | 23 (24.5) |
| Chronic myelomonocytic leukemia | 3 (2.0) | 1 (1.9) | 2 (2.1) |
| Other or unable to classify | 21 (14.2) | 9 (16.7) | 12 (12.8) |
| Time since MDS diagnosis, years | |||
| Median | 2.5 | 2.5 | 2.6 |
| Range | 0.1–20.7 | 0.1–15.3 | 0.2–20.7 |
| Karyotype, n (%) | |||
| Isolated del(5q) | 110 (74.3) | 47 (87.0) | 63 (67.0) |
| 5q− syndrome | 40 (27.0) | 19 (35.2) | 21 (22.3) |
| del(5q) plus 1 additional abnormality | 25 (16.9) | 5 (9.3) | 20 (21.3) |
| del(5q) plus ⩾2 additional abnormalities | 12 (8.1) | 2 (3.7) | 10 (10.6) |
| Unable to classifya | 1 (0.7) | 0 | 1 (1.1) |
| Number of cytopenias, n (%) | |||
| 1 | 81 (54.7) | 26 (48.1) | 55 (58.5) |
| 2 or 3 | 63 (42.6) | 27 (50.0) | 36 (38.3) |
| Missing | 4 (2.7) | 1 (1.9) | 3 (3.2) |
| Bone marrow blast count, n (%) | |||
| <5% | 101 (68.2) | 38 (70.4) | 63 (67.0) |
| ⩾5% | 28 (18.9) | 7 (13.0) | 21 (22.3) |
| Missing | 19 (12.8) | 9 (16.7) | 10 (10.6) |
| RBC transfusion burden (units/8 weeks), n (%) | |||
| <4 units/8 weeks | 35 (23.6) | 17 (31.5) | 18 (19.1) |
| ⩾4 units/8 weeks | 107 (72.3) | 33 (61.1) | 74 (78.7) |
| Missing | 6 (4.1) | 4 (7.4) | 2 (2.1) |
| Karyotype IPSS risk score (central review), n (%) | |||
| 0 | 112 (75.7) | 47 (87.0) | 65 (69.1) |
| 0.5 | 26 (17.6) | 6 (11.1) | 20 (21.3) |
| 1.0 | 10 (6.8) | 1 (1.9) | 9 (9.6) |
| IPSS risk category (central review), n (%) | |||
| Low-risk (0) | 49 (33.1) | 19 (35.2) | 30 (31.9) |
| Intermediate-1-risk (0.5–1.0) | 69 (46.6) | 23 (42.6) | 46 (48.9) |
| Intermediate-2-risk (1.5–2.0) | 7 (4.7) | 2 (3.7) | 5 (5.3) |
| High-risk (⩾2.5) | 2 (1.4) | 1 (1.9) | 1 (1.1) |
| Unable to classifyb | 21 (14.2) | 9 (16.7) | 12 (12.8) |
| Minimum hemoglobin level,c g/dl | |||
| Median | 7.8 | 7.9 | 7.7 |
| Range | 3.6–11.8 | 4.3–10.0 | 3.6–11.8 |
| Platelet count, × 109/l | |||
| Median | 223.0 | 246.0 | 204.5 |
| Range | 48.0–1200.0 | 63.0–919.0 | 48.0–1200.0 |
| Absolute neutrophil count, × 109/l | |||
| Median | 2.0 | 1.6 | 2.2 |
| Range | 0.3–20.7 | 0.4–4.8 | 0.3–20.7 |
| Neutropenia, n (%) | 43 (29.1) | 23 (42.6) | 20 (21.3) |
| Thrombocytopenia, n (%) | 24 (16.2) | 9 (16.7) | 15 (16.0) |
Abbreviations: del(5q), chromosome 5q deletion; FAB, French–American–British; IPSS, International Prognostic Scoring System; MDS, myelodysplastic syndromes; RBC, red blood cell.
del(5q) was confirmed by fluorescence in situ hybridization in one patient with normal metaphase karyotype.
Central review of cytogenetic data was unavailable.
Minimum hemoglobin concentration during the 8-week baseline period.
Erythroid response
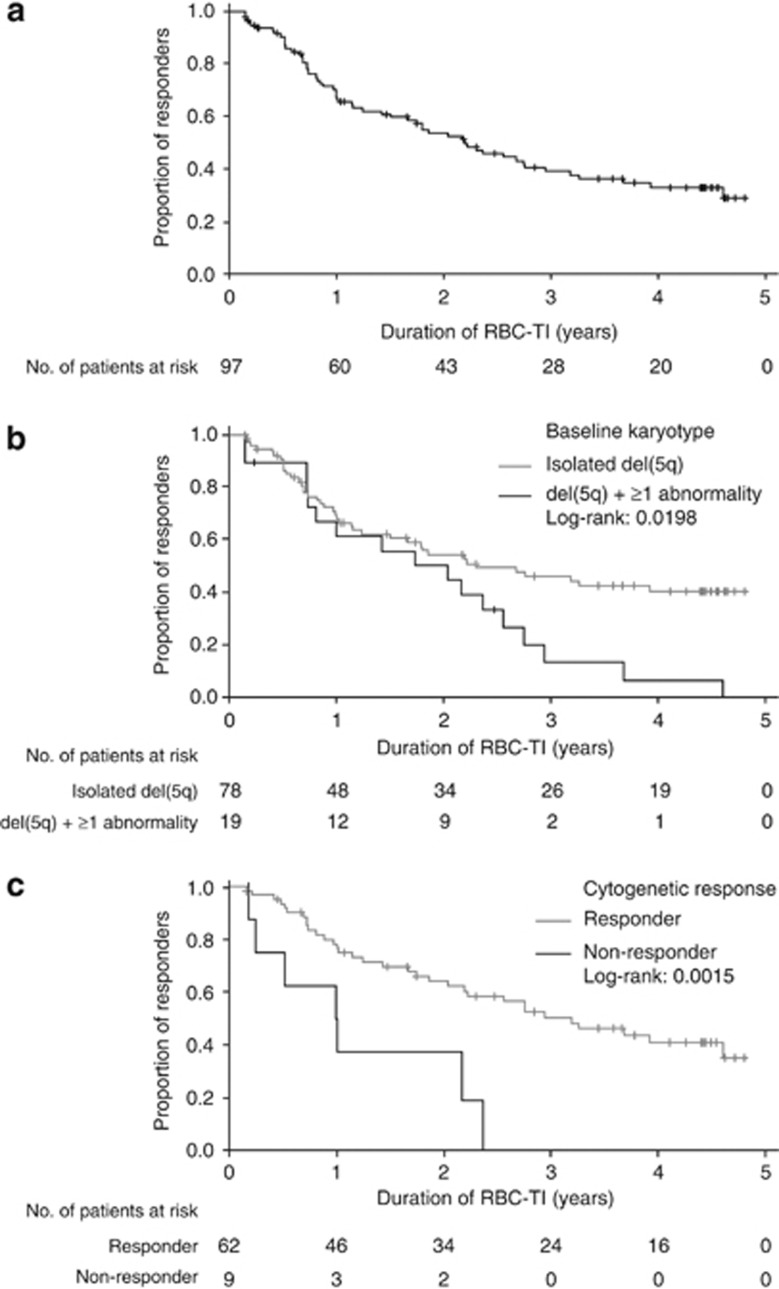
In this updated analysis, RBC-TI⩾8 weeks with hemoglobin improvement was achieved in 97 patients (65.5%) (Table 2). Sixteen additional patients (10.8%) had a minor erythroid response, yielding an overall erythroid response rate of 76.4%. Median time to RBC-TI ⩾8 weeks was 1.3 months (95% CI, 1.1–1.5). RBC-TI ⩾8 weeks rates were similar when analyzed by IPSS risk (P=0.205) or karyotype complexity (P=0.155). Rates of RBC-TI ⩾8 weeks appeared to be higher and time to response shorter in patients who were included in the follow-up phase than in the overall population (85.2% vs 65.5% and 0.8 vs 1.3 months, respectively) (Table 2). Median duration of RBC-TI ⩾8 weeks in the overall population was 2.2 years (range: 1.5–2.9) (Figure 1, panel a). Response was maintained for ⩾1 year in 66 patients (68.0%) and ⩾2 years in 52 patients (53.6%), while 28 patients (29.0%) remained TI and on-study at data cutoff. Median duration of RBC-TI ⩾8 weeks was longer in patients with isolated del(5q) than in those with del(5q) plus additional abnormalities (2.3 vs 2.0 years; P=0.020) (Figure 1, panel b). In patients evaluable for cytogenetic response, median duration of RBC-TI was longer in those who achieved a cytogenetic response compared with non-responders (3.2 vs 1.0 years; P=0.0015) (Figure 1, panel c).
Table 2. Erythroid and cytogenetic responses to lenalidomide.
| Response | All patients (N=148) | Patients in follow-up phase (n=54) | Patients not in follow-up phase (n=94) |
|---|---|---|---|
| Erythroid response, n/N (%) | |||
| RBC-TI ⩾8 weeks | 97/148 (65.5) | 46/54 (85.2) | 51/94 (54.3) |
| Minor responsea | 16/148 (10.8) | 6/54 (11.1) | 10/94 (10.6) |
| RBC-TI ⩾8 weeks+minor response | 113/148 (76.4) | 52/54 (96.3) | 61/94 (64.9) |
| RBC-TI ⩾8 weeks by IPSS, n/N (%)b | |||
| Low-risk | 34/49 (69.4) | 15/19 (78.9) | 19/30 (63.3) |
| Intermediate-1-risk | 47/69 (68.1) | 21/23 (91.3) | 26/46 (56.5) |
| Intermediate-2-risk/High-risk | 3/9 (33.3) | 2/3 (66.7) | 1/6 (16.7) |
| Unable to classify | 13/21 (61.9) | 8/9 (88.9) | 5/12 (41.7) |
| RBC-TI ⩾8 weeks by baseline karyotype, n/N (%)c | |||
| Isolated del(5q) | 78/110 (70.9) | 40/47 (85.1) | 38/63 (60.3) |
| del(5q) plus 1 additional abnormality | 12/25 (48.0) | 4/5 (80.0) | 8/20 (40.0) |
| del(5q) plus ⩾2 additional abnormalities | 7/12 (58.3) | 2/2 (100) | 5/10 (50.0) |
| Unable to classifyd | 0/1 (0) | 0/0 (0) | 0/1 (0.0) |
| Median time to RBC-TI ⩾8 weeks, months (95% CI) | 1.3 (1.1–1.5) | 0.8 (0.6–1.3) | 1.9 (1.3–2.5) |
| Cytogenetic response,e n/N (%) | |||
| Complete | 40/88 (45.5) | 20/40 (50.0) | 20/48 (41.7) |
| Partial | 23/88 (26.1) | 12/40 (30.0) | 11/48 (22.9) |
| Complete plus partial | 63/88 (71.6) | 32/40 (80.0) | 31/48 (64.6) |
Abbreviations: CI, confidence interval; del(5q), chromosome 5q deletion; IPSS, International Prognostic Scoring System; RBC-TI, red blood cell-transfusion independence.
Minor erythroid response was defined as ⩾50% reduction in the number of transfusions over 8 weeks.
P-value for RBC-TI ⩾8 weeks across subgroups was 0.205.
P-value for RBC-TI ⩾8 weeks across subgroups was 0.155.
del(5q) was confirmed by fluorescence in situ hybridization in one patient with normal metaphase karyotype.
Complete cytogenetic response evaluation required ⩾2 abnormal metaphases at baseline and was defined as the absence of abnormal metaphases after treatment. Partial cytogenetic response was defined as ⩾50% reduction in the proportion of abnormal metaphases.
Figure 1.
Duration of red blood cell-transfusion independence (RBC-TI) ⩾8 weeks response to lenalidomide in: (a) the overall population; (b) by baseline karyotype and (c) by cytogenetic response. Symbols indicate censored patients who remain transfusion independent at the time of data cutoff or at the time of study discontinuation. Abbreviation: del(5q), chromosome 5q deletion.
Cytogenetic response
Among 88 patients evaluable for cytogenetic response, 40 (45.5%) achieved a complete response and 23 (26.1%) achieved a partial response as best response, yielding an overall response rate of 71.6% (Table 2). Rates of cytogenetic responses appeared to be comparable in patients who were included in the follow-up phase (Table 2).
Among the 63 cytogenetic responders, 38 (60.3%) patients achieved an initial complete response. Of the patients with a complete response, 25 (39.7%) patients maintained this response during follow-up, 6 (9.5%) had subsequent partial response only, 6 (9.5%) relapsed and 1 (1.6%) had subsequent partial response followed by complete response and finally treatment failure. Fourteen (22.2%) patients who achieved a partial response maintained their response during follow-up, while 9 (14.3%) patients with a partial response relapsed. Furthermore, 2 patients who achieved an initial partial response improved to a complete response, yielding an overall study complete response in 40 (45.5%) evaluable patients: one patient with a partial response at day 58 had a complete response at day 143 and 1 patient had a partial response at day 149 and achieved a complete response at day 324.
Among patients who achieved a cytogenetic response, median time to best cytogenetic response (PR or CR) was 5.0 months (95% CI, 4.8–5.3) and median time to complete or partial cytogenetic response was 4.9 months (95% CI, 4.7–5.1) and 5.4 months (95% CI, 5.0–5.8), respectively.
Most cytogenetic responders also achieved RBC-TI ⩾8 weeks (62/63 (98.4%)) or RBC-TI ⩾26 weeks (57/63 (90.5%)). Among all 97 patients who achieved RBC-TI ⩾8 weeks, 62 (63.9%) were also cytogenetic responders, whereas the cytogenetic status of 26 (26.8%) patients was unknown.
Overall survival
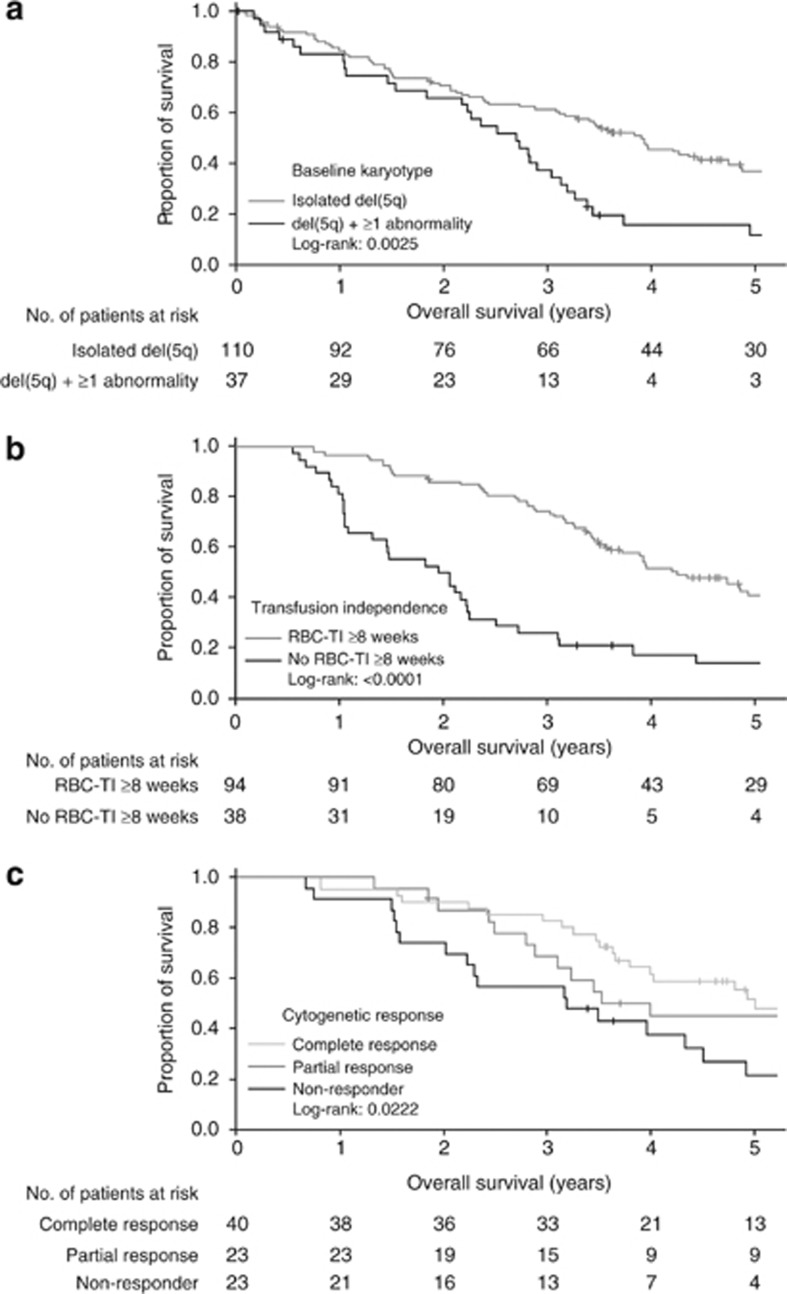
Median OS was 3.3 years (95% CI, 2.7–3.9). Cumulative 1- and 5-year OS rates were 84.2% and 30.4%, respectively. Patients with isolated del(5q) at baseline had longer median OS (3.9 years; 95% CI, 3.1–4.7) than patients with additional cytogenetic abnormalities (2.7 years; 95% CI, 1.8–3.0; P=0.003) (Figure 2, panel a).
Figure 2.
Kaplan–Meier estimates of overall survival according to: (a) baseline karyotype (1 patient had no baseline karyotype available and was excluded from the analysis); (b) red blood cell-transfusion independence (RBC-TI) ⩾8 weeks response (by the 6-month landmark analysis) and (c) partial or complete cytogenetic response (by the 6-month landmark analysis). Abbreviation: del(5q), chromosome 5q deletion.
Fewer patients with RBC-TI ⩾8 weeks died than non-responders (58.8% vs 86.3%). Similarly, fewer RBC-TI ⩾26 weeks responders died than non-responders (54.7% vs 87.1%). By the 6-month landmark analysis, median OS was longer in patients who achieved RBC-TI ⩾8 weeks (4.3 years; 95% CI, 3.6–5.9) than in non-responders (2.0 years; 95% CI, 1.3–2.2; P<0.0001) (Figure 2, panel b). Cumulative 1- and 5-year OS rates were higher in patients with RBC-TI ⩾8 weeks (96.8% and 41.1%, respectively) than in non-responders (81.6% and 14.0%, respectively). In a Cox proportional hazards model for OS by RBC-TI ⩾8 weeks response, increased risk of death was associated with lower platelet count (P=0.019) and higher absolute neutrophil count (P=0.030) in patients with RBC-TI ⩾8 weeks and with advanced age (P=0.007) in patients without RBC-TI ⩾8 weeks.
Fewer cytogenetic responders died (50.8%) than non-responders (80.0%) or patients not evaluable for cytogenetic response (81.7%). By the 6-month landmark analysis, median OS was significantly longer in patients with a cytogenetic response (4.9 vs 3.1 years in non-responders; P=0.010). Similarly, median OS was significantly longer in patients with a partial cytogenetic response (3.9 years; 95% CI, 2.8 to not evaluable) and in patients with complete cytogenetic response (4.9 years; 95% CI, 3.9 to not evaluable) than in non-responders (3.1 years; 95% CI, 2.0–4.3; P=0.022) (Figure 2, panel c). No significant difference in median OS was reported between patients achieving partial or complete cytogenetic response (3.9 vs 4.9 years; P=0.369). Cumulative 1- and 5-year OS rates were higher in cytogenetic responders (96.8% and 46.7%, respectively) than in non-responders (91.3% and 24.2%, respectively). The incidence of death was lowest in the 57 patients who achieved both cytogenetic response and RBC-TI ⩾26 weeks (47.4%), and highest in the 17 patients who achieved neither RBC-TI ⩾26 weeks nor cytogenetic response (82.4% P=0.001).
Cox proportional hazards model results are shown in Table 3. By the 6-month landmark analysis, RBC-TI ⩾8 weeks was associated with a 73% reduction in the relative risk of death (P<0.001). Baseline factors that negatively affected OS were advanced age (P<0.001), male sex (P=0.012), bone marrow blast count ⩾5% (P=0.015), lower platelet count (P=0.030) and lower minimum hemoglobin level in the 8-week baseline period (not correlated with transfusion burden) (P=0.035). The association between increasing karyotype complexity and death was of borderline significance (65% increased risk; P=0.065). Similar results were observed when the overall population was analyzed, except that bone marrow blast count and platelet count did not significantly affect OS, whereas unfavorable FAB subtype (RAEB or CMML) negatively affected OS (P=0.009).
Table 3. Results of Cox proportional hazards model for OS and AML progression excluding patients who died in the first 6 months (landmark analysis) and including all patients.
| Model factors | Hazard ratio (95% CI) | P-value |
|---|---|---|
| 6-Month landmark analysis: OS (N=132) | ||
| Age, years | 1.05 (1.03–1.08) | <0.001 |
| Sex (male vs female) | 1.95 (1.16–3.28) | 0.012 |
| del(5q) (plus ⩾1 abnormality vs isolated) | 1.65 (0.97–2.81) | 0.065 |
| Bone marrow blasts (⩾5% vs <5%) | 2.03 (1.15–3.58) | 0.015 |
| Platelet count, × 109/l | 1.00 (0.99–1.00) | 0.030 |
| Absolute neutrophil count, × 109/l | 1.14 (0.97–1.34) | 0.119 |
| Minimum hemoglobin level,a g/dl | 0.78 (0.63–0.98) | 0.035 |
| RBC-TI ⩾8 weeks (time dependent) | 0.27 (0.16–0.46) | <0.001 |
| 6-Month landmark analysis: AML progression (N=131)b | ||
| Time since diagnosis, years | 0.89 (0.76–1.04) | 0.129 |
| Number of cytopenias (2 or 3 vs 1) | 2.82 (1.10–7.27) | 0.031 |
| IPSS risk score (High, Int-2, Int-1 vs Low)c | 5.29 (1.82–15.41) | 0.002 |
| RBC-TI ⩾8 weeks (time dependent) | 0.44 (0.17–1.10) | 0.080 |
| Overall population: OS (N=148) | ||
| Age, years | 1.05 (1.03–1.08) | <0.001 |
| Sex (male vs female) | 1.75 (1.07–2.89) | 0.027 |
| FAB (RAEB or CMML vs RA or RARS) | 1.95 (1.18–3.20) | 0.009 |
| Platelet count, × 109/l | 1.00 (0.99–1.00) | 0.124 |
| Absolute neutrophil count, × 109/l | 1.12 (1.00–1.31) | 0.150 |
| Minimum hemoglobin level,a g/dl | 0.81 (0.66–0.99) | 0.044 |
| RBC-TI ⩾8 weeks (time dependent) | 0.30 (0.18–0.49) | <0.001 |
| Overall population: AML progression (N=147)b | ||
| del(5q) (plus ⩾1 abnormality vs isolated) | 3.25 (1.46–7.23) | 0.004 |
| RBC-TI ⩾8 weeks (time dependent) | 0.48 (0.21–1.10) | 0.083 |
Abbreviations: AML, acute myeloid leukemia; CMML, chronic myelomonocytic leukemia; del(5q), chromosome 5q deletion; FAB, French–American–British; Int, intermediate; IPSS, International Prognostic Scoring System; OS, overall survival; RA, refractory anemia; RAEB, RA with excess blasts; RARS, RA with ringed sideroblasts; RBC-TI, red blood cell-transfusion independence.
Notes: Patients without a death date were censored at last known date of being alive. Only factors with P<0.15 are presented.
Minimum hemoglobin concentration in the 8-week baseline period.
One patient was diagnosed with AML at baseline and therefore excluded from the analysis.
Most patients had Low or Intermediate-1 risk IPSS (33.1 and 46.6%, respectively).
Disease progression
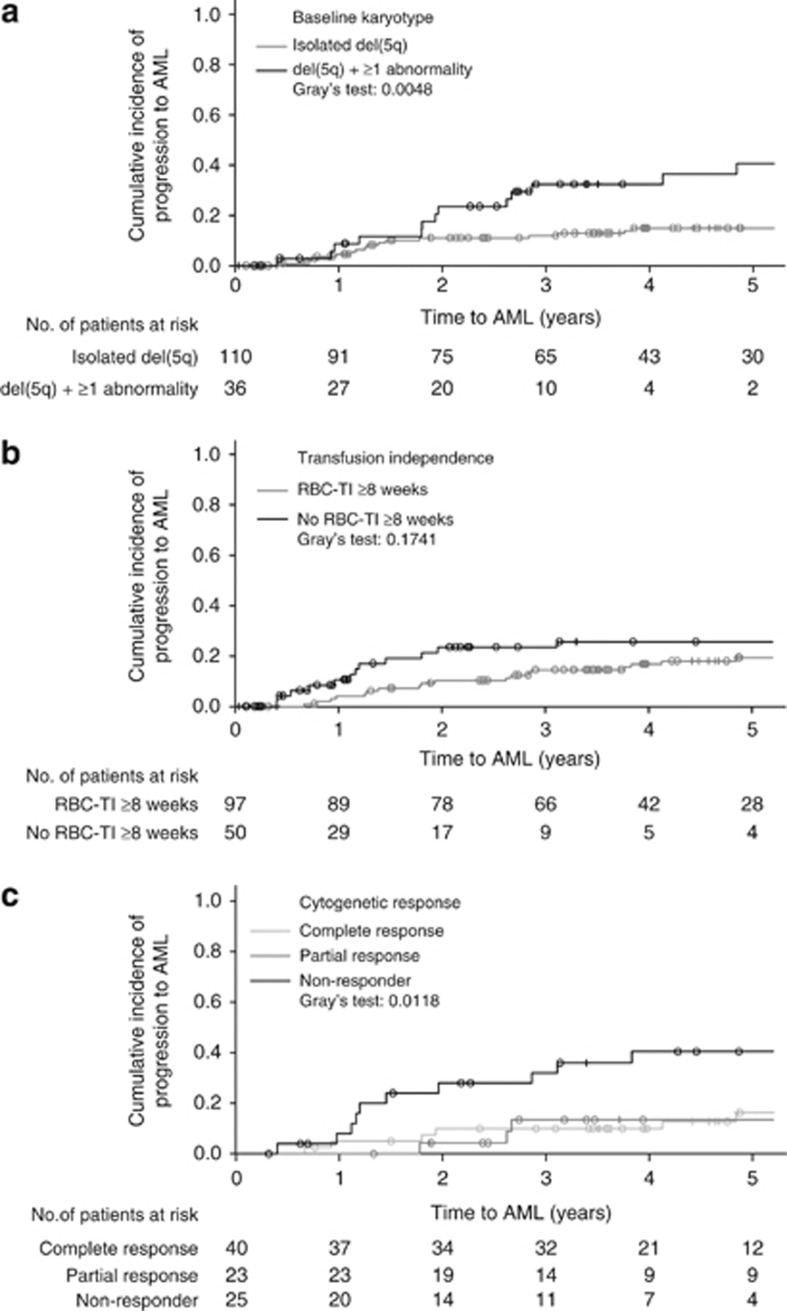
Seven patients (4.8%) progressed to a more advanced FAB subtype and 36 patients (24.5%) progressed to AML. Cumulative 1- and 5-year AML progression rates were 6.9% and 28.6%, respectively. AML progression occurred in 7 of 49 (14.3%) IPSS Low-risk patients (including 6 of 40 (15.0%) with 5q− syndrome), 20 of 69 (29.0%) Int-1-risk patients, 3 of 9 (33.3%) Int-2- or High-risk patients and 6 of 21 (28.6%) unclassified patients. Median follow-up duration for AML progression was 3.1 years. Median time to AML progression was not reached in patients with isolated del(5q) and was 4.1 years (95% CI, 2.6 to not evaluable) in patients with additional cytogenetic abnormalities (P=0.001). When analyzed with death as a competing risk, time to AML progression was longer in patients with isolated del(5q) compared with patients with additional cytogenetic abnormalities (P=0.005) (Figure 3, panel a).
Figure 3.
Time to acute myeloid leukemia (AML) progression with death as a competing risk according to: (a) baseline karyotype (one patient had no baseline karyotype available and was excluded from the analysis); (b) red blood cell-transfusion independence (RBC-TI) ⩾8 weeks response and (c) partial or complete cytogenetic response. Symbols indicate censored patients (+) or patients who died without AML (∘). Abbreviation: del(5q), chromosome 5q deletion.
Fewer patients with RBC-TI ⩾8 weeks progressed to AML than non-responders (20.6% vs 32.0%). Similarly, fewer RBC-TI ⩾26 weeks responders progressed to AML than non-responders (20.9% vs 29.5%). Median time to AML progression was not reached in patients with RBC-TI ⩾8 weeks (95% CI, not evaluable) and was 5.2 years (95% CI, 1.8 to not evaluable) in non-responders (P=0.001). Cumulative 1- and 5-year AML progression rates were lower in patients with RBC-TI ⩾8 weeks (4.3% and 23.7%, respectively) than in non-responders (13.1% and 42.5%, respectively). When analyzed with death as a competing risk, time to AML progression in patients with a RBC-TI ⩾8 weeks response was no longer significantly different from non-responders (P=0.174) (Figure 3, panel b). Fewer cytogenetic responders progressed to AML than non-responders (15.9% (10/63) vs 52.0% (13/25)). Median time to AML progression was not reached in patients who achieved partial or complete cytogenetic response and was 3.8 years (95% CI, 1.5 to not evaluable) in non-responders (P=0.002). Time to AML progression remained significant when analyzed with death as a competing risk (P=0.012) (Figure 3, panel c). There was no significant difference in time to AML progression between patients who achieved partial or complete cytogenetic response (P=0.847). Cumulative 1- and 5-year AML progression rates were lower in cytogenetic responders (3.2% and 14.9%, respectively) than in non-responders (8.7% and 60.2%, respectively).
The incidence of AML progression was lowest in patients who achieved both RBC-TI ⩾26 weeks and cytogenetic response and highest in patients who achieved neither RBC-TI ⩾26 weeks nor cytogenetic response (14.0% vs 52.9% P=0.009).
Cox proportional hazards model results in the overall population are presented in Table 3. By the 6-month landmark analysis, achievement of RBC-TI ⩾8 weeks was associated with a trend for reduced risk of AML progression (56% risk reduction; P=0.080). The relative risk of AML progression was increased approximately threefold in patients with 2 or 3 cytopenias at baseline (P=0.031) and fivefold in patients with IPSS defined Int-1-risk or higher risk MDS (P=0.002). In the overall population, RBC-TI ⩾8 weeks was also associated with a trend for reduced relative risk of AML progression (52% risk reduction; P=0.083), and del(5q) plus additional abnormalities was associated with an approximately threefold higher relative risk of AML progression (P=0.004); however, neither number of cytopenias nor IPSS category were significant.
Discussion
Lenalidomide yields high rates of RBC-TI and cytogenetic response in MDS patients with del(5q).10, 12, 14 The concordance between cytogenetic response and RBC-TI, and the selective action of lenalidomide on clones containing the del(5q) abnormality may be necessary for sustained hematologic improvement.15 Also, the ability of lenalidomide to suppress the del(5q) clone in heavily transfusion-dependent patients distinguishes it from recombinant erythropoiesis-stimulating agents. Analysis of the MDS-003 phase II trial suggests that induction of sustained RBC-TI and/or suppression of the del(5q) clone with lenalidomide may improve survival and reduce the risk of AML progression in transfusion-dependent MDS patients with del(5q).
Transfusion dependence is associated with decreased OS and greater risk of AML progression proportionate to monthly transfusion burden in MDS patients with <5% bone marrow blasts.16, 17 Similar findings were reported for patients with del(5q) in a recent analysis from the Mayo Clinic.8 In the current study, median OS was significantly prolonged in patients who achieved RBC-TI ⩾8 weeks compared with those who did not achieve RBC-TI (4.3 and 2.0 years, respectively; P<0.0001). Furthermore, the median OS reported in responders was approximately 1 year longer than that achieved with best supportive care only (3.3 years) in a similar patient population.18 Despite the significant differences in outcome, the data should be viewed with recognition of the inherent potential bias. For example, patients had to survive long enough to be classified as responders, whereas non-responders may have died at any time. The landmark analysis, which excluded patients who died in the first 6 months, was performed to reduce this bias. Consistent with results reported previously for the prospective, randomized, placebo-controlled MDS-004 study,12 multivariate analysis indicated that achievement of RBC-TI ⩾8 weeks was a significant predictor for OS and reduced the risk of AML progression in the landmark analysis. Achievement of RBC-TI with lenalidomide may improve the chance of survival in lower-risk MDS patients with del(5q) or it may be a surrogate factor indicating better underlying bone marrow function. Baseline factors affecting OS included age, sex, bone marrow blast and platelet counts, and minimum hemoglobin concentration. In addition, consistent with previous reports, complex karyotype at baseline was associated with reduced OS (P=0.065).9
Achievement of RBC-TI was closely associated with cytogenetic response, with 98.4% of cytogenetic responders achieving RBC-TI ⩾8 weeks (the only cytogenetic responder who did not achieve RBC-TI had isolated del(5q)). Consistent with this, cytogenetic response was associated with improved OS and a lower risk of AML progression. Given the small proportion of patients with 5q− syndrome (27.0%), it is unlikely that selective sensitivity of del(5q) subsets with more favorable features accounts for the significant difference in survival and AML risk between cytogenetic responders and non-responders. Interestingly, we found no significant difference in OS between patients achieving a partial or complete cytogenetic response, indicating that partial suppression of the del(5q) clone may be sufficient to impact survival.
Several disease characteristics were shown to predict AML progression in untreated patients, including karyotype complexity (P<0.001), platelet count (P=0.001) and bone marrow blast count (P=0.016).9 Results of the current study showed that the number of cytopenias (P=0.031) and IPSS category (P=0.002) were significant predictors of AML progression. AML progression was delayed in patients who achieved RBC-TI ⩾8 weeks (P=0.001) or cytogenetic response (P=0.002). Achievement of RBC-TI ⩾8 weeks was also shown to be an independent predictor of risk for AML progression. These long-term follow-up data corroborate previous observations that achievement of cytogenetic response and RBC-TI with lenalidomide is associated with reduced risk of AML progression in MDS patients with del(5q).12 However, when analyzed with death as a competing risk, time to AML progression in patients with a RBC-TI ⩾8 weeks response was no longer different from non-responders. In a competing risk model, death of any cause before transformation to AML is mathematically included in the statistical analysis to adjust for possible bias. A patient who dies due to an AML-unrelated event cannot develop AML. It should be noted, however, that since MDS patients are elderly and the disease itself leads to increased non-leukemic mortality, assessment is statistically challenging.
Achievement of RBC-TI or cytogenetic response with lenalidomide is a crucial determinant of long-term survival and AML progression in transfusion-dependent patients with lower-risk MDS and del(5q). Optimizing RBC-TI and cytogenetic response may, therefore, maximize the disease-modifying potential of lenalidomide in these patients.
Acknowledgments
This study was supported by Celgene Corporation. The authors received editorial and writing support in the preparation of this manuscript from Christian Geest, PhD, of Excerpta Medica, sponsored by Celgene Corporation. The authors wish to express their appreciation of the assessment of the bone marrow cytogenetics conducted in this study by Dr Gordon Dewald, who passed away on 26 February 2010.
Disclaimer
The authors are fully responsible for content and editorial decisions for this manuscript. No authors received honoraria for writing.
Author contributions
AFL designed the research; AFL, JMB, MAS, JMS, SDN and AG performed research and collected data; TF performed the statistical analysis; AFL wrote the manuscript. All authors were involved in analyzing and interpreting data and approved the final version of the manuscript.
AFL is a consultant for and has received honoraria and research funding from Celgene Corporation. MAS is a consultant for and has received honoraria from Celgene Corporation. BS, TF and RDK are employees of and hold equity in Celgene Corporation. JMS has received honoraria and research funding from Celgene Corporation. SDN has received honoraria from Celgene Corporation. AG is a consultant for and has received honoraria from Celgene Corporation. JMB declares no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on the Leukemia website (http://www.nature.com/leu)
Supplementary Material
References
- Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292–2302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- Nimer SD. Myelodysplastic syndromes. Blood. 2008;111:4841–4851. doi: 10.1182/blood-2007-08-078139. [DOI] [PubMed] [Google Scholar]
- Ebert BL, Pretz J, Bosco J, Chang CY, Tamayo P, Galili N, et al. Identification of RPS14 as a 5q- syndrome gene by RNA interference screen. Nature. 2008;451:335–339. doi: 10.1038/nature06494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giagounidis AA, Germing U, Haase S, Hildibrandt B, Schlegelberger B, Schoch C, et al. Clinical, morphological, cytogenetic, and prognostic features of patients with myelodysplastic syndromes and del(5q) including band q31. Leukemia. 2004;18:113–119. doi: 10.1038/sj.leu.2403189. [DOI] [PubMed] [Google Scholar]
- Howe RB, Porwit-MacDonald A, Wanat R, Tehranchi R, Hellström-Lindberg E. The WHO classification does make a difference. Blood. 2004;103:3265–3270. doi: 10.1182/blood-2003-06-2124. [DOI] [PubMed] [Google Scholar]
- Kelaidi C, Park S, Brechignac S, Mannone L, Vey N, Dombret H, et al. Treatment of myelodysplastic syndromes with 5q deletion before the lenalidomide era; the GFM experience with EPO and thalidomide. Leuk Res. 2008;32:1049–1053. doi: 10.1016/j.leukres.2007.11.037. [DOI] [PubMed] [Google Scholar]
- Van den Berghe H, Cassiman JJ, David G, Fryns JP, Michaux JL, Sokal G. Distinct haematological disorder with deletion of the long arm of no. 5 chromosome. Nature. 1974;251:437–438. doi: 10.1038/251437a0. [DOI] [PubMed] [Google Scholar]
- Patnaik MM, Lasho TL, Finke CM, Gangat N, Caramazza D, Holtan SG, et al. WHO-defined 'myelodysplastic syndrome with isolated del(5q)' in 88 consecutive patients: survival data, leukemic transformation rates and prevalence of JAK2, MPL and IDH mutations. Leukemia. 2010;24:1283–1289. doi: 10.1038/leu.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallo M, Cervera J, Schanz J, Such E, Garaa-Manero G, Luño E, et al. Impact of adjunct cytogenetic abnormalities for prognostic stratification in patients with myelodysplastic syndrome and deletion 5q. Leukemia. 2011;25:110–120. doi: 10.1038/leu.2010.231. [DOI] [PubMed] [Google Scholar]
- List A, Dewald G, Bennett J, Giagounidis A, Raza A, Feldman E, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355:1456–1465. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- List AF, Wride K, Dewald GW, Bennett JM, Giagounidis A, Kurtin S, et al. Cytogenetic response to lenalidomide is associated with improved survival in patients with chromosome 5q deletion Leuk Res 200731(Suppl 1S38(abstract C028). [Google Scholar]
- Fenaux P, Giagounidis A, Selleslag D, Beyne-Rauzy O, Mufti G, Mittelman M, et al. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion-dependent patients with Low-/Intermediate-1-risk myelodysplastic syndromes with del5q. Blood. 2011;118:3765–3776. doi: 10.1182/blood-2011-01-330126. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Bennett JM, Kantarjian H, Pinto A, Schiffer CA, Nimer SD, et al. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood. 2000;96:3671–3674. [PubMed] [Google Scholar]
- List A, Kurtin S, Roe DJ, Buresh A, Mahadevan D, Fuchs D, et al. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med. 2005;352:549–557. doi: 10.1056/NEJMoa041668. [DOI] [PubMed] [Google Scholar]
- Wei S, Chen X, Rocha K, Epling-Burnette PK, Djeu JY, Liu Q, et al. A critical role for phosphatase haplodeficiency in the selective suppression of deletion 5q MDS by lenalidomide. Proc Natl Acad Sci USA. 2009;106:12974–12979. doi: 10.1073/pnas.0811267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcovati L, Porta MG, Pascutto C, Invernizzi R, Boni M, Travaglino E, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol. 2005;23:7594–7603. doi: 10.1200/JCO.2005.01.7038. [DOI] [PubMed] [Google Scholar]
- Malcovati L, Della Porta MG, Cazzola M. Predicting survival and leukemic evolution in patients with myelodysplastic syndrome. Haematologica. 2006;91:1588–1590. [PubMed] [Google Scholar]
- Germing U, Lauseker M, Hildebrandt B, Symeonidis A, Cermak J, Pfeilstöcker M, et al. Survival, prognostic factors, and rates of leukemic transformation in a multicenter study of 303 untreated patients with MDS and del(5q) [abstract] Blood 2009114Abstract 945. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.