Abstract
Purpose
Deregulation of IGF signaling plays an important role in prostate cancer and contributes to invasion and metastasis. We determined the effect of apigenin, a plant flavone, on IGF signaling and its downstream targets in TRAMP mice.
Methods
Mice received p.o. apigenin at 20 and 50µg/day dose for 20 weeks. ELISA, Western blotting and immunohistochemistry were performed to examine the IGF-axis and its regulated pathway in response to apigenin intake.
Results
Increased serum levels of IGF-I, VEGF, uPA and concomitant decrease in IGFBP3 were observed; p-Akt (Ser473), p-ERK1 (T202/Y204) and p-ERK2 (T185/Y187) expression increased in the dorso-lateral prostate of TRAMP mice during the course of cancer progression as a function of age. P.o. administration of apigenin resulted in substantial reduction in the levels of IGF-I and increase in the levels of IGFBP-3 in the serum and the dorso-lateral prostate. This modulation of IGF/IGFBP-3 was associated with an inhibition of p-Akt and p-ERK1/2. Apigenin intake resulted in marked inhibition of VEGF, uPA, MMP-2 and MMP-9 which coincided with tumor growth inhibition and complete absence of metastasis in TRAMP mice.
Conclusions
Our results indicate that apigenin effectively suppressed prostate cancer progression in TRAMP mice by attenuating IGF-I/IGFBP-3 signaling and inhibiting angiogenesis and metastasis.
Keywords: insulin-like growth factor, prostate cancer, apigenin, angiogenesis, metastasis
INTRODUCTION
IGF-I signaling plays an important role during tumor development and its upregulation has been observed in many human tumors including prostate cancer (1, 2). Epidemiological evidence suggests that patients with elevated serum IGF-I levels in the upper quartile of the reference range had a 4-fold increase risk of developing prostate cancer when compared with patients whose serum IGF-I levels were in the lower quartile (3–5). Furthermore, studies indicate that circulating IGF-I levels may be a better predictor of prostate cancer than serum PSA (6). Studies demonstrate that levels of IGFBP2 and IGFBP3 are also altered in the serum and prostate tissue of prostate cancer patients (7, 8), suggesting that the IGF-I signaling axis could be an important contributor in the development and progression of prostate cancer.
Two IGF ligands are known viz. IGF-I and IGF-II; these ligands are primarily synthesized by the liver (1, 2). The biological functions of these ligands are mediated mainly by the IGF-I receptor, which binds IGF-I with higher affinity than IGF-II (2). IGF-I binds to the IGF-IR, which is a heterdimeric transmembrane protein comprised of two subunits -α and -β. The β subunit expresses intrinsic tyrosine kinase activity and is activated upon ligand binding to the α-subunit (2). Tyrosine kinase activation results in autophosphorylation within the kinase domain, leading to downstream signaling. The activated IGF-IR then phosphorylates adapter proteins, such as IRS-1, which in turn activates phosphatidylinositol 3-kinase (PI3K)/Akt and the mitogen-activated protein kinase signaling pathways. Activation of the latter pathways has been shown to contribute to cancer progression (2).
Studies indicate that IGF-I production in the normal prostate is by the stromal cells, whereas IGF-IR is expressed by normal prostate epithelial cells; these features play a role in the maintenance of paracrine regulation (9). In vivo studies demonstrate that IGF-I may increase proliferation of prostate cancer cells, whereas antisense-mediated inhibition of IGF-IR suppresses cell invasiveness and in vivo tumor growth (10). Deregulated expression of IGF-I in prostate epithelium leads to neoplasia in transgenic mice (11). Upregulation of IGF-I expression has been found in neoplastic prostate epithelial cells; it has been postulated that this is an adaptive response that may contribute to the evolution of androgen-independent prostate cancer. Increased IGF-I signaling induces vascular endothelial growth factor (VEGF) and triggers ‘angiogenic switch’ leading to prostatic neovascularization (12). It is also known that type I IGF-receptor is a regulator of matrix metalloproteinase-2 synthesis, which together with urokinase-type plasminogen activator (uPA) activation and VEGF production leads to increased tumor angiogenesis and metastasis (13, 14). Because this signaling is activated and contributes to cancer progression, inhibition of IGF-IR actions may be achievable through the IGF axis, thereby offering another rational approach to the prevention and/or therapy of prostate cancer.
Apigenin (4, 5, 7 trihydroxyflavone) is a natural bioflavonoid which has been shown to possess potent cancer preventive and therapeutic properties (15 and references therein). Apigenin inhibits the growth of various human cancer cells and induces apoptosis (16). Apigenin is a potent inhibitor of several protein kinases, including epidermal growth factor receptor and src tyrosine kinase (17, 18). Apigenin has been shown to modulate the expression of PI3K-Akt, MAPKs (ERK1/2, c-jun-N-terminal kinase, and p38), casein kinase-2 and other upstream kinases involved in the development and progression of cancer (19, 20). Our group has demonstrated that apigenin induces apoptosis in prostate cancer cells, both in vitro and in vivo, through upregulation of IGFBP-3 and suppression of IGF-I signaling (21, 22). Transgenic adenocarcinoma of the mouse prostate (TRAMP) mice spontaneously develops prostatic adenocarcinoma which simulates progressive form of human disease and therefore serves as a useful model for the study of prostate cancer (9). We have recently shown that oral intake of apigenin by TRAMP mice, at doses equivalent to human consumption of a healthy diet of flavonoids (6–64 mg/day of flavones and flavonols), inhibits the development of prostate cancer and its subsequent progression by blocking β-catenin signaling (23, 24). Understanding the molecular mechanism(s) of apigenin-mediated inhibition of prostate cancer is essential in developing rational mechanism-based approaches to the prevention and treatment of prostate cancer. In this report, we demonstrate that apigenin-mediated suppression of prostate cancer progression, metastasis and angiogenesis in TRAMP mice appears to be mediated through inhibition of IGF-I and its downstream signaling pathways.
MATERIALS AND METHODS
Materials
Antibodies for anti-IGF-I (sc-9013), anti-IGFBP-3 (sc-9028), anti-Akt (sc-8312), anti-MMP-2 (sc-13595), anti-MMP-9 (sc-10737) and anti-β-Actin (sc-47778) were procured from Santa Cruz Biotechnology, CA; whereas, total anti-ERK (4695), anti- p-ERK1/2 (4370), anti-p-Akt (Ser-473) and anti-p-Akt (Thr-308) were purchased from Cell Signaling Technology, MA. Anti-VEGF antibody was procured from Lab Vision Corporation, CA. Mouse IGFBP-3 (MGB300) and mouse/rat IGF-I (MG100) Quantikine ELISA Kit was obtained from R&D Systems, MN. Mouse VEGF (MMV00) Quantikine ELISA Kit, p-Akt (S473) (SUV887) pan-specific surveyor IC; p-ERK1 (T202/Y204)/ERK2 (T185/Y187) (SUV1018) surveyor IC and mouse uPA total antigen ELISA kits were purchased from Cell Sciences, MA.
Animals
Male and female heterozygous C57BL/TGN TRAMP mice, Line PB Tag 8247NG were purchased as breeding pairs from The Jackson Laboratory (Ann Arbor, MI). The animals were bred and maintained at the AAALAC-accredited Animal Resource Facility of Case Western Reserve University. Housing and care of the animals was in accordance with the guidelines established by the University’s Animal Research Committee and with the NIH Guidelines for the Care and Use of Laboratory Animals. Transgenic males for the studies were routinely obtained as [TRAMP×C57BL/6] F1 or as [TRAMP×C57BL/6] F2 offspring. Identity of transgenic mice was established by PCR based DNA-screening as previously described (23).
Study design and apigenin intake
The animals were fed with the autoclaved Teklad 8760 high-protein diet and tap water ad libitum throughout the study. In the first set of experiments, TRAMP and non-transgenic mice were divided into 4 groups and each group consisted of 8–10 mice. The 4 groups were sacrificed at 8, 16, 24 and 32 weeks of age, respectively. Blood and the dorso-lateral prostate tissue were collected for further investigation.
In the second experiment, eight week-old male TRAMP mice were equally divided into 3 groups consisting of 12 mice per group. Equal number of non-transgenic mice was considered for comparison. Apigenin (10 mg) was suspended in 1 ml vehicle material (0.5% methyl cellulose and 0.025% Tween 20) by sonication for 30 s at 4°C and further diluted for appropriate concentration. Apigenin, 20- and 50- µg/mouse/day (w/v) was administered by gavage in 0.2 ml of a vehicle consisting of 0.5% methyl cellulose and 0.025%Tween 20, daily for 6 days per week for 20 weeks, starting at 8 weeks of age. The per-oral route was chosen in order to simulate dietary consumption of flavonoids in humans. These doses are comparable to the daily consumption of flavonoid in humans as reported in previously published studies (24). It is important to emphasize that apigenin reaches its maximum concentration in blood 24 h post ingestion; therefore, we opted for daily dosing of apigenin in order to maintain peak blood levels in mice during these studies. Animal were sacrificed at specified ages; serum was isolated from blood and dorso-lateral prostate tissues were excised and weighed. Total prostate tissue lysates were prepared for ELISA and Western blotting.
Preparation and analysis of tissue
The dorsolateral prostates were excised and weighed, and a small portion was fixed overnight in 10% zinc–buffered formalin and then transferred to 70% ethanol. Sections (4µm) from 8 prostate tissues were cut from paraffin-embedded tissue and mounted on slides. The sections were stained with H&E as previously described (23) and were evaluated for the presence or absence of the following lesions: prostatic intraepithelial neoplasia, well-differentiated adenocarcinoma, moderately differentiated adenocarcinoma, and poorly differentiated adenocarcinoma. The histologic characteristics of these lesions have been well established and described in a previous publication (25).
ELISA assay
ELISA for IGF-I, IGFBP-3, VEGF and uPA were performed in serum samples of both TRAMP and non-transgenic littermates at various ages of sacrifice and analyses were reported in units/mL of serum. ELISA for p-Akt (Ser-473) and p-ERK (T202/Y204)/ (T185/Y187) were performed in the lystaes obtained from the dorso-lateral prostates of TRAMP and non-transgenic mice. The analyses were performed according to vendor’s protocol and results were reported in unit/per mg protein.
Metastases examination
Microscopic examinations of lymph nodes, liver, and lungs were done to evaluate for the presence of metastases. The India ink method was used to examine the lungs for metastasis as previously described (23).
Immunohistochemistry
IHC for PCNA was done on formalin-fixed, paraffin-embedded prostate tissue sections using a standard protocol as previously described using 3,3′-diaminobenzidene and counterstaining with Mayer's hematoxylin (23, 25).
Western Blot Analysis
Prostate tissues excised from mice were stored at −80°C. For Western blotting, 25µg protein was resolved over 4–20% Tris-glycine polyacrylamide gel and then transferred onto the nitrocellulose membrane. The blots were blocked using 5% nonfat dry milk and probed using appropriate primary antibodies overnight at 4°C. The membrane was then incubated with appropriate secondary antibody horseradish peroxidase conjugate (Santa Cruz Biotech) followed by detection using chemiluminescence ECL kit (GE Healthcare Biosciences). For equal loading of proteins, the membrane was probed with appropriate loading controls. Densitometric measurements of the bands in Western blot analysis were done using digitalized scientific software program using Kodak 2000R imaging system.
Statistical analysis
Changes of various biomarkers in prostate during the course of the experiments were analyzed by analysis of variance (ANOVA) using Kruskal-Wallis test, a nonparametric test based on Wilcoxon scores followed by pair-wise comparison in which P values were not adjusted for multiple comparisons. All tests were two sided and P<0.05 was considered to be statistically significant.
RESULTS
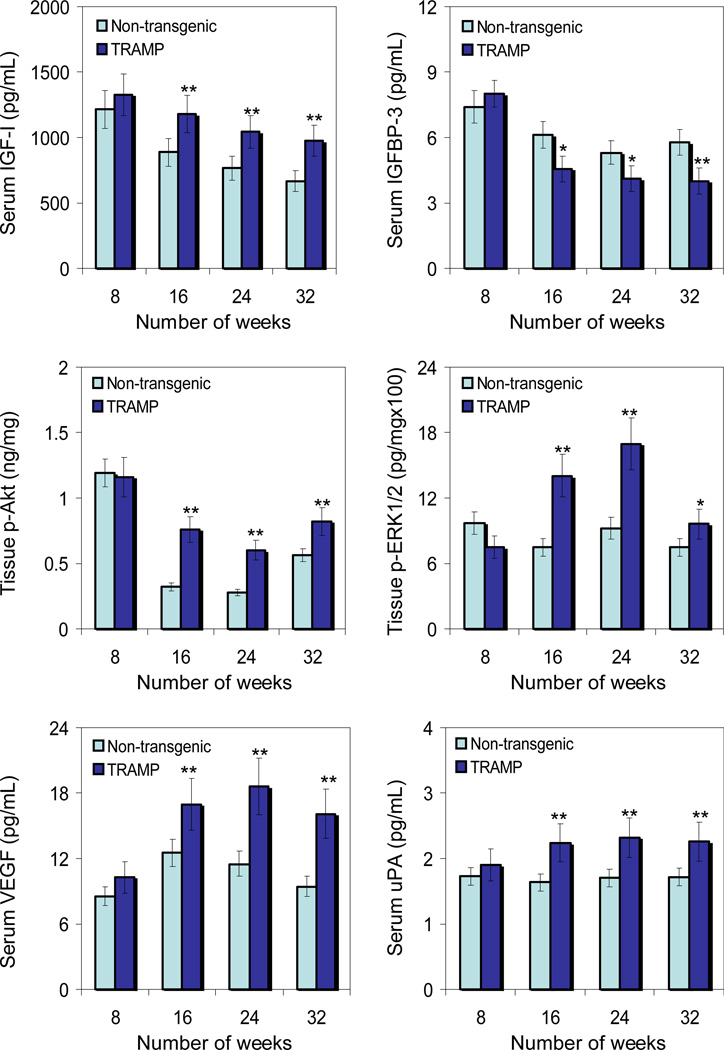
Earlier studies have demonstrated an increase in serum IGF-I levels in prostate cancer patients (3–6). Because IGFs are locally produced by most tissues and act in an autocrine and paracrine manner, we determined the serum levels of IGF-I and IGFBP3 in TRAMP and non-transgenic littermates with progressive age. As shown in figure 1, serum IGF-I levels were significantly higher in TRAMP mice, starting at 8 weeks of age and remained elevated throughout 32 weeks, compared to non-transgenic littermates. An increase of 109% to146% was noted from 8–32 weeks in TRAMP mice compared to non-transgenic littermates. In contrast, a decreasing trend in serum IGFBP-3 was noted in TRAMP mice compared to non-transgenic littermates with progressive age. A decrease of 25.5% to 30.8% between 16–32 weeks in TRAMP mice was noted compared to non-transgenic littermates, whereas the levels of serum IGFBP3 were almost similar at 8 weeks of age in both TRAMP and non-transgenic mice.
Fig.1.
Levels of insulin like growth factor-I(IGF-I), insulin like growth factor binding protein-3 (IGFBP-3), phosphorylated forms of Akt and extracellular signal-regulated kinases1/2 (ERK1/2), vascular endothelial growth factor (VEGF) and urokinase plasminogen activator (uPA) in the serum and dorso-lateral prostate during progressive stages of prostate cancer development in TRAMP mice and age-matched non-transgenic littermates. Serum levels of IGF-I, IGFBP-3, VEGF and uPA; whereas phosphorylated levels of Akt (Ser473) and ERK1 (T202/Y204)/ ERK2 (T185/Y187) were detected in the tissue lysates obtained from the dorso-lateral prostates by Enzyme-linked immunosorbant assay (ELISA) at 8, 16, 24 and 32 weeks of age in non-transgenic and TRAMP mice. Data represents the mean ± SE of 6 mice. *P < 0.05 and **P < 0.001 versus age-matched non-transgenic control. Details are described in ‘Materials and Method’ section.
Intrinsic induction of IGF-I triggers multiple signaling pathways that include PI3K-Akt and MAPK-ERK pathways, which are implicated in increased cell survival and proliferation (26). We therefore analyzed the levels of p-Akt (Ser473) and p-ERK1 (T202/Y204)/ERK2 (T185/Y187) in the lysates obtained from the dorso-lateral prostates of TRAMP and non-transgenic littermates of various age groups. As shown in figure 1, similar to an increase in the expression of IGF-I in the serum, significant increases in p-Akt and p-ERK1/2 levels were observed in the tissue lysates of TRAMP mice, compared to non-transgenic littermates. A 237% increase in p-Akt was noted at 16 weeks, 214% at 24 weeks and 146% at 32 weeks of age in TRAMP mice compared to non-transgenic littermates. Similarly, an increase of 186% was noted in p-ERK at 16 weeks, 184% at 24 weeks and 128% at 32 weeks of age in the dorsolateral prostates of TRAMP mice compared to non-transgenic littermates. No significant difference in the p-Akt levels were observed at 8 weeks of age; although 33% decrease in p-ERK was noted in TRAMP mice compared to non-transgenic littermates.
Another consequence of increased IGF-I is an increase in cancer cell invasiveness and angiogenesis, related to the fact that IGF-I can induce VEGF and uPA (12, 13). We next determined the serum levels of VEGF and uPA in TRAMP and non-transgenic littermates in age-dependent manner. Similar to the observed increases in IGF-I levels, progressive increases in serum VEGF and uPA levels were noted in TRAMP mice in an age-dependent manner, compared to non-transgenic littermates. Compared to non-transgenic littermates, an increase of 120% to 170% was observed in serum VEGF and 102% to 136% was noted in serum uPA in TRAMP mice between 8–32 weeks of age.
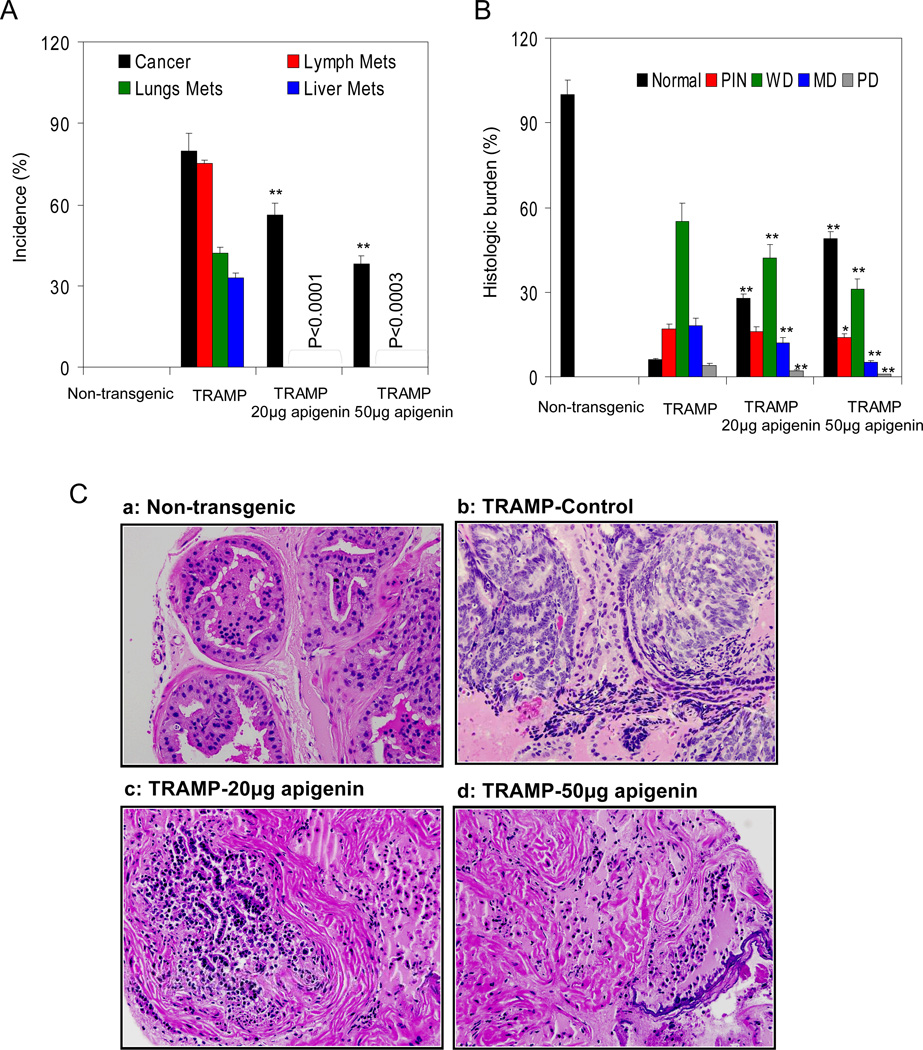
To investigate the effects of apigenin intake on prostate tumor growth and progression in TRAMP mice, experiments were conducted using a control group and administering apigenin at doses of 20 and 50µg/day to two other groups of TRAMP mice, starting at 8 weeks of age and continuing for 20 weeks. All TRAMP mice receiving vehicle only developed advanced prostate tumors that extensively infiltrated the abdominal region. Apigenin administration to TRAMP mice resulted in significant reductions in the incidence of palpable tumors: 56% of animals receiving 20µg/day of apigenin developed palpable tumors, whereas 38% of animals receiving 50µg/day of apigenin developed palpable tumors. We also studied the effects of apigenin intake on the development of systemic metastases. At the end of the 28 week experiment, 100% of the control group of TRAMP mice developed invasive cancers; 75% of these animals showed metastases to lymph nodes, 42% showed metastases to lungs, and 33% showed metastases to liver. In sharp contrast, none of the TRAMP mice that received apigenin exhibited metastases to any of the distant organs studied (Figure 2A).
Fig.2.
Effect of apigenin intake on cancer progression and metastasis in TRAMP mice. A Apigenin administration to mice at 20 and 50µg/day represented no metastasis, whereas, lung, lymph node and liver metastasis was observed in TRAMP control mice. B Distribution of pathologic findings after apigenin intake in the dorsolateral lobes of TRAMP mice. H&E-stained slides were evaluated by three independent scientists. Prostatic lobe was scored for percentage of each pathologic finding present in that lobe. The scores of the evaluators were averaged from 8 mice. Columns, average percentage of each pathologic finding in the dorsolateral prostate in TRAMP mice at 28 week of age; pathologic findings: PIN, prostatic intraepithelial neoplasia; WD, well-differentiated cancer; MD, moderately differentiated cancer; PD, poorly differentiated cancer. *P < 0.05; **P < 0.001, TRAMP apigenin versus TRAMP control (Kruskal-Wallis test). Bars ± SE of 8 mice C Representative haematoxylin and eosin stained photomicrographs (magnification ×40) of 28 weeks dorso-lateral prostates of non-transgenic control, TRAMP control, TRAMP (20µg/day apigenin) and TRAMP (50µg/day apigenin) representing various pathologic findings. A typical dorso-lateral prostate from a non-transgenic mouse exhibited acini with abundant eosinophilic intra-lumenal secretions. TRAMP mice (control) exhibited well-differentiated cancer with extensive epithelial stratification, crowded cribriform structures accompanied with marked thickening, remodeling, and hyper-cellularity of the fibromuscular stroma. Apigenin administration to TRAMP mice resulted in a marked reduction in epithelial stratification and cribriform structures. Details are described in ‘Materials and Method’ section.
Next we evaluated the dorso-lateral prostates of TRAMP mice in control and apigenin-receiving groups of animals at 28 weeks of age (Figure 2B). Prostates of vehicle-treated control TRAMP mice exhibited ~17% prostatic intraepithelial neoplasia and cancers of variable size, >50% of which were composed of well-differentiated adenocarcinoma, >18% composed of moderately differentiated cancer and <4% composed of poorly differentiated cancer. About 5% of the prostate tissue was nonneoplastic in these animals. The histologic findings in the prostates of 20µg/day apigenin-treated TRAMP mice at 28 weeks were notably different from findings in vehicle-treated TRAMP mice, showing a >25% proportion of nonneoplastic prostate tissue with concomitant decreases in prostatic intraepithelial neoplasia (<15%) and well-differentiated (<40%), moderately differentiated (<12%), and poorly differentiated (<2%) cancers. The prostates of mice receiving a higher dose of 50µg/day apigenin showed the following findings: more than 50% of the prostate tissue was non-neoplastic, and there were with significant reductions in the proportions of prostatic intraepithelial neoplasia (<4%) and well-differentiated (<30%), moderately differentiated (<5%), and poorly differentiated (<1%) cancers, respectively, as compared to the vehicle-treated control group. Examples of the typical histology of the dorso-lateral prostates of age-matched non-transgenic, TRAMP, apigenin-treated mice are shown in Figure 2C.
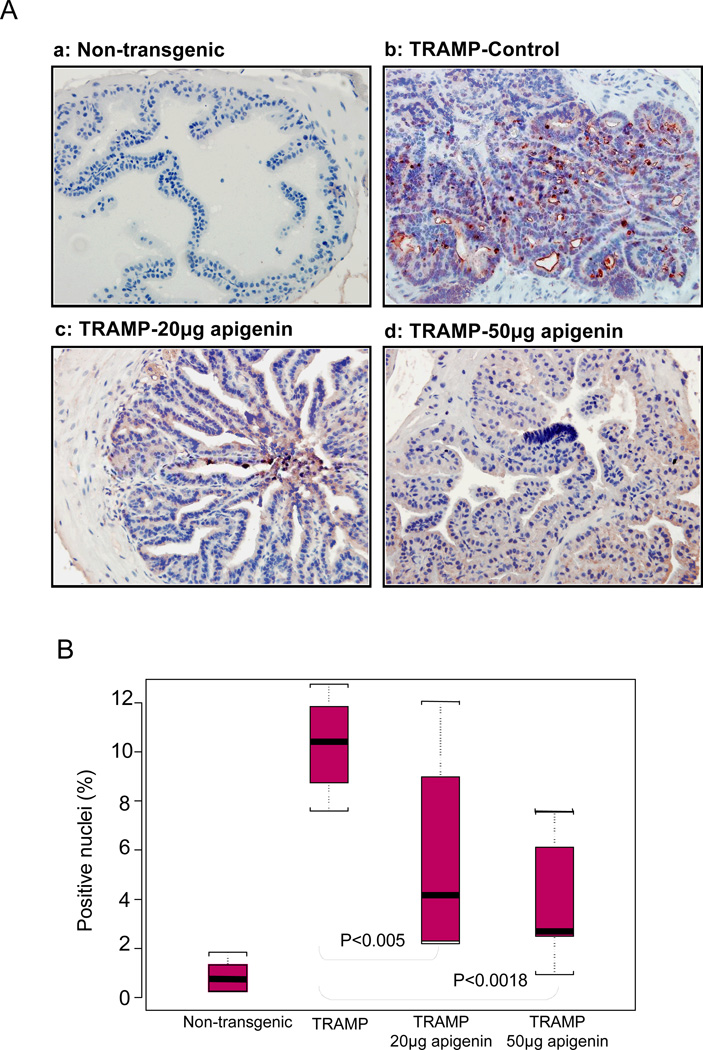
We next determined the effects of apigenin intake on cellular proliferation in mouse prostates by assessing the expression of a proliferation-related protein, PCNA. PCNA is a requisite auxiliary protein for DNA polymerase δ–driven DNA synthesis and is cell cycle regulated (27). As shown in figure 3A, p.o. administration of apigenin markedly suppressed proliferation and PCNA protein expression in the dorso-lateral prostates of TRAMP mice. A significant decrease of 59% and 78% in PCNA expressing epithelial cells was observed after intake of 20 and 50µg/day apigenin (Figure 3B).
Fig.3.
Effect of apigenin intake on the extent of proliferation in the dorso-lateral prostates of TRAMP mice. A Apigenin intake in TRAMP mice at 20 and 50µg/day exhibited decreased nuclear expression of proliferating cell nuclear antigen (PCNA) in a dose-dependent manner as ascertained by immunohistochemical analyses. In vehicle-treated TRAMP mice, extensive PCNA staining was observed in the nuclei of epithelial cells compared with non-transgenic mice. B Statistical box plot analysis of PCNA nuclear presence was performed by counting PCNA positive nuclear stained cells from various locations in non-transgenic control, TRAMP control, TRAMP (20µg/day apigenin) and TRAMP (50µg/day apigenin). Box plot for positive nuclei (%), black bar = median, red box = 25th to 75th percentiles, Bars = entire range from 5 mice. Details are described in ‘Materials and Method’ section.
Next we sought to determine the effect of apigenin on transgene expression, as apigenin might be a consequence of direct suppression of the probasin promoter resulting in reduced expression of the Tag transgene. Apigenin treatment to TRAMP mice did not alter the expression of the PB-Tag transgene (T and t antigen protein) because it was readily detectable in both apigenin and control group (data not shown).
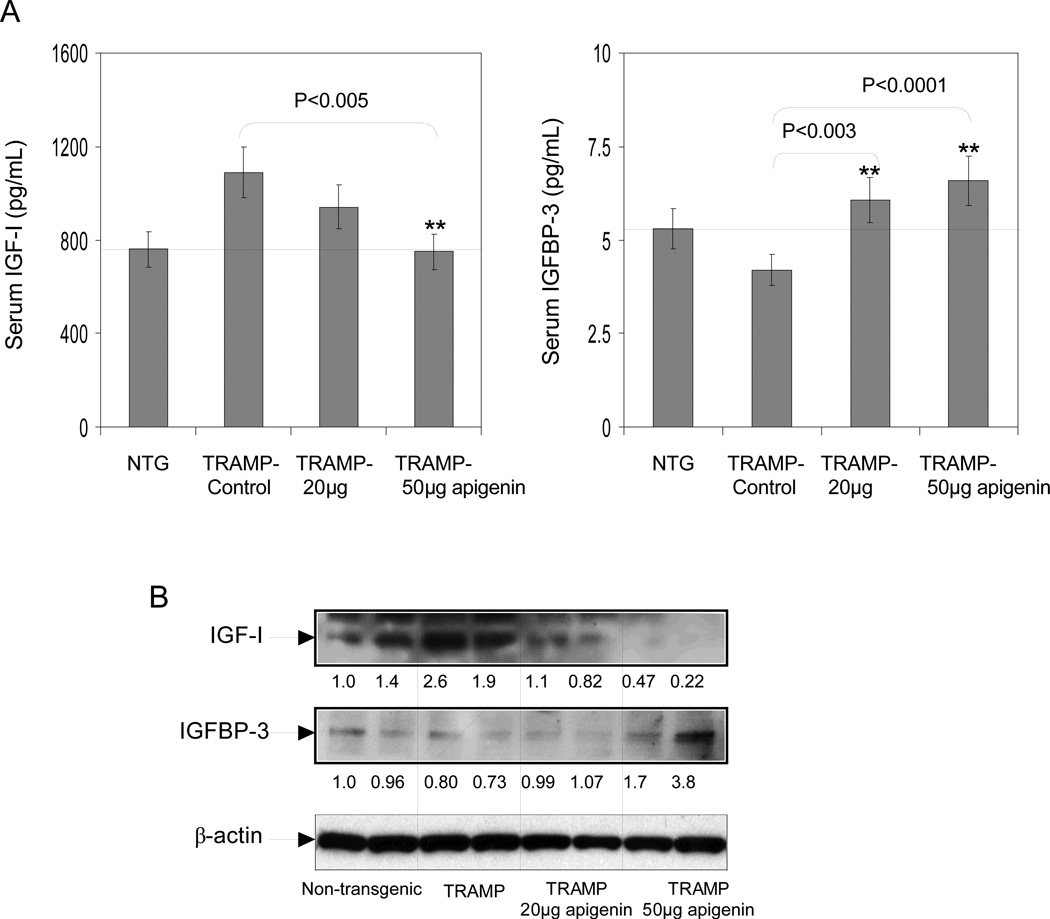
We have observed that apigenin intake reduces the incidence of prostate tumors in TRAMP mice. Next we sought to determine whether this reduction correlates with serum IGF-I and IGFBP3 levels. As shown in figure 4A, apigenin administration for 20 weeks to TRAMP mice resulted in lowering of serum IGF-I and restored serum IGFBP3 levels. Analysis of serum data suggested that the IGF-I to IGFBP3 ratio was inhibited by 40% and 45% at 20 and 50µg/day, respectively. Furthermore, apigenin feeding resulted in significant inhibition in the protein expression of IGF-I and concomitant restoration of IGFBP3 levels in the dorso-lateral prostate tissue of TRAMP mice (Figure 4B).
Fig.4.
Effect of apigenin intake on IGF-I and IGFBP-3 levels in serum and dorsolateral prostates of TRAMP mice. A Serum levels of IGF-I and IGFBP-3 were detected through ELISA assay as provided by the vendor’s protocol. Apigenin intake caused dose-dependent increase in IGFBP-3 level whereas, IGF-I levels significantly decreased. **P < 0.001, TRAMP apigenin versus TRAMP control (Kruskal-Wallis test). Bars ± SE of 6 mice B Protein expressions IGF-I and IGFBP-3 in the total lysate of the dorsolateral prostates of non-transgenic control, TRAMP control, TRAMP (20µg/day apigenin) and TRAMP (50µg/day apigenin) was detected by Western blotting. A significant decrease in IGF-I protein expression was observed after apigenin feeding, conversely IGFBP-3 protein expression were increased. Representative data from two mice per group. Equal loading of protein in the lanes was confirmed by stripping the membrane and reprobing it with β-actin, a housekeeping protein. Details are described in ‘Materials and Method’ section.
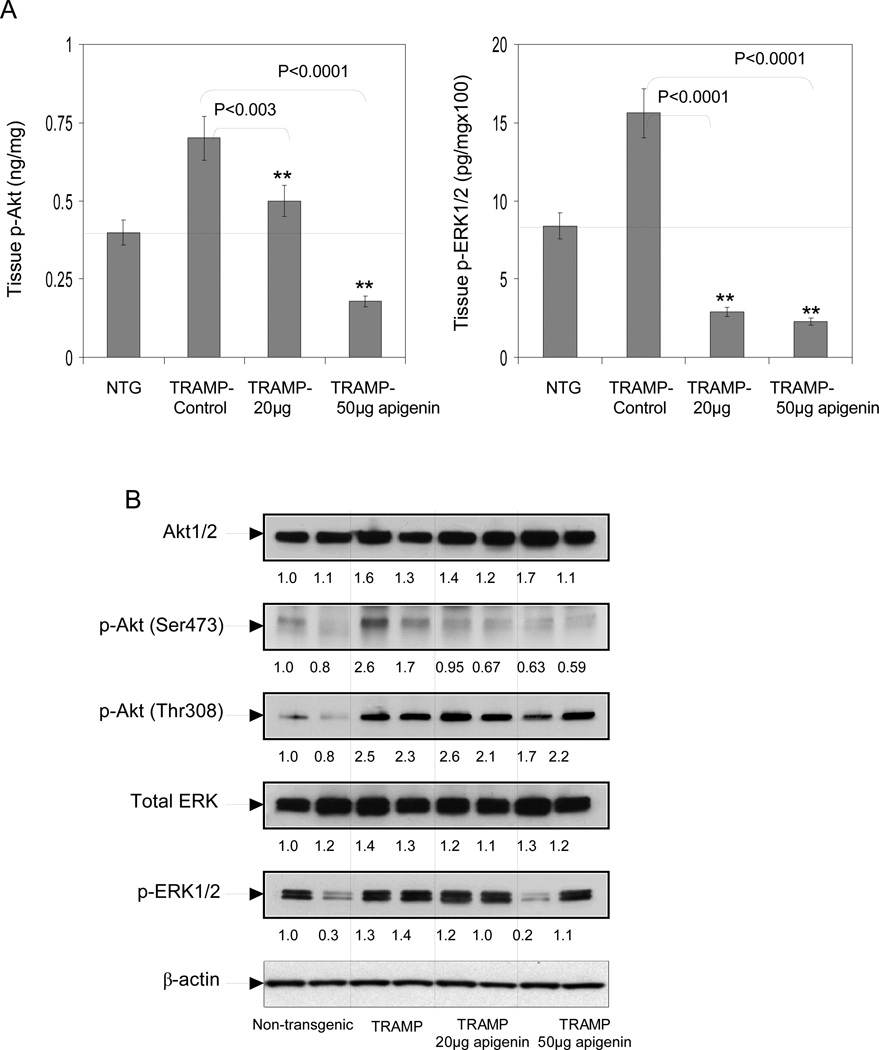
We also determined the effect of apigenin intake on the PI3K-Akt and MAPK-ERK pathways. As shown in figure 5A, apigenin intake for 20 weeks to TRAMP mice resulted in significant reductions in p-Akt (29% at 20µg/day and 74% at 50µg/day) and ERK1/2 (81% at 20µg/day and 86% at 50µg/day) levels in the tissue lysates obtained from the dorso-lateral prostates. Western blot analysis demonstrated that phosphorylation of Akt at Ser473 was inhibited in apigenin-fed animals compared to control animals; a modest decrease in Akt at Thr308 was observed in animals receiving a higher dose of apigenin (50µg/day). No changes were observed in the total Akt levels. We also observed significant inhibition in phosphorylation of ERK1/2 in the dorso-lateral prostates of TRAMP mice after apigenin intake. The effect was dose-dependent; however, no significant changes were observed in the total ERK protein expression (Figure 5B).
Fig.5.
Effect of apigenin intake on total ERK, p-ERK1/2, total Akt and p-Akt (Ser473), (Thr-308) levels in the dorsolateral prostates of TRAMP mice. A Dorso-lateral prostate tissues levels of phosphorylated-Akt (Ser473), phosphorylated-ERK1 (T202/Y204)/ ERK2 (T185/Y187) were detected through ELISA method as provided by the vendor’s protocol. Apigenin intake caused dose-dependent decrease in p-Akt (Ser473) and p-ERK1/2 expression. **P < 0.001, TRAMP apigenin versus TRAMP control (Kruskal-Wallis test). Bars ± SE of 6 mice B Protein expressions of Akt1/2, p-Akt (Ser473) and (Thr-308); total ERK and p-ERK1/2 in the total lysate of dorsolateral prostates of non-transgenic control, TRAMP control, TRAMP (20µg/day apigenin) and TRAMP (50µg/day apigenin) was detected by Western blotting. A significant decrease in p-Akt (Ser473) and p-ERK1/2 protein expression was observed after apigenin feeding, whereas, Akt1/2, total ERK1/2 and p-AKT (Thr-308) protein expressions were unaffected in all TRAMP groups. Representative data from two mice per group. Equal loading of protein in the lanes was confirmed by stripping the membrane and reprobing it with β-actin, a housekeeping protein. Details are described in ‘Materials and Method’ section.
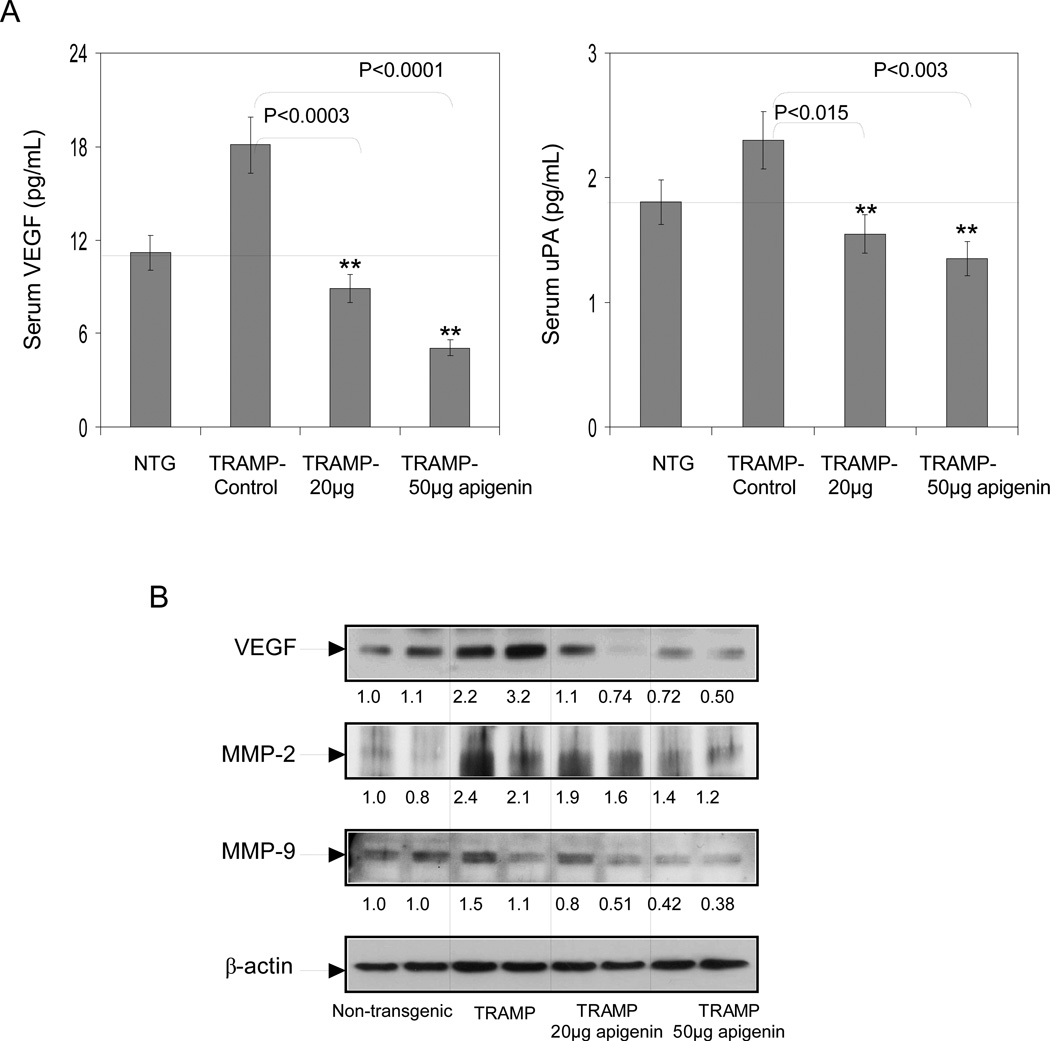
Next we determined the effect of apigenin intake in TRAMP mice on VEGF and uPA levels. As shown in figure 6A, a significant decrease in serum VEGF and uPA levels were observed after apigenin feeding to TRAMP mice. A decrease of 51% was noted at 20µg/day and 72% at 50µg/day in serum VEGF, whereas a decrease of 34% was noted at 20µg/day and 41% at 50µg/day in serum uPA levels after apigenin feeding. Similar results were noted in the VEGF protein in the tissue lysates obtained from dorso-lateral prostates of TRAMP apigenin-fed mice (Figure 6B). We also investigated the protein expression of MMP-2 and MMP-9 in the dorsolateral prostates of control and apigenin-fed TRAMP mice. As shown in figure 6B, a significant decrease in protein expression of MMP-2 and MMP-9 was observed after apigenin feeding to TRAMP mice in a dose-dependent manner.
Fig.6.
Effect of apigenin intake on VEGF and uPA in the serum; and MMP-2, MMP-9 and VEGF levels in the dorsolateral prostates of TRAMP mice. A Serum levels of VEGF and uPA were detected through ELISA assay as provided by the vendor’s protocol. Apigenin intake caused a dose-dependent decrease in serum VEGF and uPA levels. **P < 0.001, TRAMP apigenin versus TRAMP control (Kruskal-Wallis test). Bars ± SE of 6 mice B Protein expression of VEGF, MMP-2 and MMP-9 in the dorso-lateral prostate tissues of non-transgenic control, TRAMP control, TRAMP (20µg/day apigenin) and TRAMP (50µg/day apigenin) was detected by Western blotting. A significant decrease in VEGF, MMP-2 and MMP-9 protein expression was observed after apigenin intake. Representative data from two mice per group. Equal loading of protein in the lanes was confirmed by stripping the membrane and reprobing it with β-actin, a housekeeping protein. Details are described in ‘Materials and Method’ section.
DISCUSSION
IGF-I is a peptide growth factor that is primarily produced in the liver along with IGF binding protein, IGFBP3 (1, 2). In men with prostate cancer, elevated serum IGF-I levels have been observed at least 5 years prior to the clinical diagnosis of prostate cancer (6). Based on these findings, it has been proposed that serum IGF-I may serve as a marker for early detection of prostate cancer, although the role and origin of the elevated serum IGF-I was not well characterized. Furthermore, it has been difficult to follow changes in the IGF axis in prostate tissue at the molecular level during clinical disease progression. We began by evaluating the levels of IGF-I and its binding protein IGFBP-3 in the serum of TRAMP mice and non-transgenic littermates at varying ages. The serum IGF-I levels were significantly higher as cancer progressed from undetectable cancer at 8 weeks to prostate adenocarcinoma at 32 weeks, compared to age-matched non-transgenic mice. This increase in IGF-I was associated with a concomitant decrease in its binding protein IGFBP-3, and relative assessment of the ratios of IGF-I and IGFBP-3 suggested a progressive and significant shift that favored increasing IGF-I levels. These results were consistent with previous observations in which prostate-specific IGF-I was found to be increased during prostate cancer progression in TRAMP mice (28). Our findings were similar to previous studies as the increase in serum IGF-I was probably due to progressive disease response of the prostate rather than due to a systemic response and further support the notion that progression of prostate cancer in TRAMP mice is IGF-I dependent (9, 28).
We have previously demonstrated that oral intake of apigenin at levels comparable to human consumption results in significant inhibition in the development and progression of prostate cancer along with increased survival of TRAMP mice (23). In this study, we examined the underlying pathways to understand whether IGF-I-induced signaling pathways are altered by apigenin intake and further determined whether this feeding regimen inhibits the expression of molecules involved in angiogenesis and subsequent metastasis. It is widely accepted that IGF-I is involved in multiple cellular responses related to growth, including synthesis of DNA, RNA and cellular proteins (1, 2). Binding of IGF-I to its receptor IGF-1R causes activation of the receptor tyrosine kinase and its autophosphorylation, stimulating cell proliferation in most tissues including normal and malignant prostate cancer cells (2). Our previous studies have demonstrated that apigenin suppresses autophosphorylation in prostate cancer cells both in constitutively expressed cell lines and after IGF-I stimulation, and induces IGFBP3, the binding protein for IGF-I, thereby reducing the amount of ligand available for interaction with IGF-1R (21). Our present studies in TRAMP mice demonstrate that apigenin feeding resulted in inhibition of IGF-I and elevation of IGFBP3 levels in the serum and prostate gland during age progression.
Evidence suggests that PI3K-Akt and MAPK are important pathways in transmitting IGF-I mitogenic and anti-apoptotic signals (26). Activation of the catalytic subunit of PI3K results in the production of phosphatidyl inositols and causes membrane translocation, phosphorylation, and activation of the Ser/Thr kinase Akt/PKB, a major transducer of the PI3K signal (29). The mitogenic effects of IGF-I in prostate cancer cells converge at the level of the MAPK-ERK (29, 30). Examining the expression of these proteins revealed increasing levels of phosphorylation of Akt at Ser473 and ERK1 at T202/Y204 and ERK2 at T185/Y187 in an age-dependent manner in the prostates of TRAMP mice. Our previous studies using specific inhibitors of MEK1/2 and p38 demonstrated an inhibition of PC-3 cell proliferation in parallel with inhibition of phosphorylation of ERK1/2 and p38 (19). Interestingly, high phosphorylation of ERK1/2 was observed after treatment of cells with apigenin which usually do not activate the downstream signaling molecules that favors cell proliferation (31). Akt is another major influence in IGF-I signaling, and a number of factors regulated by Akt have been shown to be involved in regulating cell survival and proliferation (32). We previously observed that apigenin blocked constitutive as well as IGF-I induced activation of Akt in human prostate cancer cells (33). In the present study, apigenin administration to TRAMP mice resulted in the inhibition of downstream signaling cascades, including both the PI3K-Akt and the MAPK-ERK signaling pathways.
Studies demonstrate that serum IGF-I levels correlate with the increase in mean vessel density associated with prostate cancer progression, suggesting a relationship between IGF-I and the induction of prostatic neovascularization (8). In human prostate cancer, VEGF expression has been related to a more aggressive and metastatic phenotype (34, 35). Parallel to human studies, our studies in TRAMP mice demonstrate a significant increase in serum VEGF which correlates with disease progression. We therefore investigated whether oral intake of apigenin would alter levels of the angiogenesis marker, VEGF. Our earlier observations had shown that apigenin administration to TRAMP mice reduces the incidence of invasion and metastasis of prostate cancer in these animals. In the current study, apigenin intake by TRAMP mice resulted in significant reductions in serum and prostate tissue levels of VEGF. Previous studies have demonstrated that apigenin inhibits tumor angiogenesis through decreasing HIF-1α and VEGF expression (13). The effect of apigenin administration on HIF-1α levels in TRAMP mice has not been evaluated, but may be of considerable interest.
IGF-I activates the urokinase plasminogen activator (uPA) and uPA receptor (uPAR) systems in various malignancies (36). Because tumors need proteolytic enzymes to invade surrounding tissue and metastasize, we investigated whether tumor progression in TRAMP mice leads to increase in uPA expression. Our studies demonstrate significant higher serum uPA levels in TRAMP mice throughout the 32 weeks that we studied them, compared to levels in age-matched non-transgenic mice. We then evaluated the effect of apigenin intake on uPA levels in TRAMP mice. Previous studies have demonstrated that apigenin has the ability to block the generation of uPA in HUVEC cells (37). In the present study we observed that serum uPA levels were significantly reduced in apigenin-fed mice, in a dose-dependent manner. Oral intake of apigenin in TRAMP mice was associated with reduced levels of both MMP-2 and MMP-9 in the dorso-lateral prostate, compared to controls. It is known that VEGF stimulates endothelial cells to secrete several MMPs and uPA, resulting in the degradation of the vessel basement membrane, which in turn allows the cells to invade the surrounding matrix and facilitate migration process (38). The absence of metastases in apigenin-fed TRAMP mice may reasonably be attributed to reduced production of VEGF, uPA and matrix degradation enzymes (MMP-2 and MMP-9), which all appear to be necessary and intimately involved in the process of cell invasion and metastasis.
There is growing evidence from epidemiologic and case-control studies that higher intake of plant flavonoids reduces the risk of certain chronic diseases including cancer (39). Reports have shown a strong inverse association between flavone intake and risk of breast, colorectal, and epithelial ovarian cancer (39–41). Our studies on the TRAMP mouse prostate cancer model have shown that apigenin, a plant flavone, is capable of suppressing prostate carcinogenesis at physiologically achievable concentrations. The dose of 20 and 50µg/day apigenin used in our studies corresponds to consumption of approximately 50 and 120 mg/day of flavonoid by an adult human, an intake that results in effective physiologically attainable serum concentrations in humans.
In conclusion, the present studies clearly demonstrate that apigenin effectively blocks the IGF signaling axis in TRAMP mice, thereby inhibiting the invasion and progression of prostate cancer. Our studies support the notion that apigenin may be worthy of further development as an anticancer agent, exploiting its effectiveness in inhibiting the IGF signaling axis in prostate cancer.
ACKNOWLEDGEMENTS
This work was supported by grants from United States Public Health Services RO1 CA108512 and RO1 AT002709 and funds from Prevent Cancer Foundation to SG and RO3 CA1376676 to SS.
ABBREVIATIONS
- nIGF-1
Insulin-like growth factor 1
- IGFBP-3
Insulin-like growth factor-binding protein 3
- uPA
Urokinase-type plasminogen activator
- VEGF
Vascular endothelial growth factor
- ERK
Extracellular signal-regulated kinases
- TRAMP
Transgenic adenocarcinoma of the mouse prostate
- MMP
Matrix metalloproteinases
- IGF-IR
Insulin-like growth factor 1 receptor
- PI3K
Phosphoinositide 3-kinase
- IRS-1
Insulin receptor substrate 1
- JNK
c-Jun N-terminal kinases
- PCNA
Proliferating Cell Nuclear Antigen
- uPAR
Urokinase receptor
REFERENCES
- 1.Lima GA, Corrêa LL, Gabrich R, Miranda LC, Gadelha MR. IGF-I, insulin and prostate cancer. Arq Bras Endocrinol Metabol. 2009;53(8):969–975. doi: 10.1590/s0004-27302009000800010. [DOI] [PubMed] [Google Scholar]
- 2.Meinbach DS, Lokeshwar BL. Insulin-like growth factors and their binding proteins in prostate cancer: cause or consequence? Urol Oncol. 2006;24(4):294–306. doi: 10.1016/j.urolonc.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Wolk A, Mantzoros CS, Andersson SO, Bergström R, Signorello LB, Lagiou P, Adami HO, Trichopoulos D. Insulin-like growth factor 1 and prostate cancer risk: a population-based, case-control study. J Natl Cancer Inst. 1998;90(12):911–915. doi: 10.1093/jnci/90.12.911. [DOI] [PubMed] [Google Scholar]
- 4.Ismail AH, Pollak M, Behlouli H, Tanguay S, Begin LR, Aprikian AG. Insulin-like growth factor-1 and insulin-like growth factor binding protein-3 for prostate cancer detection in patients undergoing prostate biopsy. J Urol. 2002;168(6):2426–2430. doi: 10.1016/S0022-5347(05)64160-2. [DOI] [PubMed] [Google Scholar]
- 5.Allen NE, Key TJ, Appleby PN, Travis RC, Roddam AW, Rinaldi S, Egevad L, Rohrmann S, Linseisen J, Pischon T, Boeing H, Johnsen NF, Tjønneland A, Grønbaek H, Overvad K, Kiemeney L, Bueno-de-Mesquita HB, Bingham S, Khaw KT, Tumino R, Berrino F, Mattiello A, Sacerdote C, Palli D, Quirós JR, Ardanaz E, Navarro C, Larrañaga N, Gonzalez C, Sanchez MJ, Trichopoulou A, Travezea C, Trichopoulos D, Jenab M, Ferrari P, Riboli E, Kaaks R. Serum insulin-like growth factor (IGF)-I and IGF-binding protein-3 concentrations and prostate cancer risk: results from the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2007;16(6):1121–1127. doi: 10.1158/1055-9965.EPI-06-1062. [DOI] [PubMed] [Google Scholar]
- 6.Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Hennekens CH, Pollak M. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279(5350):563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 7.Tennant MK, Thrasher JB, Twomey PA, Birnbaum RS, Plymate SR. Insulin-like growth factor-binding protein-2 and-3 expression in benign human prostate epithelium, prostate intraepithelial neoplasia, and adenocarcinoma of the prostate. J Clin Endocrinol Metab. 1996;81(1):411–420. doi: 10.1210/jcem.81.1.8550786. [DOI] [PubMed] [Google Scholar]
- 8.Shariat SF, Lamb DJ, Kattan MW, Nguyen C, Kim J, Beck J, Wheeler TM, Slawin KM. Association of preoperative plasma levels of insulin-like growth factor I and insulin-like growth factor binding proteins-2 and-3 with prostate cancer invasion, progression, and metastasis. J Clin Oncol. 2002;20(3):833–841. doi: 10.1200/JCO.2002.20.3.833. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan PJ, Mohan S, Cohen P, Foster BA, Greenberg NM. The insulin-like growth factor axis and prostate cancer: lessons from the transgenic adenocarcinoma of mouse prostate (TRAMP) model. Cancer Res. 1999;59(9):2203–2209. [PubMed] [Google Scholar]
- 10.Furukawa J, Wraight CJ, Freier SM, Peralta E, Atley LM, Monia BP, Gleave ME, Cox ME. Antisense oligonucleotide targeting of insulin-like growth factor-1 receptor (IGF-1R) in prostate cancer. Prostate. 2010;70(2):206–218. doi: 10.1002/pros.21054. [DOI] [PubMed] [Google Scholar]
- 11.DiGiovanni J, Kiguchi K, Frijhoff A, Wilker E, Bol DK, Beltrán L, Moats S, Ramirez A, Jorcano J, Conti C. Deregulated expression of insulin-like growth factor 1 in prostate epithelium leads to neoplasia in transgenic mice. Proc Natl Acad Sci U S A. 2000;97(7):3455–3460. doi: 10.1073/pnas.97.7.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang YZ, Wong YC. Sex hormone-induced prostatic carcinogenesis in the noble rat: the role of insulin-like growth factor-I (IGF-I) and vascular endothelial growth factor (VEGF) in the development of prostate cancer. Prostate. 1998;35(3):165–177. doi: 10.1002/(sici)1097-0045(19980515)35:3<165::aid-pros2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 13.Slomiany MG, Rosenzweig SA. Hypoxia-inducible factor-1-dependent and -independent regulation of insulin-like growth factor-1-stimulated vascular endothelial growth factor secretion. J Pharmacol Exp Ther. 2006;318(2):666–675. doi: 10.1124/jpet.106.104158. [DOI] [PubMed] [Google Scholar]
- 14.Soulitzis N, Karyotis I, Delakas D, Spandidos DA. Expression analysis of peptide growth factors VEGF, FGF2, TGFB1, EGF and IGF1 in prostate cancer and benign prostatic hyperplasia. Int J Oncol. 2006;29(2):305–314. [PubMed] [Google Scholar]
- 15.Shukla S, Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm Res. 2010;27(6):962–978. doi: 10.1007/s11095-010-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta SC, Kim JH, Prasad S, Aggarwal BB. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010;29(3):405–434. doi: 10.1007/s10555-010-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geahlen RL, Koonchanok NM, McLaughlin JL, Pratt DE. Inhibition of protein-tyrosine kinase activity by flavanoids and related compounds. J Nat Prod. 1989;52(5):982–986. doi: 10.1021/np50065a011. [DOI] [PubMed] [Google Scholar]
- 18.Franzen CA, Amargo E, Todorovic V, Desai BV, Huda S, Mirzoeva S, Chiu K, Grzybowski BA, Chew TL, Green KJ, Pelling JC. The chemopreventive bioflavonoid apigenin inhibits prostate cancer cell motility through the focal adhesion kinase/Src signaling mechanism. Cancer Prev Res (Phila) 2009;2(9):830–841. doi: 10.1158/1940-6207.CAPR-09-0066. [DOI] [PubMed] [Google Scholar]
- 19.Shukla S, Gupta S. Apigenin-induced cell cycle arrest is mediated by modulation of MAPK, PI3K-Akt, and loss of cyclin D1 associated retinoblastoma dephosphorylation in human prostate cancer cells. Cell Cycle. 2007;6(9):1102–1114. doi: 10.4161/cc.6.9.4146. [DOI] [PubMed] [Google Scholar]
- 20.Wang G, Ahmad KA, Harris NH, Ahmed K. Impact of protein kinase CK2 on inhibitor of apoptosis proteins in prostate cancer cells. Mol Cell Biochem. 2008;316(1–2):91–97. doi: 10.1007/s11010-008-9810-9. [DOI] [PubMed] [Google Scholar]
- 21.Shukla S, Mishra A, Fu P, MacLennan GT, Resnick MI, Gupta S. Up-regulation of insulin-like growth factor binding protein-3 by apigenin leads to growth inhibition and apoptosis of 22Rv1 xenograft in athymic nude mice. FASEB J. 2005;19(14):2042–2044. doi: 10.1096/fj.05-3740fje. [DOI] [PubMed] [Google Scholar]
- 22.Shukla S, Gupta S. Apigenin suppresses insulin-like growth factor I receptor signaling in human prostate cancer: an in vitro and in vivo study. Mol Carcinog. 2009;48(3):243–252. doi: 10.1002/mc.20475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shukla S, MacLennan GT, Flask CA, Fu P, Mishra A, Resnick MI, Gupta S. Blockade of beta-catenin signaling by plant flavonoid apigenin suppresses prostate carcinogenesis in TRAMP mice. Cancer Res. 2007;67(14):6925–6935. doi: 10.1158/0008-5472.CAN-07-0717. [DOI] [PubMed] [Google Scholar]
- 24.Hollman PC, Katan MB. Dietary flavonoids: intake, health effects and bioavailability. Food Chem Toxicol. 1999;37(9–10):937–942. doi: 10.1016/s0278-6915(99)00079-4. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan-Lefko PJ, Chen TM, Ittmann MM, Barrios RJ, Ayala GE, Huss WJ, Maddison LA, Foster BA, Greenberg NM. Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. Prostate. 2003;55(3):219–237. doi: 10.1002/pros.10215. [DOI] [PubMed] [Google Scholar]
- 26.Rho O, Kim DJ, Kiguchi K, Digiovanni J. Growth factor signaling pathways as targets for prevention of epithelial carcinogenesis. Mol Carcinog. 2011;50(4):264–279. doi: 10.1002/mc.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelman Z. PCNA: structure, functions and interactions. Oncogene. 1997;14(6):629–640. doi: 10.1038/sj.onc.1200886. [DOI] [PubMed] [Google Scholar]
- 28.Adhami VM, Siddiqui IA, Ahmad N, Gupta S, Mukhtar H. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Res. 2004;64(23):8715–8722. doi: 10.1158/0008-5472.CAN-04-2840. [DOI] [PubMed] [Google Scholar]
- 29.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8(8):627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarker D, Reid AH, Yap TA, de Bono JS. Targeting the PI3K/AKT pathway for the treatment of prostate cancer. Clin Cancer Res. 2009;15(15):4799–4805. doi: 10.1158/1078-0432.CCR-08-0125. [DOI] [PubMed] [Google Scholar]
- 31.Llorens F, Miró FA, Casañas A, Roher N, Garcia L, Plana M, Gómez N, Itarte E. Unbalanced activation of ERK1/2 and MEK1/2 in apigenin-induced HeLa cell death. Exp Cell Res. 2004;299(1):15–26. doi: 10.1016/j.yexcr.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Levine AJ, Feng Z, Mak TW, You H, Jin S. Coordination and communication between the p53 and IGF-1-AKT-TOR signal transduction pathways. Genes Dev. 2006;20(3):267–275. doi: 10.1101/gad.1363206. [DOI] [PubMed] [Google Scholar]
- 33.Kaur P, Shukla S, Gupta S. Plant flavonoid apigenin inactivates Akt to trigger apoptosis in human prostate cancer: an in vitro and in vivo study. Carcinogenesis. 2008;29(11):2210–2217. doi: 10.1093/carcin/bgn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Latil A, Bièche I, Pesche S, Valéri A, Fournier G, Cussenot O, Lidereau R. VEGF overexpression in clinically localized prostate tumors and neuropilin-1 overexpression in metastatic forms. Int J Cancer. 2000;89(2):167–171. doi: 10.1002/(sici)1097-0215(20000320)89:2<167::aid-ijc11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 35.Chen J, De S, Brainard J, Byzova TV. Metastatic properties of prostate cancer cells are controlled by VEGF. Cell Commun Adhes. 2004;11(1):1–11. doi: 10.1080/15419060490471739. [DOI] [PubMed] [Google Scholar]
- 36.Dunn SE, Torres JV, Oh JS, Cykert DM, Barrett JC. Up-regulation of urokinase-type plasminogen activator by insulin-like growth factor-I depends upon phosphatidylinositol-3 kinase and mitogen-activated protein kinase kinase. Cancer Res. 2001;61(4):1367–1374. [PubMed] [Google Scholar]
- 37.Kim MH. Flavonoids inhibit VEGF/bFGF-induced angiogenesis in vitro by inhibiting the matrix-degrading proteases. J Cell Biochem. 2003;89(3):529–538. doi: 10.1002/jcb.10543. [DOI] [PubMed] [Google Scholar]
- 38.Kumar R, Yoneda J, Bucana CD, Fidler IJ. Regulation of distinct steps of angiogenesis by different angiogenic molecules. Int J Oncol. 1998;12(4):749–757. doi: 10.3892/ijo.12.4.749. [DOI] [PubMed] [Google Scholar]
- 39.Bosetti C, Spertini L, Parpinel M, Gnagnarella P, Lagiou P, Negri E, Franceschi S, Montella M, Peterson J, Dwyer J, Giacosa A, La Vecchia C. Flavonoids and breast cancer risk in Italy. Cancer Epidemiol Biomarkers Prev. 2005;14(4):805–808. doi: 10.1158/1055-9965.EPI-04-0838. [DOI] [PubMed] [Google Scholar]
- 40.Simons CC, Hughes LA, Arts IC, Goldbohm RA, van den Brandt PA, Weijenberg MP. Dietary flavonol, flavone and catechin intake and risk of colorectal cancer in the Netherlands Cohort Study. Int J Cancer. 2009;125(12):2945–2952. doi: 10.1002/ijc.24645. [DOI] [PubMed] [Google Scholar]
- 41.Gates MA, Tworoger SS, Hecht JL, De Vivo I, Rosner B, Hankinson SE. A prospective study of dietary flavonoid intake and incidence of epithelial ovarian cancer. Int J Cancer. 2007;121(10):2225–2232. doi: 10.1002/ijc.22790. [DOI] [PubMed] [Google Scholar]