In this issue of the Biophysical Journal, Kumar and Grubmüller (1) report their studies on the physical and structural properties of the channel of a double-stranded (ds) DNA translocation motor for bacteriophage ϕ29 DNA packaging. In living organisms, transportation and translocation of dsDNA between two cellular compartments or components is a ubiquitous phenomenon critical for cell mitosis, bacterial binary fission, DNA replication, repair, holliday junction resolution, homologous recombination, viral genome packaging, or trafficking (2). These DNA motion events are accomplished by a biomotor using ATP as its energy source. Bacteriophage is a virus that infects bacteria. One of the important steps during the replication of dsDNA viruses is the translocation of their lengthy DNA genome into the preformed viral protein shell, called the “procapsid”. This translocation event is driven by a biomotor. The protein hub of the bacteriophage ϕ29 DNA packaging motor, called the “connector”, allows dsDNA to enter into the procapsid during the late stage of replication, and exit into target cells during infection. The central channel of the connector is formed by 12 subunits of the gp10 protein (Fig. 1).
Figure 1.
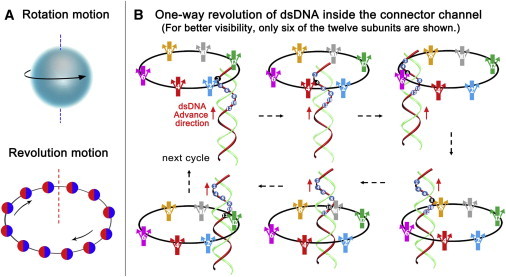
Illustration of the revolution mechanism without rotation. (A) Cartoon showing that rotation is like the Earth rotating around its own axis, and revolution motion is analogous to the Earth revolving around the Sun. (B) Diagram of dsDNA one-way revolution inside the 12 subunits of the connector channel wall (5). The loops (arrows on each subunit) within the channel serve as one-way valves that allow the dsDNA to advance into the procapsid, but do not allow movement in the opposite direction (8). To see this figure in color, go online.
Biomotors were once classified into two categories: linear and rotation. For decades, the DNA-packaging motor of dsDNA viruses has been popularly believed to be a five-fold rotation motor, which is seemingly supported by the spiral configuration of the motor connector channel from structure studies. However, biotechnological and biophysical studies revealed that the connector does not rotate during DNA packaging (3–5). Tethering of the DNA end to the bead demonstrated that DNA translocation by the motor was active, although no detectable rotation of the bead or bead clusters was observed (6,7). These results led to a puzzle concerning how the spiral-shape motor connector acting as a nut can drive the helical dsDNA bolt without rotation of either the bolt or the nut. This puzzle has since been solved by the recent discovery of a third type of biomotor mechanism of revolution without rotation (5,8). By analogy, rotation resembles the Earth rotating on its own axis for one cycle every 24 h, whereas revolution resembles the Earth revolving around the Sun, one circle per 365 days (Fig. 1 A).
Kumar and Grubmüller (1), based on their recent biophysical studies, report new evidence supporting the one-way revolution mechanism of biomotors. The authors probe the elasticity and stiffness of the connector channel of the ϕ29 DNA packaging motor at the atomic level by dynamic simulations, revealing the mechanical properties of the connector (1). They use solid biophysical data to rule out the untwist-twist model, which was reported in the past, and in turn shed light on the mechanism of the packaging motor. In the untwist-twist model, the connector untwists at 12°, utilizing one ATP, and grasps the DNA. Then the connector twists back to its original position, thus translocating DNA into the capsid. However, equilibrium and nonequilibrium (force-probe) molecular-dynamics simulations show that the connector as a whole is softer than the procapsid, while the middle region of the connector is one of the known stiffest protein materials (1). The calculations indicate that the proposed 12° untwisting would require 10 times more energy than is available from the hydrolysis of one ATP molecule derived from empirical data (1).
Moreover, the umbrella-sampling simulations suggest that 100 kJ/mol is required to untwist the whole connector by only 4°. Their data also explain how the connector can withstand a large pressure difference because the pressure primarily acts on the rigid regions of the connector, with the channel stabilized by this high stiffness to maintain its inner diameter without dsDNA in the channel. The authors elucidate that the overall heterogeneity of the connector mechanical properties serves to favor the one-way revolution model (5,8,9), in which the connector acts as a one-way valve restricting the DNA leakage during packaging, facilitated by the inner flexible connector loop serving as a check-valve for unidirectional transport of dsDNA (Fig. 1 B) (9,10). The independent study by Kumar and Grubmüller (1), and the one-way revolution model, is also in accordance with the recent finding of Guo et al. (11), who studied the bacteriophage T7 DNA packaging motor by cryo-electron microscopy, and discovered a small tilt of the core stack within the T7 connector to assist DNA spooling without tangling during packaging.
In the past, many biophysical studies have been carried out to interpret various biological phenomena. Biophysical studies can be extremely valuable in elucidating the mechanisms in living systems if employed in combination with perceptive interpretation. However, life systems are complicated. If a physical finding is misinterpreted, it can also be very misleading. The value and novelty of this report lies not only in the solid data, but also in the authors’ insightful view of the mechanism after data were acquired. They did not simply follow the literature; instead, they used their own logical and insightful view to infer their findings. Nevertheless, it would be more interesting in the future to carry out the theoretical, computational, and empirical studies for the connector in the presence of dsDNA to show that the DNA actually tilts and binds to one side, rather than in the center of the connector channel, further proving the revolution motion of DNA around the channel. In addition, the motor contains a ring of ATPase gp16. Revolution requires the sequential action of the subunits of ATPase ring. The mechanism of how the motor regulates the sequential action is still unknown.
It would be desirable if the mechanism of the sequential action among the channel subunits is elucidated in the future. Revolution without rotation involves using the left-handed connector channel to facilitate the entry of the dsDNA during packaging (8,10). It would be reasonable to believe that, after DNA packaging is completed, a conformational change will occur to convert the left-handed connector into the right-handed configuration for facilitating the exit of the DNA to infect host cells. Indeed, three steps of conformational changes of the ϕ29 connector have been reported. It is expected that a small right-handed twisting of the dsDNA will be observed because the dsDNA is aligned with the wall of the connector channel and the left- to right-handed conformational change. Biophysical studies to distinguish the very-small-angle, right-handed twisting from the 360°/10.5 basepairs’ left-handed rotation, would be very interesting.
The use of revolution for a biomotor is an energy-effective process. The genome in humans and many other living systems consists of long dsDNA. If a rotation mechanism is involved, rotation with the helical nature of dsDNA will result in coiling and twisting of the dsDNA. Such supercoiling can be resolved by applying topoisomerase or helicase. However, the use of enzymes to remove or prevent knotting is extremely expensive in energy consumption, especially when the lengthy DNA chromosome is concerned. Nature has elegantly evolved a revolution mechanism (5,8) without rotation, coiling, or torque, and with reduced friction. The novelty in nature is sometimes beyond our expectation!
Acknowledgments
P.G. thanks Ashwani Sharma and Zhengyi Zhao for their constructive comments. The author is a co-founder of Kylin Therapeutics, Inc., and Biomotor and RNA Nanotech Development Co. Ltd.
The research in the author’s lab/center was supported by National Institutes of Health/National Cancer Institute grants No. R01 EB012135, No. R01EB003730, and No. U01CA151648. Funding to the author’s Endowed Chair in Nanobiotechnology position is by the William Fairish Endowment Fund.
References
- 1.Kumar R., Grubmüller H. Elastic properties and heterogeneous stiffness of the ϕ29 motor connector channel. Biophys. J. 2014;106:1338–1348. doi: 10.1016/j.bpj.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crozat E., Grainge I. FtsK DNA translocase: the fast motor that knows where it’s going. ChemBioChem. 2010;11:2232–2243. doi: 10.1002/cbic.201000347. [DOI] [PubMed] [Google Scholar]
- 3.Baumann R.G., Mullaney J., Black L.W. Portal fusion protein constraints on function in DNA packaging of bacteriophage T4. Mol. Microbiol. 2006;61:16–32. doi: 10.1111/j.1365-2958.2006.05203.x. [DOI] [PubMed] [Google Scholar]
- 4.Hugel T., Michaelis J., Bustamante C. Experimental test of connector rotation during DNA packaging into bacteriophage ϕ29 capsids. PLoS Biol. 2007;5:e59. doi: 10.1371/journal.pbio.0050059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz C., De Donatis G.M., Guo P. Revolution rather than rotation of AAA+ hexameric ϕ29 nanomotor for viral dsDNA packaging without coiling. Virology. 2013;443:28–39. doi: 10.1016/j.virol.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang C.L., Zhang H., Savran C.A. Bright-field analysis of ϕ29 DNA packaging motor using a magnetomechanical system. Appl. Phys. Lett. 2008;93:153902–153903. doi: 10.1063/1.3000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shu D., Zhang H., Guo P. Counting of six pRNAs of ϕ29 DNA-packaging motor with customized single-molecule dual-view system. EMBO J. 2007;26:527–537. doi: 10.1038/sj.emboj.7601506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Z., Khisamutdinov E., Guo P. Mechanism of one-way traffic of hexameric ϕ29 DNA packaging motor with four electropositive relaying layers facilitating antiparallel revolution. ACS Nano. 2013;7:4082–4092. doi: 10.1021/nn4002775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang H., Jing P., Guo P. Role of channel lysines and the “push through a one-way valve” mechanism of the viral DNA packaging motor. Biophys. J. 2012;102:127–135. doi: 10.1016/j.bpj.2011.11.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jing P., Haque F., Guo P. One-way traffic of a viral motor channel for double-stranded DNA translocation. Nano Lett. 2010;10:3620–3627. doi: 10.1021/nl101939e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo F., Liu Z., Jiang W. Visualization of uncorrelated, tandem symmetry mismatches in the internal genome packaging apparatus of bacteriophage T7. Proc. Natl. Acad. Sci. USA. 2013;110:6811–6816. doi: 10.1073/pnas.1215563110. [DOI] [PMC free article] [PubMed] [Google Scholar]


