Abstract
Given the difficulty in finding a cure for HIV/AIDS, a promising prevention strategy to reduce HIV transmission is to directly block infection at the portal of entry. The recent Thai RV144 trial offered the first evidence that an antibody-based vaccine may block heterosexual HIV transmission. Unfortunately, the underlying mechanism(s) for protection remain unclear. Here we theoretically examine a hypothesis that builds on our recent laboratory observation: virus-specific antibodies (Ab) can trap individual virions in cervicovaginal mucus (CVM), thereby reducing infection in vivo. Ab are known to have a weak—previously considered inconsequential—binding affinity with the mucin fibers that constitute CVM. However, multiple Ab can bind to the same virion at the same time, which markedly increases the overall Ab-mucin binding avidity, and creates an inheritable virion-mucin affinity. Our model takes into account biologically relevant length and timescales, while incorporating known HIV-Ab affinity and the respective diffusivities of viruses and Ab in semen and CVM. The model predicts that HIV-specific Ab in CVM leads to rapid formation and persistence of an HIV concentration front near the semen/CVM interface, far from the vaginal epithelium. Such an HIV concentration front minimizes the flux of HIV virions reaching target cells, and maximizes their elimination upon drainage of genital secretions. The robustness of the result implies that even exceedingly weak Ab-mucin affinity can markedly reduce the flux of virions reaching target cells. Beyond this specific application, the model developed here is adaptable to other pathogens, mucosal barriers, and geometries, as well as kinetic and diffusional effects, providing a tool for hypothesis testing and producing quantitative insights into the dynamics of immune-mediated protection.
Introduction
Antibodies (Ab) produced by our immune system can bind specifically to foreign pathogens, facilitating numerous mechanisms of immune protection against infections. Although more Ab are secreted into mucus that coats exposed organs than into blood and lymph (1), how Ab mediate protection in cervicovaginal mucus (CVM) coating the female reproductive tract remains insufficiently understood (2,3). Virus-specific Ab molecules can accumulate on the surface of virions and directly inhibit them from binding and infecting target cells, a process known as “neutralization”. However, whereas previous animal studies showed that topical Ab molecules offer robust protection against vaginal infections (4,5), protection was also found with antibodies and at vaginal antibody titers that are unlikely to adequately neutralize (6,7). Ab can elicit other protective functions, such as ingestion and destruction of the pathogens (opsonization) or infected cells (using antibody-dependent cellular cytotoxicity (ADCC)) by specialized immune cells, as well as activation of a cascade of enzymes that lead to direct lysis of the pathogen membrane (complement) (8). However, healthy female genital secretions typically have little complement activity and few—if any—active leukocytes, due to the low pH environment created by lactobacilli in the vaginal flora (9–11). The aforementioned classical mechanisms of immune protection, therefore, do not adequately explain many instances of Ab-mediated protection found in the female reproductive tract, including the landmark Thai RV144 HIV vaccine trial, the first HIV vaccine to show significant protection in humans (12,13). The RV144 vaccination regimen reduced the risk of HIV acquisition by ∼30% over 3 years despite inducing primarily nonneutralizing Ab, and offering otherwise little to no protection against systemic progression of HIV infections in vaccinated subjects infected with HIV (13).
The extremely promising yet puzzling findings of the RV144 trial, along with similarly confounding observations from different in vivo studies, compel the exploration of additional mechanisms of immune protection at mucosal surfaces. Recently, we began focusing on an alternative mechanism of vaginal immunity, whereby an array of virion-bound Ab collectively imparts to the individual virion multiple weak Ab-mucin bonds, generating sufficient avidity to slow-down or even immobilize individual virions in mucus. We note that the coupling of weak Ab-mucin affinity to Ab-virion binding kinetics as a mechanism of mucosal immunity has been previously explored but generally dismissed, in part because repeated efforts have generally failed to detect (i.e., high affinity) binding of individual Ab to mucins (14–17). Olmsted et al. (14) and Saltzman et al. (17) showed that both Immunoglobulin G and A (IgG and IgA) antibodies exhibit rapid diffusion in human cervical mucus, and slowed only slightly (∼5–20%) compared to their diffusion in water, with no immobilized (strongly bound) fraction detected. Because Ab are much smaller than the mesh pores (14,18), any reduced mobility must be due to very short-lived (<1 s) binding interactions with the mucin mesh that are readily broken by thermal excitation. Such low affinity of Ab molecules to mucins clearly implies that a single virion-bound Ab cannot adequately slow a virion. However, because many Ab molecules can bind to an individual virion, the cumulative Ab-mucin affinity can be inherited by the host virion, assuming that the binding of IgG-Fab to virion does not interfere with the overall IgG-mucin interactions. Based on this assumption, virion diffusion may be increasingly hindered with each additionally bound Ab (refer to Fig. 1 A). Although initially postulated nearly 10 years ago (9) as of this writing, this hypothesis was not substantiated experimentally until Wang et al. (19) recently confirmed that Herpes simplex virus serotype 1 (HSV-1) readily penetrated human CVM with low or no detectable endogenous anti-HSV-1 IgG, and became trapped in samples with modest (i.e., subneutralizing) levels of endogenous or exogenously-added anti-HSV-1 IgG (19).
Figure 1.
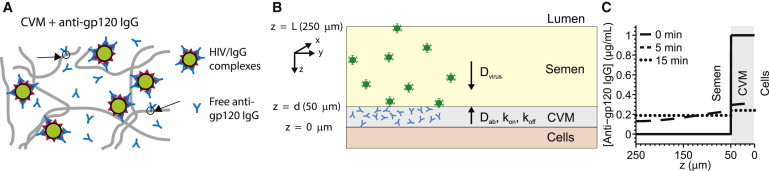
(A) Schematic depicting multiple HIV-bound IgG immobilizing HIV virions in CVM via multiple low-affinity IgG-mucin bonds. (B) Schematic of our model for diffusion of HIV from seminal secretions across a cervicovaginal mucus (CVM) layer supplemented with anti-HIV IgG en route to the underlying vaginal epithelium. To reduce infection, IgG must bind to HIV in sufficient quantities to neutralize or to trap the virions in mucus before HIV virions successfully penetrate CVM and reach the vaginal epithelium. (C) Predicted concentration profiles of IgG in the semen and CVM layers at various times postejaculation. To see this figure in color, go online.
Furthermore, the IgG-mucin interactions appear to be markedly influenced by N-glycans on IgG-Fc. A nonneutralizing but binding IgG facilitated effective protection against vaginal Herpes infection in mice; interestingly, the protection was abolished when vaginal mucus was removed, indicating that virus-specific IgG can indeed work in tandem with mucus to reduce infection. We are now making similar observations of IgG-induced trapping with a variety of mucus secretions from different animal species against different viruses, highly motile bacterial pathogens, and synthetic nanoparticles (see Movie S1 and Movie S2 in the Supporting Material). Because our experiments are based on particle tracking of individual pathogens, we avoid potential pitfalls observed previously by Pando et al. (20) and ten Wolde and Tănase-Nicola (21), where fluorescence-recovery-after-photobleaching was used to infer diffusion coefficients of tagged particles in the presence of traps (where Ab-mucin binding events are the traps) can lead to potentially inaccurate results.
These results raise the possibility that this tandem effect of weak Ab-mucin affinity, coupled with rapid virion-binding kinetics of vaccine-elicited Ab, can hinder the diffusion of HIV across CVM and limit the flux of virions reaching target cells. It is important to note that HIV deposited in the female reproductive tract can rapidly penetrate genital secretions (22,23); thus, there is a very limited window of opportunity for Ab to bind and accumulate on the virus surface and hinder virions before they penetrate the CVM and reach target cells in the submucosa (S. A. McKinley, A. Chen, F. Shi, S. Wang, P. J. Mucha, M. G. Forest, and S. K. Lai, unpublished). In addition, HIV virions are typically characterized by a sparse density of surface antigen sites, making it even more difficult to achieve a sufficient number of virion-bound Ab to hinder their diffusive transport across the CVM.
To better understand the subtle yet potentially significant interplay between these kinetic and diffusive processes associated with CVM laden with IgG molecules that have weak mucin affinities, the introduction of HIV-laden semen, and the unfolding of events in vivo, we developed a mathematical model to incorporate measured kinetic and diffusion constants and parameterize the least-understood rate constants (e.g., the putative weak Ab-mucin affinity). This model allows us to simulate the transmission of HIV in seminal secretions through Ab-laden CVM just after sexual intercourse under physiologically relevant conditions, with Ab concentration, Ab-antigen binding kinetics, and Ab-mucin affinity as tunable model parameters (Fig. 1 A). We can then use numerical simulations to explore the impact of varying degrees of weak Ab-mucin affinity on the expected potency of Ab-mediated protection against vaginal HIV infection. We find that the coupling of weak Ab-mucin affinity to Ab-HIV binding kinetics has two important consequences: 1. The interactions between pathogen-bound Ab and mucins directly reduce the flux of HIV reaching the vaginal epithelium. We can then predict the extent of flux reduction based on specified IgG-mucin affinity, IgG concentration, IgG-antigen binding affinity, and semen/CVM thicknesses. 2. There is a rapid formation of a surprisingly highly localized concentration of virions (a virion concentration front) near the semen/CVM interface most distant from the epithelial cells. This virion concentration front not only contributes to our calculated reduction in virion flux, but presumably can also facilitate rapid elimination of trapped virions by natural mucus clearance mechanisms.
Materials and Methods
Modeling the physiology of HIV transmission in the female reproductive tract
Typically, the vaginal epithelium is highly folded into collapsed rugae coated with a layer of viscoelastic and adhesive CVM gel. During coitus, the epithelium becomes somewhat stretched and exposed. For simplicity, we thus model the vaginal epithelial surface as the inner surface of a simple cylinder coated with a roughly d = 50-μm-thick CVM layer containing HIV-specific Ab (see Fig. 1 A). The thickness of the CVM coating is estimated based on total volumes of mucus that can be collected in the absence of coital stimulation, and assumed to be constant and uniform (S. A. McKinley, A. Chen, F. Shi, S. Wang, P. J. Mucha, M. G. Forest, and S. K. Lai, unpublished; (25,26)). After ejaculation, the seminal fluid is assumed for simplicity to evenly overlay the CVM layer with a thickness of ∼200 μm (27), with virions uniformly dispersed within the seminal secretions at a density of 2.8 × 105 virions/mL (28).
Once virions penetrate CVM and reach the epithelial lumen, virions must still access target cells in the submucosa, and intact stratified vaginal epithelia has long been believed to serve as a mechanical barrier excluding virus access. Nevertheless, HIV virions have been observed to quickly penetrate the superficial layers of the stratified epithelium in human cervical explants and the female rhesus macaque genital tract, thereby gaining access to superficial Langerhans cells and CD4+ T cells (29,30). The timescale required for successful cellular penetration of HIV may be further reduced by any preexisting micro or macro lesions in the epithelium as well as abrasions upon coital stirring (31,32). Thus, in the absence of an established mathematical model that can accurately recapitulate HIV penetration of the squamous epithelium, we chose virion passage through the CVM layer as the timescale to evaluate Ab-mediated trapping of virions, up to a maximum of 2 h. Similar assumptions were previously made by Geonnotti and Katz (33) and Lai et al. (34) to model the efficacy of microbicides against HIV and the role of the thickness of the microbicide gel layer. Thermal degradation based on gp120 shedding (T1/2 ∼ 30 h) and thermal degradation from RNA polymerase decay (T1/2 ∼ 40 h) are not incorporated because of the substantial difference in the rate of these processes from the timescale of interest (35).
Accumulation of antibodies on HIV
The rates with which broadly neutralizing Ab, such as NIH45-46, accumulate on HIV virions are dependent on Ab-antigen affinity, the number of available antigen sites, and the local antibody concentration. The binding affinity for NIH45-46 to gp120 Env spikes of HIV virions is described previously in Scheid et al. (36). The number of Env spikes N∗ on individual HIV virions are variable, and was estimated to follow a negative binomial distribution with N∗ = 14 ± 7 (range 4–35) based on cryo-electron microscopy of HIV virions (37). Each Env spike is composed of trimeric gp120 molecules that can be bound by up to three monoclonal IgGs without significant steric obstruction in IgG binding. Ab bind and unbind to individual sites at rates kon and koff, and the overall binding/unbinding rates are dependent on the number of unoccupied binding sites (3N∗ − n) and the local antibody concentration u(z,t). Denoting IgG concentration in CVM as u(z,t), the viral load as a vector
with vn representing the concentration of virions with n Ab bound at (z,t), we can estimate the rates with which IgG can accumulate on HIV virions over t ε [0,T] up to maximum time T of 2 h (S. A. McKinley, A. Chen, F. Shi, S. Wang, P. J. Mucha, M. G. Forest, and S. K. Lai, unpublished). Hence,
| (1) |
where (Ab) denotes an unbound Ab and (Ab)n Z (t) denotes a virion at Z(t) with n bound antibodies.
Diffusion of viruses, antibodies, and antibody-virus complexes in CVM
Due to rapid diffusivity of protons, semen-mediated neutralization of CVM is assumed to occur instantaneously, in good agreement with prior measurements of vaginal pH during and after coitus (38,39). Thus, for HIV virions without bound Ab, we adopted the measured diffusivity of individual HIV virions in pH-neutralized CVM (22) as the virion diffusivity in CVM. This diffusivity is only a fewfold reduced compared with the theoretical virion diffusivity in water/buffer; we therefore assumed the same diffusivity value for HIV virions in semen. We further assume coital stirring motion does not influence the movement of virions into the epithelial layer, due to the viscoelastic nature of CVM and semen. When mucus is sheared between two surfaces, adhesive contacts and entanglements between mucin fibers are drawn apart and a slippage plane forms parallel to the two surfaces, which is reflected by the shear-thinning rheological profile of mucus (9,35). Thus, although the viscous drag between the surfaces drops considerably, enabling mucus to function as an effective lubricant, the gel layers adhering to both surfaces remain unstirred even in the presence of vigorous shearing actions from copulation. This suggests CVM and semen can be largely modeled as immiscible layers over the timescale of the simulation, and viruses in semen are unlikely to be easily stirred into the mucus layer adhering to the vaginal epithelium.
Our mathematical model describes the dynamics of male-to-female HIV transmission from the instant semen is ejaculated into the vaginal lumen (time t = 0), tracking the diffusion of HIV virions from the semen layer (d < z < L) into the CVM layer (0 < z < d) and finally to the epithelium (z = 0). (See Fig. 1 B for schematic; Table 1 lists the various input parameters.) The process continues for each virion for 2 h or until the virion reaches the vaginal epithelium, whichever occurs first (see above for further details). IgG is the predominant Ab subclass found in CVM (rather than IgA) (40,41). The diffusion of IgG in mucus may be slowed by interactions with mucins (9,42); fluorescence-recovery-after-photobleaching studies showed that IgG is slowed only slightly (∼5–20%) by low-affinity interactions with mucins in midcycle human cervical mucus compared with its diffusion in water, with no permanently bound fractions (14,17). Given the far lower mucin content in semen, we assume the diffusivity of IgG in semen is comparable with its diffusivity in water. Because of its rapid diffusivity, we expect IgG to be uniformly distributed in CVM at t = 0, rapidly enter the semen layer when t > 0, and achieve a steady-state distribution within minutes, with a slightly higher steady-state concentration in CVM versus that in semen (Fig. 1 C).
Table 1.
Parameters and values incorporated into the model
| Parameter | Symbol | Value |
|---|---|---|
| HIV-1 | ||
| Radius | rHIV | 50 nm |
| Diffusivity in semen | Dv0 | 1.27 μm2/s |
| Diffusivity in CVM | Dvn | αnDv0 |
| Viral load in semen | 8.4 × 105 copies/ejaculate | |
| Number of Env trimer spikes | N∗ | 14 ± 7 (SD) |
| bnAb (IgG) | ||
| Ab-mucin affinity | α | Variable |
| Diffusivity in semen | DAb | 40 μm2/s |
| Diffusivity in CVM | αDAb | Variable |
| Initial Ab concentration in CVM (NIH45-46) | [NIH45-46] | Variable |
| Ab-Env affinity (NIH45-46) | kon, koff | 4.26 × 104, 2.87 × 10−4 |
| Vagina | ||
| Surface area of lumen | SAvagina | 145 cm2 |
| Volume of luminal CVM | VCVM | 750 μL |
| Thickness of CVM Layer | HCVM | 50 μm |
| Volume of semen | Vsemen | 3.0 mL |
| Thickness of semen layer | Hsemen | 200 μm |
To model whether weak affinity between each individual IgG with mucins can facilitate HIV trapping when multiple IgG are bound to the same virion (Fig. 1 A), we define α to be the ratio of the diffusivity of IgG in mucus versus in water (= Dmucus/Dwater ∼ 0.8–0.95). The quantity α is therefore a phenomenological parameter that represents the fraction of time that an IgG molecule can freely diffuse in the mucin network: α = 1 corresponds to no affinity; smaller α implies greater affinity; and α = 0 indicates permanent binding between an individual IgG molecule and mucins. This parameter can be written directly in terms of Ab-mucin binding kinetics. Let mD = moff/mon denote the ratio of the Ab-mucin unbinding and binding rates, respectively, and let [M] denote the effective concentration of Ab binding sites that are available in the mucin network. Then, by standard Markov chain kinetics, α = 1/(1 + [M]/mD). This estimate is consistent with previous analyses (20,43). Assuming each HIV-bound IgG binds and unbinds to the mucin mesh independently, we can estimate the diffusivity of an HIV-IgG complex in terms of the number n of IgG bound to the virion and α: an HIV virion is assumed to be mobile during the αn fraction of time that all of its n bound IgG molecules are not bound to the mucin mesh, giving a time-averaged diffusivity Dvn = αnDv0.
See Table 2 for a definition of terms used in this article.
Table 2.
Abbreviations used in the text
| Abbreviation | Name/explanation |
|---|---|
| Ab | Antibody |
| ADCC | Antibody-dependent cellular cytotoxicity |
| CVM | Cervicovaginal mucus |
| HSV | Herpes simplex virus |
| HIV | Human immunodeficiency virus |
| HPV | Human papillomavirus |
| IgA, IgG | Immunoglobulin A, Immunoglobulin G (two common classes of Ab molecules) |
| IC50, IC80 | Ab concentrations to inhibit viral infectivity by 50 and 80%, respectively |
Results
Low-affinity IgG-mucin interactions alone can effectively trap HIV in mucus
We have previously shown that HIV virions diffuse readily in pH-neutralized CVM (mimicking semen-induced neutralization of acidic CVM), and a substantial fraction of HIV virions can penetrate CVM and reach the vaginal epithelium within minutes (22). We now examine whether Ab can accumulate on HIV in sufficient quantities within this narrow time window to retard virus diffusion, using the formula for Dvn, and thereby reduce the flux of virions reaching target cells. We choose NIH45-46, a recently discovered Ab from the lab of Diskin et al. (44) that targets the gp120 molecules present on the envelope (Env) spikes of HIV, because it has the following characteristics: 1. Very high affinity to Env; 2. High neutralization potency (i.e., low IC50 and IC80 values (see Table 1)); and 3. Wide breadth of neutralization against diverse HIV strains (36,44). At an initial concentration of 5 μg/mL in CVM, which would represent ∼1% of all secreted IgG found in CVM, NIH45-46 quickly accumulates on the surface of HIV; within 10 and 30 min, respectively, we estimate an average HIV virion is bound by ∼10 and 20 NIH45-46, respectively (Fig. 2, A and B).
Figure 2.
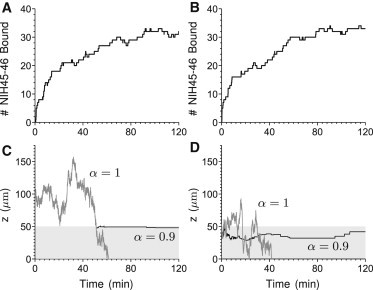
Dynamics of HIV virions diffusing across CVM supplemented with NIH45-46. (A and B) The kinetics of accumulation of NIH45-46 IgG on example HIV virions, and their spatial location over time (C and D). Virion paths start at either 50 μm (A and C) or 0 μm (B and D) from the semen/CVM interface. Paths were chosen so that the passage time was within one standard deviation of the expected mean passage time (α = 1). The α = 0.9 path is derived from the same realization as for α = 1, with the modification that a virion in the CVM has a probability αn of free diffusion.
To illustrate the impact of the weak IgG-mucin interactions, we directly compare selected virion diffusion paths without IgG-mucin interactions (α = 1) versus paths under identical Brownian realizations, where each bound NIH45-46 interacts with mucins ∼10% of the time (i.e., α = 0.9) (Fig. 2, C and D). Virions initially located at a greater distance away from the semen/CVM interface appear to become trapped near the interface (Fig. 2 C). In contrast, virions that start off near the semen/CVM interface are able to diffuse some distance into the CVM layer before becoming trapped (Fig. 2 D). In both cases, the number of HIV-bound NIH45-46 effectively suppresses the probability that any HIV/NIH45-46 complex can be simultaneously free of all potential IgG-mucin interactions for extended periods of time. This in turn leads to rapid and sustained sequestration of viruses in CVM despite the extremely weak affinity of each IgG-mucin bond (Fig. 2, C and D, and see the Supporting Material for Fig. S1 A, inset). Because of the fast timescale of the Ab-mucin affinities relative to that of virion diffusion across the CVM, we obtain nearly identical results whether modeling virion-bound Ab-mucin interactions as preventing the motion of the Ab-virion complex for an αn fraction of time or by utilizing an average-reduced (by αn) diffusion coefficient.
We further analyze the mean number of bound NIH45-46 Ab on HIV virions that possess the following characteristics: 1. Remain in semen, 2. Remain in CVM, or 3. Penetrate across the CVM layer and reach the vaginal epithelium. Not surprisingly, virions that successfully penetrated CVM have a markedly lower number of NIH45-46 molecules bound to them (see Fig. S1 B; ∼3–5-fold lower). Interestingly, virions sequestered in CVM possess a somewhat greater number of NIH45-46 molecules bound than virions that remain in semen; this likely reflects a combination of marginally higher concentration of free NIH45-46 in CVM than in semen, and the retarded mobility of HIV/NIH45-46 complexes in CVM.
HIV-specific IgG in CVM induces rapid formation and pinning of an HIV concentration front near the semen/CVM interface
We next evaluate how small changes in α influence viral flux and spatial distribution within the CVM layer. Interestingly, rather than a uniform distribution of virions throughout the CVM layer, the model suggests HIV-binding IgG quickly induces formation of a stationary virion concentration front, with HIV virions concentrated in a thin CVM layer close to the semen/CVM interface (Fig. 3, and see Movie S3 and Movie S4). An HIV concentration front forms even when individual IgG-mucin affinity is weak (Fig. 3, B and C, versus Fig. 3 A), and becomes markedly more pronounced with further subtle increases in IgG-mucin affinity (Fig. 3 D). The effectiveness of this IgG-mediated molecular shield against HIV diffusion is reflected by several features: the peak virus concentration at the semen/CVM interface (Fig. 3 E); the fast rate at which virions concentrate at the interface (Fig. 3 E); and the fraction of virions becoming trapped within the first 5 μm from the semen/CVM interface (Fig. 3 F). Indeed, even at the weakest affinity simulated (α = 0.95), we observe a >30% drop in flux of HIV virions arriving at the vaginal epithelium after 2 h (Fig. 4, A–D). The flux of HIV virions reaching target cells can be reduced by >90% if the IgG-mucin affinity is slightly stronger (α = 0.8), or if the NIH45-46 concentration is further increased (Fig. 4 E).
Figure 3.
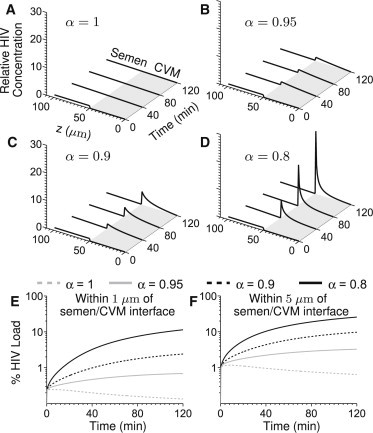
HIV concentration profiles across genital secretions coating the vaginal epithelium for (A) α = 1, (B) α = 0.95, (C) α = 0.9, and (D) α = 0.8, at an initial NIH45-46 concentration of 5 μg/mL. The virus concentration curves are shown at 0, 40, 80, and 120 min. (E and F) Percentage of virus in CVM within 1 and 5 μm, respectively, of the semen/CVM interface over time.
Figure 4.
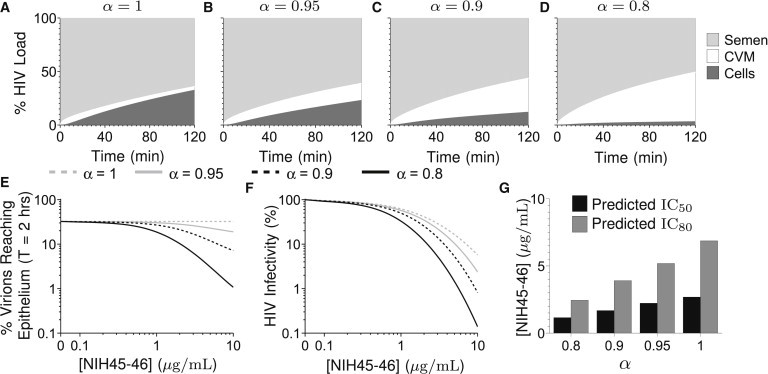
(A–D) Percentage of viral load in semen, CVM, and cells over time for (A) α = 1, (B) α = 0.95, (C) α = 0.9, and (D) α = 0.8 and an initial Ab concentration of [NIH45-46] = 5 μg/mL. (E) Percent of viral load arriving at epithelium in 2 h for various values of α, initial Ab concentration [NIH45-46]. (F) HIV infectivity (defined as the fraction of Ab-free Env spikes on virions arriving at the epithelium compared with [NIH45-46] = 0 μg/mL, α = 1) for various α, initial Ab concentration [NIH45-46]. (G) Predicted IC50, IC80 (see Table 1), [NIH45-46] = 5 μg/mL.
IgG-mediated trapping enhances protection by neutralizing antibodies
It is unclear the extent to which reducing the flux of HIV virions to the vaginal epithelium can improve protection naturally afforded by broadly neutralizing antibodies. Conceptually, virion trapping in CVM enhances protection by neutralizing antibodies only if trapping directly reduces the flux of virions with substantial amounts of infectious (i.e., Ab-free) Env spikes; trapping does not enhance protection against virions that are already completely neutralized at the time of cellular encounters. The latter is particularly true for virions with few Env spikes natively, which may not generate sufficient IgG-mucin avidity to become trapped in mucus yet only require a small number of bound Ab to become effectively neutralized. Nevertheless, it is likely difficult to neutralize all Env spikes on HIV virions, especially on virions that have relatively more Env spikes and/or quickly traverse the mucus layer and reach target cells. In these instances, which may represent the majority of the virions responsible for vaginal HIV transmission, the reduced flux due to trapping viruses by IgG-mucin interactions may markedly enhance protection. We thus seek to quantify protection by Ab-neutralization without IgG-mucin affinity (α = 1) as well as with varying degrees of Ab-mucin affinity.
It is generally assumed that the binding of a single IgG molecule to an Env spike is sufficient to inactivate the infectivity associated with that spike, and that each additional bound IgG to a previously IgG-free Env incrementally reduces the likelihood of infection (45–47). By modeling the number of IgG-free and IgG-bound Env on virions that arrive at the vaginal epithelium compared to IgG-null control, we can estimate any improved protection (reduction in infectivity) afforded by IgG-mediated trapping of HIV. Our model suggests that NIH45-46, with mucin affinity in the α = 0.8–0.95 range, can substantially improve the protective efficacy of NIH45-46 by neutralization alone, especially at higher NIH45-46 concentrations (Fig. 4, F and G). These results strongly underscore the importance of harnessing IgG-mucin interactions in the context of vaccine and prophylactic antibody development.
Discussion
During sexual transmission, pathogens are first exposed to CVM coating the female reproductive tract, and viruses and obligate intracellular bacteria (e.g., Chlamydia) must penetrate CVM to reach target cells in the vaginal epithelium or submucosa. Thus, an effective CVM barrier should exclude and eliminate pathogens directly at the portal of entry. Unfortunately, pathogens have evolved numerous mechanisms to quickly penetrate across mucus, such as surfaces that evade adhesive interactions with sticky mucins, and/or by evolving directed motility apparatus (e.g., beating flagella). Indeed, many pathogens that readily infect mucosal surfaces, including HIV, HSV, Norwalk virus, and Human Papillomavirus (HPV), have all been shown to readily penetrate human mucus secretions (14,22,23). IgG-mucin interactions that trap pathogens in mucus therefore represent an attractive and potentially important mechanism by which the immune system can readily adapt and reinforce the body’s first line of defense against diverse and rapidly evolving pathogens. Trapping virions in genital tract mucus may markedly reduce heterosexual transmission of viral infections. Women acquire many of the major sexually transmitted viral infections (e.g., HIV, HPV, and HSV) at rates of ∼1 per 100–1000 sex acts on average (48–52), suggesting that few, if any, of these infectious virions are able to infect target cells per intercourse. Consequently, any reduction in the flux of virions that reach target cells should proportionally reduce transmission rates. Blocking infections directly at the portal of entry may be especially important against infections that are difficult if not impossible to cure, including HIV, Herpes, and other polymicrobial infections (e.g., bacterial vaginosis).
Due to the inability to detect permanent, high-affinity bonds between individual Ab molecules and mucins, the notion that Ab can work in tandem with mucus to block infections has received little attention to date. Surprisingly, our model suggests that even exceedingly weak IgG-mucin interactions are capable of rapidly inducing a concentration-front formation of highly concentrated virions at the semen/CVM interface. This concentration front formation is likely critically dependent on the ability of IgG to undergo rapid diffusion in genital secretions. Because HIV virions are uniformly distributed within seminal fluids, and there is substantially greater volume of semen than CVM, the majority of virions in semen are actually located at substantial distances away from the semen/CVM interface just after semen deposition. Because IgG possesses diffusivity nearly 30-fold greater than that for HIV virions in genital secretions, they can diffuse into semen much more quickly than HIV can diffuse into CVM. Thus, HIV-specific IgGs such as NIH45-46 likely accumulate on the virions in substantial quantities before the HIV/NIH45-46 complexes enter the CVM layer. As soon as they reach the CVM layer, HIV/NIH45-46 complexes with a sufficient number of bound NIH45-46s experience a dramatic downshift in their diffusivity, as captured by the diffusion coefficient Dvn, and finally become immobilized near the semen/CVM interface by a sufficient number (n) of IgG-mucin bonds. Mucus is a non-Newtonian fluid that is continuously secreted and shed; in this process, the most superficial mucus layers and semen should be cleared much more quickly than mucus immediately adjacent to the epithelium (35). Indeed, seminal discharge is typically observed flowing out of the introitus within minutes of ejaculation. Thus, the formation of a virion concentration front at the semen/CVM interface—the maximum distance away from the vaginal epithelium—not only should minimize the flux of virions reaching target cells in the submucosa, but also likely enable most rapid elimination of viruses by natural mucus clearance mechanisms.
Despite evidence that IgG can trap both viruses and highly motile bacteria with remarkable potency, the actual biophysical mechanism underscoring this muco-trapping phenomenon remains not well understood. Indeed, it was unclear whether the exceedingly weak affinity of each IgG-mucin crosslink (α = 0.8–0.95 range, as suggested in the literature (14,17,19)) can collectively produce sufficient avidity to trap HIV virions, or if HIV could only be immobilized by individually high affinity and stable IgG-mucin bonds, as suggested in Fahrbach et al. (42). There are a number of factors that may potentially alter IgG-mucin affinity, including variations in the N-glycans on IgG-Fc (19). High-affinity bonds between individual pathogen-bound IgG and mucins are also conceptually possible if a conformational change in the Fc domain of IgG upon binding to antigen targets can expose a moiety with substantially greater muco-affinity than native, unbound IgG. Because this question is difficult to directly address experimentally, we developed our mathematical model and simulation to explore HIV diffusional dynamics incorporating known antibody binding kinetics and IgG-mucin affinity. Although our findings do not disprove the possibility of antigen-binding-induced increase in muco-affinity of IgG, we clearly demonstrate that very weak IgG-mucin interactions alone can theoretically generate sufficient avidity to immobilize HIV and likely other sexually transmitted viruses in CVM.
In our model with NIH45-46, one of the most potent broadly neutralizing IgGs against HIV, trapping viruses in CVM appears to only modestly improve protection compared with neutralization. Nevertheless, trapping virions in mucus can likely play a greater role in protection afforded by other less potently neutralizing IgGs. This includes IgGs that readily bind virions but are not neutralizing or even IgGs that bind relatively weakly to HIV, because trapping virions in mucus require only binding and not necessarily neutralizing IgG. Nonneutralizing IgG are commonly found in nature; indeed, many of the naturally produced IgG against HIV found in HIV+ patients associate with either the lipid membrane of HIV virions, or other parts of the gp120 site on the Env spike not directly involved in HIV infection of immune cells (53). Likewise, virtually all of the IgGs detected in the moderately successful RV144 trial were nonneutralizing, and thus must depend on other immune protective mechanisms to block HIV transmission. In line with this hypothesis, we recently found that a nonneutralizing monoclonal IgG against HSV is able to mediate substantial vaginal protection in mice, but the protection is abolished in the absence of an intact mucus layer (19). Efforts to harness IgG-mucin interactions for improved mucosal protection, by maximizing virion-binding IgG, may further increase the repertoire of antigen sites that can be utilized for vaccine or prophylaxis development.
Our model is only a first step toward an improved quantitative understanding of the dynamics of mucosal immunity in the female reproductive tract. We expect additional iterations, and future improvements to our model will provide further predictive insights into reinforcing vaginal mucosal immunity against HIV and other sexually transmitted infections.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or National Science Foundation. The authors declare no conflicts of interest.
This work was supported by the National Institutes of Health grants No. R21AI093242 and No. U19AI096398 (both to S.K.L.); National Science Foundation grants No. DMS-1122483 and DMS-1100281 (both to M.G.F.), grant No. DMS-0645369 (to P.J.M.), and grant No. DMS-1127914 (to A.C.); Simons Foundation Project No. 245653 (to S.A.M.); and Packard Foundation and NSF CAREER award No. DMR-1151477, and startup funds from the Eshelman School of Pharmacy and Lineburger Cancer Center at University of North Carolina at Chapel Hill (these last all to S.K.L.).
Contributor Information
M. Gregory Forest, Email: forest@unc.edu.
Samuel K. Lai, Email: lai@unc.edu.
Supporting Material
References
- 1.Chipperfield E.J., Evans B.A. Effect of local infection and oral contraception on immunoglobulin levels in cervical mucus. Infect. Immun. 1975;11:215–221. doi: 10.1128/iai.11.2.215-221.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert-Guroff M. IgG surfaces as an important component in mucosal protection. Nat. Med. 2000;6:129–130. doi: 10.1038/72206. [DOI] [PubMed] [Google Scholar]
- 3.Hessell A.J., Hangartner L., Burton D.R. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 4.Veazey R.S., Shattock R.J., Moore J.P. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat. Med. 2003;9:343–346. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 5.Sherwood J.K., Zeitlin L., Saltzman M. Controlled release of antibodies for long-term topical passive immunoprotection of female mice against genital Herpes. Nat. Biotechnol. 1996;14:468–471. doi: 10.1038/nbt0496-468. [DOI] [PubMed] [Google Scholar]
- 6.Moog C., Dereuddre-Bosquet N., LeGrand R. Protective effect of vaginal application of neutralizing and nonneutralizing inhibitory antibodies against vaginal SHIV challenge in macaques. Mucosal Immunol. 2014;7:46–56. doi: 10.1038/mi.2013.23. [DOI] [PubMed] [Google Scholar]
- 7.Hessell A.J., Rakasz E.G., Burton D.R. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 2009;5:e1000433. doi: 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burton D.R., Woof J.M. Human antibody effector function. Adv. Immunol. 1992;51:1–84. doi: 10.1016/s0065-2776(08)60486-1. [DOI] [PubMed] [Google Scholar]
- 9.Cone R.A. Mucus. In: Mestecky J., Lamm M.E., Strober W., Bienenstock J., McGhee J.R., editors. Handbook of Mucosal Immunology. 3rd Ed. Academic Press; London, UK: 2005. pp. 49–72. [Google Scholar]
- 10.Hill J.A., Anderson D.J. Human vaginal leukocytes and the effects of vaginal fluid on lymphocyte and macrophage defense functions. Am. J. Obstet. Gynecol. 1992;166:720–726. doi: 10.1016/0002-9378(92)91703-d. [DOI] [PubMed] [Google Scholar]
- 11.Schumacher G.F.B. Immunology of spermatozoa and cervical mucus. Hum. Reprod. 1988;3:289–300. doi: 10.1093/oxfordjournals.humrep.a136698. [DOI] [PubMed] [Google Scholar]
- 12.Kresge K.J. The mysteries of protection. IAVI Rep. 2009;13:5. [Google Scholar]
- 13.Rerks-Ngarm S., Pitisuttithum P., Kim J.H. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 14.Olmsted S.S., Padgett J.L., Cone R.A. Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophys. J. 2001;81:1930–1937. doi: 10.1016/S0006-3495(01)75844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clamp J.R. The relationship between secretory immunoglobulin A and mucus. Biochem. Soc. Trans. 1977;5:1579–1581. doi: 10.1042/bst0051579. [DOI] [PubMed] [Google Scholar]
- 16.Crowther R., Lichtman S., Forstner G. Failure to show secretory IgA binding by rat intestinal mucin. Fed. Proc. 1985;66:691. [Google Scholar]
- 17.Saltzman W.M., Radomsky M.L., Cone R.A. Antibody diffusion in human cervical mucus. Biophys. J. 1994;66:508–515. doi: 10.1016/s0006-3495(94)80802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai S.K., Wang Y.-Y., Hanes J. Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses. Proc. Natl. Acad. Sci. USA. 2010;107:598–603. doi: 10.1073/pnas.0911748107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y.-Y., Kannan A., Lai S.K. IgG in cervicovaginal mucus traps HSV and prevents vaginal Herpes infections. Mucosal Immunol. 2014 doi: 10.1038/mi.2013.120. 2014 Feb 5. http://dx.doi.org/10.1038/mi.2013.120. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pando B., Dawson S.P., Pearson J.E. Messages diffuse faster than messengers. Proc. Natl. Acad. Sci. USA. 2006;103:5338–5342. doi: 10.1073/pnas.0509576103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ten Wolde P.R., Tănase-Nicola S. Biophysics: pass on the message. Nat. Phys. 2006;2:371–372. [Google Scholar]
- 22.Lai S.K., Hida K., Hanes J. Human immunodeficiency virus type 1 is trapped by acidic but not by neutralized human cervicovaginal mucus. J. Virol. 2009;83:11196–11200. doi: 10.1128/JVI.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shukair S.A., Allen S.A., Hope T.J. Human cervicovaginal mucus contains an activity that hinders HIV-1 movement. Mucosal Immunol. 2013;6:427–434. doi: 10.1038/mi.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reference deleted in proof.
- 25.Jamison P.L., Gebhard P.H. Penis size increase between flaccid and erect states: an analysis of the Kinsey data. J. Sex Res. 1998;24:177–183. doi: 10.1080/00224498809551408. [DOI] [PubMed] [Google Scholar]
- 26.Pendergrass P.B., Belovicz M.W., Reeves C.A. Surface area of the human vagina as measured from vinyl polysiloxane casts. Gynecol. Obstet. Invest. 2003;55:110–113. doi: 10.1159/000070184. [DOI] [PubMed] [Google Scholar]
- 27.Rehan N.E., Sobrero A.J., Fertig J.W. The semen of fertile men: statistical analysis of 1300 men. Fertil. Steril. 1975;26:492–502. doi: 10.1016/s0015-0282(16)41169-6. [DOI] [PubMed] [Google Scholar]
- 28.Gupta P., Mellors J., Rinaldo C.R. High viral load in semen of human immunodeficiency virus type 1-infected men at all stages of disease and its reduction by therapy with protease and nonnucleoside reverse transcriptase inhibitors. J. Virol. 1997;71:6271–6275. doi: 10.1128/jvi.71.8.6271-6275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu J., Gardner M.B., Miller C.J. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 2000;74:6087–6095. doi: 10.1128/jvi.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maher D., Wu X., Southern P. HIV binding, penetration, and primary infection in human cervicovaginal tissue. Proc. Natl. Acad. Sci. USA. 2005;102:11504–11509. doi: 10.1073/pnas.0500848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norvell M.K., Benrubi G.I., Thompson R.J. Investigation of microtrauma after sexual intercourse. J. Reprod. Med. 1984;29:269–271. [PubMed] [Google Scholar]
- 32.Shattock R.J., Moore J.P. Inhibiting sexual transmission of HIV-1 infection. Nat. Rev. Microbiol. 2003;1:25–34. doi: 10.1038/nrmicro729. [DOI] [PubMed] [Google Scholar]
- 33.Geonnotti A.R., Katz D.F. Dynamics of HIV neutralization by a microbicide formulation layer: biophysical fundamentals and transport theory. Biophys. J. 2006;91:2121–2130. doi: 10.1529/biophysj.106.086322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lai B.E., Henderson M.H., Katz D.F. Transport theory for HIV diffusion through in vivo distributions of topical microbicide gels. Biophys. J. 2009;97:2379–2387. doi: 10.1016/j.bpj.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai S.K., Wang Y.-Y., Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009;61:158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheid J.F., Mouquet H., Nussenzweig M.C. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu P., Liu J., Roux K.H. Distribution and three-dimensional structure of AIDS virus envelope spikes. Nature. 2006;441:847–852. doi: 10.1038/nature04817. [DOI] [PubMed] [Google Scholar]
- 38.Fox C.A., Meldrum S.J., Watson B.W. Continuous measurement by radio-telemetry of vaginal pH during human coitus. J. Reprod. Fertil. 1973;33:69–75. doi: 10.1530/jrf.0.0330069. [DOI] [PubMed] [Google Scholar]
- 39.Tevi-Bénissan C., Bélec L., Grésenguet G. In vivo semen-associated pH neutralization of cervicovaginal secretions. Clin. Diagn. Lab. Immunol. 1997;4:367–374. doi: 10.1128/cdli.4.3.367-374.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Z., Palaniyandi S., Zhu X. Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection. Proc. Natl. Acad. Sci. USA. 2011;108:4388–4393. doi: 10.1073/pnas.1012861108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Usala S.J., Usala F.O., Schumacher G.F. IgG and IgA content of vaginal fluid during the menstrual cycle. J. Reprod. Med. 1989;34:292–294. [PubMed] [Google Scholar]
- 42.Fahrbach K.M., Malykhina O., Hope T.J. Differential binding of IgG and IgA to mucus of the female reproductive tract. PLoS ONE. 2013;8:e76176. doi: 10.1371/journal.pone.0076176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strier D.E., Chernomoretz A., Ponce Dawson S. Slow time evolution of two-time-scale reaction-diffusion systems: the physical origin of nondiffusive transport. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2002;65:046233. doi: 10.1103/PhysRevE.65.046233. [DOI] [PubMed] [Google Scholar]
- 44.Diskin R., Scheid J.F., Bjorkman P.J. Increasing the potency and breadth of an HIV antibody by using structure-based rational design. Science. 2011;334:1289–1293. doi: 10.1126/science.1213782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schønning K., Lund O., Hansen J.-E.S. Stoichiometry of monoclonal antibody neutralization of T-cell line-adapted human immunodeficiency virus type 1. J. Virol. 1999;73:8364–8370. doi: 10.1128/jvi.73.10.8364-8370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang X., Kurteva S., Sodroski J. Stoichiometry of antibody neutralization of human immunodeficiency virus type 1. J. Virol. 2005;79:3500–3508. doi: 10.1128/JVI.79.6.3500-3508.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Magnus C., Regoes R.R. Estimating the stoichiometry of HIV neutralization. PLOS Comput. Biol. 2010;6:e1000713. doi: 10.1371/journal.pcbi.1000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hughes J.P., Baeten J.M., Celum C. Determinants of per-coital-act HIV-1 infectivity among African HIV-1-serodiscordant couples. J. Infect. Dis. 2012;205:358–365. doi: 10.1093/infdis/jir747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heffron R., Mugo N., Baeten J.M. Hormonal contraceptive use and risk of HIV-1 disease progression. AIDS. 2013;27:261–267. doi: 10.1097/QAD.0b013e32835ad473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wald A., Langenberg A.G., Corey L. Effect of condoms on reducing the transmission of Herpes simplex virus type 2 from men to women. JAMA. 2001;285:3100–3106. doi: 10.1001/jama.285.24.3100. [DOI] [PubMed] [Google Scholar]
- 51.Tobian A.A.R., Kigozi G., Wawer M.J. Male circumcision and Herpes simplex virus type 2 infection in female partners: a randomized trial in Rakai, Uganda. J. Infect. Dis. 2012;205:486–490. doi: 10.1093/infdis/jir767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Collins S.I., Mazloomzadeh S., Woodman C.B. Proximity of first intercourse to menarche and the risk of human papillomavirus infection: a longitudinal study. Int. J. Cancer. 2005;114:498–500. doi: 10.1002/ijc.20732. [DOI] [PubMed] [Google Scholar]
- 53.Mouquet H., Scheid J.F., Nussenzweig M.C. Polyreactivity increases the apparent affinity of anti-HIV antibodies by heteroligation. Nature. 2010;467:591–595. doi: 10.1038/nature09385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leveque R.J., Li Z. The immersed interface method for elliptic equations with discontinuous coefficients and singular sources. SIAM J. Numer. Anal. 1994;31:1019–1044. [Google Scholar]
- 55.Lai S.K., O’Hanlon D.E., Hanes J. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc. Natl. Acad. Sci. USA. 2007;104:1482–1487. doi: 10.1073/pnas.0608611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


