Abstract
Spinal nerve roots have a peculiar structure, different from the arrangements in the peripheral nerve. The nerve roots are devoid of lymphatic vessels but are immersed in the cerebrospinal fluid (CSF) within the subarachnoid space. The blood supply of nerve roots depends on the blood flow from both peripheral direction (ascending) and the spinal cord direction (descending). There is no hypovascular region in the nerve root, although there exists a so-called water-shed of the bloodstream in the radicular artery itself. Increased mechanical compression promotes the disturbance of CSF flow, circulatory disturbance starting from the venous congestion and intraradicular edema formation resulting from the breakdown of the blood-nerve barrier. Although this edema may diffuse into CSF when the subarachnoid space is preserved, the endoneurial fluid pressure may increase when the area is closed by increased compression. On the other hand, the nerve root tissue has already degenerated under the compression and the numerous macrophages releasing various chemical mediators, aggravating radicular symptoms that appear in the area of Wallerian degeneration. Prostaglandin E1 (PGE1) is a potent vasodilator as well as an inhibitor of platelet aggregation and has therefore attracted interest as a therapeutic drug for lumbar canal stenosis. However, investigations in the clinical setting have shown that PGE1 is effective in some patients but not in others, although the reason for this is unclear.
Keywords: Lumbar canal stenosis, Cauda equine, Nerve root, Prostaglandin E1, Blood flow
Core tip: The radicular symptoms associated with degenerative disease of the lumbar spine are reported to be attributable to a combination of mechanical nerve root compression and resultant circulatory disturbance. Disturbance of blood flow in the cauda equina and nerve roots is reported to play an important role in the mechanism of intermittent claudication in patients with lumbar canal stenosis. Prostaglandin E1 (PGE1) is a potent vasodilator as well as an inhibitor of platelet aggregation and has therefore attracted interest as a therapeutic drug for lumbar canal stenosis with intermittent claudication. However, investigations in the clinical setting have shown that lipo-PGE1 is effective in some patients but not in others, although the reason for this is unclear.
INTRODUCTION
Lumbar canal stenosis (LCS) is increasingly a common disease in the elderly. The number of patients with LCS complaining of low back pain, lower extremity pain and/or numbness, and neurogenic intermittent claudication (NIC) has increased yearly[1,2]. Compression of the cauda equina and nerve roots by LCS is a major clinical problem associated with NIC[3-5]. The development of NIC in LCS has been reported to involve circulatory disturbance due to circumferential compression of cauda equina by the surrounding tissues. When lumbar lordosis increases in the standing position, the severity of stenosis increases and the cauda equina are constricted more strongly, while the constriction decreases in the sitting position or when the trunk is flexed. As a result, NIC is often noted as a characteristic feature. The main symptoms of NIC include deep muscular pain, weakness and loss of sensation in the lower limbs. Such symptoms do not develop immediately after the start of walking, but eventually become severe enough to disturb walking. The patient can only walk from 40-50 m to 400-500 m without resting and the symptoms resolve after resting for several to 10 min. Thus, it is generally agreed that the primary cause of NIC is chronic compression of the cauda equina. In this article, we have reviewed the pathophysiology, diagnosis, and treatment of LCS associated with NIC.
DEFINITION AND CLASSIFICATION OF LCS
In 1910, Sumida gave the first description of LCS due to fetal chondrodystrophy[6]. Sarpyener reported such stenosis due to congenital partial skeletal dysplasia in 1945[7]. Thus, both of them reported congenital conditions. The concept of LCS has been known widely since Verbiest reported on idiopathic developmental stenosis in French in 1949[8], in Dutch in 1950[9], and in English in 1954[10] and 1955[11]. In 1976, Arnoldi et al[12] proposed the international definition and classification of lumbar spinal canal stenosis that is still used widely. However, confusion has arisen with regard to interpretation, because it covers both central stenosis (spinal canal stenosis) and lateral stenosis (nerve root canal and intervertebral foramen stenosis), and also covers acquired stenosis due to various degenerative diseases as well as congenital and developmental stenosis. Here, the international classification (Table 1) is interpreted and the problems with it are clarified. The word ‘stenosis’ implies narrowing of a hollow tubular structure. On this basis, LCS may be defined as any type of narrowing of the spinal canal, nerve root canals (or tunnels) or intervertebral foramina. It may be local, segmental or generalized. It may be caused by bone or by soft tissue and the narrowing may involve the bony canal alone or the dural sac or both. Herniations of the nucleus pulposus have in the past been considered as a distinct and separate entity. They are included in this classification when they occur together with other types of stenosis, which is frequently the case. Space occupying lesions due to the products of inflammation or neoplasm are in the strictest sense types of “stenosis” but are excluded. According to this definition, the symptoms caused by LCS are non-specific and very varied.
Table 1.
International classification of lumbar canal stenosis
| Congenital/developmental |
| Acquired |
| Degenerative (spondylosis) |
| Central |
| peripheral |
| Degenerative spondylolisthesis |
| Combined |
| Congenital/developmental + Degenerative |
| Congenital/developmental + Hernia |
| Degenerative IIernia |
| Congenital/developmental + Degenerative + Hernia |
| Spondylolisthetic/spondylolytic |
| Iatrogenic |
| Post-tranmatic |
| Miscellaneous |
Reproduced with permission from Arnoldi et al[12].
Figure 1 is designed to make this classification easier to understand. Among the acquired types of stenosis, spondylosis and degenerative spondylolisthesis are classified as degenerative stenosis accompanied with LCS (a, c, d, e, f in Figure 1). The condition is classified as combined stenosis (b-i) if congenital or developmental factors are also present. Disc herniation is not covered by the concept of spinal stenosis when it exists alone. However, it is classified as spinal stenosis (combined stenosis) when congenital or developmental (b-iii, iv) or degenerative (b-iii, iv) factors are also present. The success of surgical treatment for disc herniation is dependent on the presence of factors causing spinal stenosis. However, it is not always easy to determine whether there are such factors in patients with herniation and whether the stenosis is developmental or acquired. As a result, herniation accompanied by spinal canal or nerve root canal stenosis (b-ii, iii, iv) is often treated after misdiagnosis as simple herniation. On the other hand, patients with central or lateral stenosis who are treated under the diagnosis of spinal stenosis due to spondylosis or degenerative spondylolisthesis may also sometimes have combined developmental stenosis (b-i).
Figure 1.

Schematic drawing illustrating the international classification of lumbar canal stenosis.
BLOOD SUPPLY OF CAUDA EQUINA NERVE ROOT
Anatomical studies of the vasculature of the nerve roots have developed in association with studies on the vasculature of the spinal cord. In the 19th century, Adamkiewicz[13,14] and Kadyi[15,16] clarified the particularly important role of radicular arteries in the blood supply of the spinal cord. After that, much work was devoted to the vasculature of the spinal cord[17-24], but no research placed emphasis on the supply to the nerve roots until the mid-twentieth century. Corbin described anatomic details of radicular arteries and classified them into three groups: artères radiculo-grères, artères radiculo-piemeriennes, and artères radiculo-medullares[25]. The first two arteries were named as distal and proximal radicular arteries by Parke et al[26] and were thought to be nutrient arteries of the nerve roots. They described that each lumbosacral spinal nerve root receives its intrinsic blood supply from both distal and proximal radicular arteries, through which the blood flows toward a mutual anastomosis in the proximal one third of the root. They postulated that the region of relative hypovascularity formed below the conus by the combined areas of anastomoses in the cauda equina may provide an anatomic rationale for the suspected neuroischemic manifestations concurrent with degenerative changes in the lumbar spine. Crock et al[27] based on their studies, hold a different view: that there is no area of hypovascularity in the region of the middle third of the cauda equina. Kobayashi et al[28] also examined the vasculature of the cauda equina nerve root in dogs with the aid of high-speed serial photography after injecting India ink in the Aorta. Consequently, the blood flow direction of the extradural nerve root and descending in the cauda equina nerve root, and there existed a so-called watershed of the blood-stream in the radicular artery itself near the root of the dural sleeve (Figure 2A, B). When the stream of the ascending radicular artery was intercepted by compression, however, the blood flow direction changed quickly and the blood supply was compensated by the descending radicular artery. Also abundant fine intrinsic arteries form networks in every part of the nerve root, including the area of so-called watershed of the radicular artery (Figure 2B). These observations indicate that there is no relatively hypovascular region in the nerve root, which is vulnerable in the course of degenerative changes of the lumbosacral spine. Although there exists a so-called watershed of the bloodstream in the radicular artery itself, the site of which is, however, changeable due to circumstances. Microangiograms showed an abundant vascular network with repeating T-shaped branching throughout the length of the nerve root (Figure 3). This was also present near the watershed region in the radicular artery, and there was no hypovascular region as suggested by Parke et al[26]. Thus, the intraradicular vessels controlled segmentally by radicular arteries have no fixed direction of flow and there appears to be no particular clinical significance in the watershed region of arteries maintaining a high intravascular pressure. Regarding the onset of compression-induced disturbance of nerve root circulation as observed in disk herniation and spinal canal stenosis, it is improbable that obstruction of the arterial system, which has thick walls and a high pressure, precedes obstruction of the venous system[29,30].
Figure 2.

Circulatory dynamics of the radicular artery for the lumbar nerve root in a dog. A: After 3 mL of India ink was injected quickly through a catheter fixed in the aortic arch, seriograhy was performed using a motor-driven camera and repeating flash to observe the flow of India ink into vessels supplying the nerve root. After injection of India ink, at first the proximal radicular arteries (PRA) running along each root filament filled with India ink in a downward direction (↓). Next, the distal radicular arteries (DRA) filled with India ink in an upward direction (↑). A watershed region of blood flow was observed in a radicular vessel (blue arrow). At last, the radicular vein (RV) filled with India ink in the downward direction (white arrows); B: A watershed region was noted in the radicular artery, and in this region the velocity of both blood streams showed a decrease; C: A clear specimen of the nerve root from the same subject as shown in A. An abundant vascular network was noted in the root near the watershed area of the radicular artery observed by seriography. AR: Anterior root. Reproduced with permission from Kobayashi et al[28].
Figure 3.
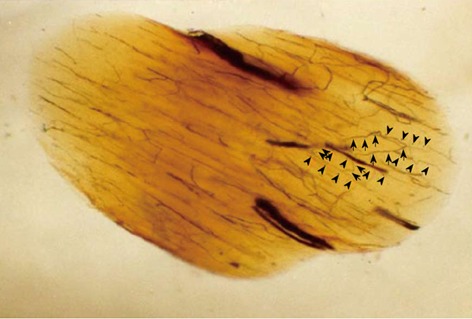
Oblique cleared section of the lumbar nerve root in a dog. There are many longitudinal (arrow head) and transverse (arrow) microvessels in the nerve root. Intraradicular vessels are abundant throughout the nerve root, and their flow is in various directions. Intraradicular vessels arising from the radicular artery as T-shaped branches rarely were affected by the direction of blood flow in the nerve root, suggesting the presence of a mechanism that maintains the blood supply to the intrinsic vessels.
EFFECT OF ARTERIAL ISCHEMIA AND VENOUS CONGESTION ON NERVE ROOT FUNCTION
Whether mechanical deformation or a circulatory disturbance plays the more prominent role in the pathogenesis of NIC with LCS has been a subject of speculation for 5 decades. Blau and Logue postulated that NIC might be evoked with ischemic neuritis of the cauda equina[31]. Evans advocated exercise-induced ischemia as the cause of NIC, which is the characteristic syndrome of this disease[32]. They supposed that reduced blood flow in the spinal nerve roots has been demonstrated during exercise, and this might contribute to the pathogenesis of NIC. Ehni et al[33] stressed the postural changes in the spinal canal on standing. He demonstrated a myelographic block in the lordotic position, but flexion permitted the contrast medium to pass. Yamada et al[34] reported the importance of intermittent constriction of the cauda equina associated with postural change. They thought that the ligamentous fulvum had a significant role in dynamic narrowing of the canal. Kavanaugh et al[35] reported that the increase of cerebrospinal fluid pressure below the blocked area might obstruct venous return and be the cause of anoxia of the cauda equina. Verbiest thought that this theory deserved further consideration, because he commonly found enlargement of the epidural venous plexus during decompression of spinal canal stenosis[36]. Although pathophysiology of the cauda equina induced by arterial ischemia or venous congestion has been an object of study for a long time, there is little agreement over which is more essential for NIC, ischemia or congestion.
Ischemic nerve root injury is a ubiquitous insult that can lead to a wide range of neuropathologic consequences, depending on the severity and duration of the ischemic event. Ischemic injury to nerve roots predominantly causes demyelination, although prolonged ischemia can also interfere with axonal transport, leading to axonal damage and Wallerian degeneration of the nerve fiber[37-40]. Vascular damage and fibrosis are common findings within the spinal canal and intervertebral foramina, and such vascular damage is significantly related to the severity of degenerative disc disease. Disc protrusion may lead to compression of epidural veins and dilation of non-compressed veins. Cooper et al[41] noted a significant relationship between evidence of venous obstruction, intraneural and perineurial fibrosis, and neural atrophy. Fibrosis may further impede nutrient transfer to endoneurial fibers, as well as predisposing to nerve stretch injury. Kobayashi et al[42,43] assessed the influence of arterial ischemia and venous congestion resulting from obstruction of blood flow without nerve root compression on intraradicular blood flow and radicular function (Figure 4). As a result, it was confirmed that nerve root ischemia had a more serious influence on blood flow, PO2, and conduction velocity than nerve root congestion. After 30 min of nerve root ischemia, recovery occurred with reperfusion, but longer ischemic periods will cause a permanent effect on radicular function due to oxygen deficiency. When changes of the femoral arterial and central venous pressures were monitored after obstruction of blood flow, both the arterial and venous pressures decreased after aortic blockade and the arterial pressure increased slightly after obstruction of the inferior vena cava. However, the central venous pressure showed an approximately 4-fold increase immediately after obstruction of the inferior vena cava, and this sudden increase in venous pressure could have a marked influence on the capillary pressure in the nerve roots. Usubiaga et al[44] demonstrated that clamping of the vena cava can be used experimentally to increase the systemic venous pressure. The same maneuver also produces congestion of the epidural veins and increases the epidural pressure[45]. But they did not describe the changes in nerve root circulation.
Figure 4.
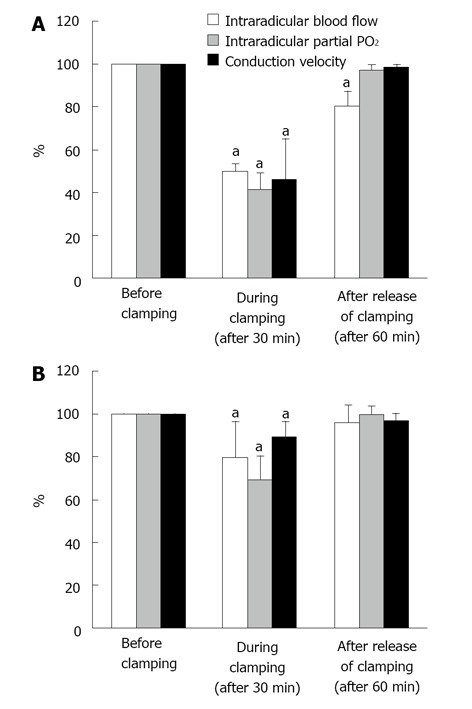
Changes of intraradicular blood flow, partial oxygen pressure and conduction velocity after Aorta (A) or inferior vena cava (B) clamp. Immediately after Aorta clamping, blood pressure in the femoral artery dropped to 26-40 mmHg and meantime, central venous pressure was slightly elevated. When the vena cava was clamped, central venous pressure increased to about 4 times of the pressure before clamping and blood pressure in the femoral artery was reduced by half. The blood flow in the seventh posterior nerve root due to Aorta and vena cava clamping fall to 50% to 60% of the blood flow before clamping in the ischemic model (aP < 0.05) and to about 20% in the congestion model (aP < 0.05). The changes of partial oxygen pressure (PO2) in the nerve root indicated a similar tendency to blood flow, 50% to 60% drop in the ischemic model (aP < 0.05) and 20% to 40% drop in the congestion model. Conduction velocity of the nerve root diminished by 40% to 50% in the ischemia model (aP < 0.05) and 10% to 20% in the congestion model. After release of clamping, both arterial and venous pressures quickly returned to the pressure before clamping. The intraradicular blood flow in the congestion model was restored within 1 h. The intraradicular blood flow in the ischemic model, however, did not recover and stayed at the reduced level (aP < 0.05). Intraradicular PO2 recovered completely in both models. The drop of conduction velocity returned almost completely within one hour after release of clamping. Reproduced with permission from Kobayashi et al[42].
The arachnoid membrane acts as a diffusion barrier for the nerve root and the blood-nerve barrier is also created by the vascular endothelial cells of the endoneurial microvessels. These nerve root barriers protect and maintain the nerve fibers in a constant environment. The capillary vessels of the nerve roots are lined by endothelial cells that contain only a few pinocytotic vesicles and are bound by tight junctions to form the blood-nerve barrier. Protein tracers that are injected intravenously do not normally leak out of the vessels due to this barrier[29,46]. When arterial ischemia was induced, protein tracers remained in the blood vessels, indicating maintenance of the integrity of the blood-nerve barrier (Figure 5A). On the other hand, venous congestion disrupted the blood-nerve barrier and there was extravasation and edema in the nerve roots (Figure 5B). Thus, the blood-nerve barrier that regulates vascular permeability in the nerve root seems to be susceptible to congestion which raises the intra vascular pressure rather than to ischemia which decreases the pressure.
Figure 5.
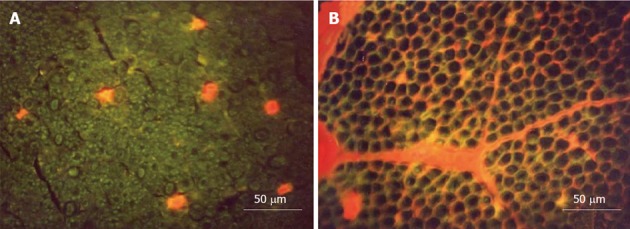
Transverse sections of the nerve root seen under a fluorescence microscope. A: Ischemia model. Evans blue albumin (EBA) emits a bright red fluorescence in clear contrast to the green fluorescence of the nerve tissue. After intravenous injection of EBA, EBA was limited inside the blood vessels, and the blood-nerve barrier was maintained; B: Congestion model. EBA emits a bright red fluorescence, which leaked outside the blood vessels, and intraradicular edema was seen under a fluorescent microscope. Reproduced with permission from Kobayashi et al[42].
PATHOMECHANISM OF INTERMITTENT CLAUDICATION
MR imaging is useful because it can noninvasively reveal the severity of LCS. It is known that sites of nerve root compression by spinal canal stenosis frequently show gadolinium enhancement on MR images, suggesting that there is breakdown of the blood-nerve barrier and edema of the nerve root (Figure 6)[29,47-50]. In LCS associated with NIC, Kobayashi et al[46] and Jinkins et al[47-49] first reported gadolinium enhancement of the cauda equina above the level of stenosis. When the nerve roots in the cauda equina are compressed in association with LCS, the pressure is distributed in a circumferential manner around the nerve root (Figure 7). Kobayashi et al[29] described that the blood-nerve barrier of the nerve root is disrupted and intraradicular edema is produced by acute compression with a microsurgical clip at more than 15 g of force for one hour or by chronic compression due to wrapping the nerve root for at least one month with a silastic tube slightly larger than the nerve root diameter[50]. They also demonstrated that the histological studies in circumferential constriction model of cauda equina revealed congestion and dilation of the intraradicular veins and Wallerian degeneration at site of constriction (Figure 8)[46]. These changes were considered to be attributed to intraradicular edema and were thought to explain the enhancement effect at the site of canal stenosis on gadolinium-enhanced MR images in LCS patients. These results suggest that NIC in LCS is caused by the following mechanism. Stenosis of the lumbar canal is aggravated by posterior flexion during walking, and circumferential mechanical compression of the cauda equina occurs repeatedly and increases in severity (Figure 7). As a result, the subarachnoid space is occluded, and congestion as well as degeneration of nerve fibers occurs in the cauda equina. Elevation of the capillary pressure induced by venous stasis is thought to cause intraradicular edema and the inflammatory response produced by compression, as well as mechanical damage to the blood-nerve barrier, because venous blood flow is stopped by compression at a very low pressure. An experiment performed by Olmarker demonstrated that the capillaries and venules of the nerve root could be occluded by mild compression of around 30-40 mmHg[30]. Takahashi et al[51] found that the epidural pressure is only 15 to 18 mmHg during lumbar flexion in LCS patients, but reaches 80 to 100 mmHg during lumbar extension. The epidural pressure increases with walking and the patient then stops walking because of leg pain and/or NIC. The pressure decreases immediately after walking is stopped and leg pain then subsides. There is a repeated pattern of increasing and decreasing pressure during walking. Although, these pressure changes are not great enough to disturb arterial blood flow, the epidural venous system may become congested if the pressure is higher than 10 to 30 mmHg. Ikawa et al[52] demonstrated that ectopic firing was elicited by venous stasis in a rat model of lumbar canal stenosis. As a result, the subarachnoid space is occluded, and congestion as well as nerve fiber degeneration occurs in the cauda equina. Efflux of excess fluid into the subarachnoid space becomes impaired by the breakdown of the blood-nerve barrier, leading to an increase in endoneurial pressure[53,54]. Although such a pressure rise is reversible, a compartment syndrome may occur in the cauda equina at the site of stenosis, blood flow[55,56] and axonal flow disturbance[38,39], provoking ectopic discharge or conduction disturbance[57,58] that is essentially responsible for NIC in nerve fibers which have been chronically damaged. Thus, venous congestion may be an essential factor precipitating circulatory disturbance in compressed nerve roots and inducing neurogenic intermittent claudication (Figure 9).
Figure 6.
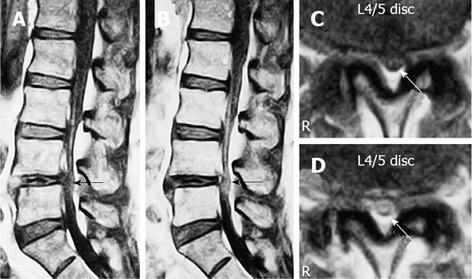
Gadolinium-enhanced magnetic resonance imaging of the cauda equina edema in lumbar canal stenosis. A 73-year-old man complained of weakness and numbness of the lower extremities after walking about 300 m, but no obvious sensory loss and muscle weakness was noted. Precontrast T1-weighted (500/35) sagittal (A) and axial (C) conventional spin echo MR image indicated a diagnosis of LCS at L4/5 disc level (arrows). T1-weighted (500/35) sagittal (B) and axial (D) Magnetic resonance image acquired at L4/5 disc level obtained after 0.1 mmol/kg intravenous Gd-DTPA administration showing the generalized central canal stenosis as well as punctuate areas of intrathecal enhancement (arrows) indicating a breakdown in the blood-nerve barrier. Reproduced with permission from Kobayashi et al[46].
Figure 7.
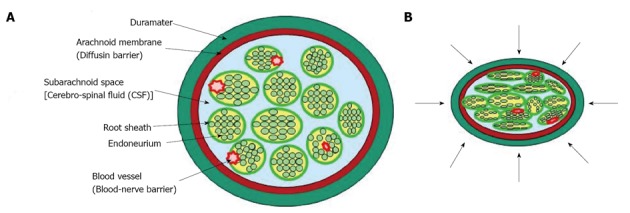
Diagram that illustrates possible mechanical effects on cauda equine. A: Normal state; B: Lumbar canal stenosis. The pressure is applied to the cauda equina with many nerve roots in a circumferential manner.
Figure 8.
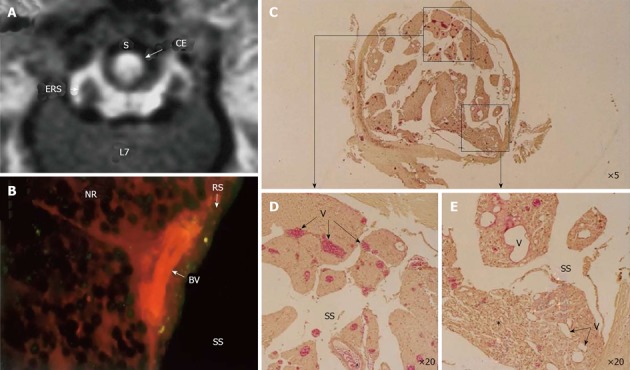
Circumferential compression of the dog cauda equine. The cauda equina was constricted outside the dura mater using a silicone tube (S), which caused 30% constriction of the diameter of the dura mater using a silicone tube at L6/7 disc level. After 3 wk constriction, clear enhancement was seen inside the cauda equina constricted by a silicon tube (S) as seen on gadolinium-enhanced magnetic resonance (MR) image [T1-weighted spin-echo (SE) image, 600/25 (TR/TE)] (A). No enhancement of epidural root sleeves (ERS) was found on this image. In the cauda equina, where enhancement was found on MR imaging, Evans blue albumin emits a bright red fluorescence which leaked outside the blood vessels, and intraradicular edema was seen under a fluorescent microscope (B). Light microscopy revealed congestion and dilation of the radicular veins (C-E) inside the cauda equina, inflammatory cells infiltration, and Wallerian degeneration (E, asterisk) was observed in the entrapped region. This situation was reflected as breakdown of blood nerve barrier on fluorescent microscopy and high intensity on gadolinium- enhanced MR imaging. BV: Blood vessel; CE: Cauda equina; ENS: Epidural root sleeves; NR: Nerve root; RS: Root sheath; S: Silicon tube; SS: Subarachnoid space.
Figure 9.
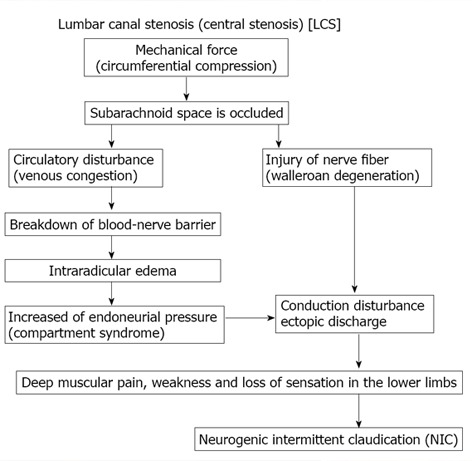
Pathogenesis of neurogenic intermittent claudication in lumbar canal stenosis.
EFFECT OF PROSTAGLANDIN-E1 TO NORMAL NERVE ROOT AND COMPRESSED NERVE ROOT
Prostaglandin E1 (PGE1), a potent vasodilator and platelet aggregation inhibitor, is well known as a useful drug for peripheral arterial disease, such as Raynaud’s and Burerger’s diseases[59], and diabetic neuropathy[60]. PGE1 has been reported to relax the contraction of vascular smooth muscle cells[61] and increase blood flow in peripheral arteries[62], followed by improvement of endothelial function[63]. Since PGE1 is rapidly inactivated in the lungs, PGE1 must be administered into the obstructed artery or a large amount of it has to be given intravenously. The distribution of PGE1 in-vivo induces the systemic effects of diarrhea, hypotension, fever and hepatic dysfunction. PGE1 also causes irritation in blood vessels near the site of injection. To avoid these problems, PGE1 was mixed with microparticles 0.2 μm in diameter made of soybean oil. Because PGE1 is incorporated into the lipid particles in this preparation, it is less susceptible to inactivation in the lung[64]. In addition, this preparation characteristically becomes concentrated and acts selectively in lesions because the lipid particles adhere to the endothelial cells of injured blood vessels[65]. Lipid microspheres incorporating prostaglandin E1 (lipo-PGE1) has therefore attracted interest as a therapeutic drug for LCS. However, investigations in the clinical setting have shown that lipo-PGE1 is effective in some patients but not in others[66-75], although the reason for this is unclear.
So far, some experimental studies of the effect of lipo-PGE1 on blood flow in normal[68,70] and compressed[76-80] nerve roots have reported an increase in flow, but none have examined the effect of lipo-PGE1 on compressed sections with apparently Wallerian degeneration after nerve root compression. After investigating the changes of nerve root blood flow caused by bolus intravenous injection of Lipo-PGE1, Toribatake et al[68] reported a 59% increase of blood flow at a dose of 0.1 μg/kg, while Murakami et al[70] reported a 37.8% increase at a dose of 0.15 μg/kg. These experimental studies of the effect of lipo-PGE1 on blood flow in normal nerve roots revealed an increase in flow, but did not examine the effect of lipo-PGE1 on compressed nerve roots. Subsequently, the effect of PGE1 on intraradicular blood flow was assessed in a rat cauda equina compression model, and PGE1 was reported to increase blood flow. However, the extent of compression applied to the nerve root and its duration were both insufficient, and the response was not compared with tissue changes of the nerve root after compression[76-80]. Kobayashi et al[81] firstly demonstrated that intravenous injection of lipo-PGE1 significantly increased intraradicular blood flow in the normal nerve root without compression, but the increase of blood flow observed in the compressed region of the nerve root exhibiting Wallerian degeneration was transient and not sustained (Figures 10 and 11). It is therefore concluded that lipo-PGE1 has less effect on severely damaged nerve roots than it does on normal nerve roots.
Figure 10.
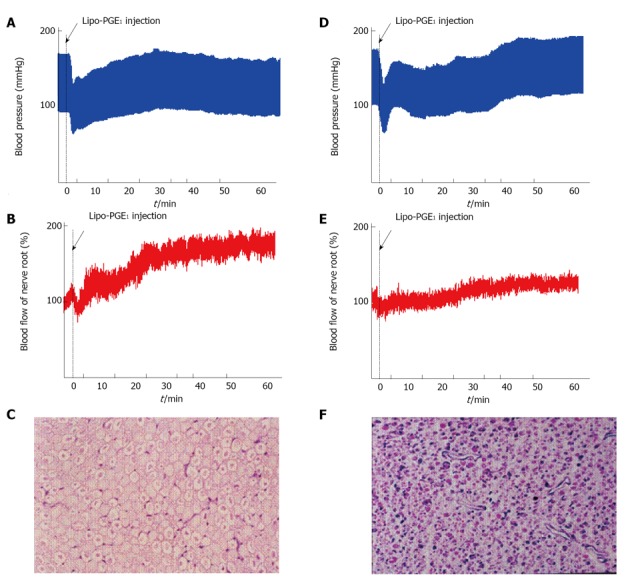
Effect of prostaglandin E1 on normal (A-C) and compressed nerve root (D-F). The seventh lumbar nerve root was clamped with a clip for 3 wk using dogs. After release of clipping, the intraradicular blood flow was measured before and after intravenous injection of lipo-prostaglandin E1 (PGE1) (D-F). As the control group, animals were evaluated at 3 wk after laminectomy. The nerve root was only exposed and wasn’t clamped with a clip (A-C). In the control (A) group, the mean blood pressure fell immediately after intravenous injection of lipo-PGE1 due to the peripheral vasodilation, but then gradually increased and recovered after 20 min. The changes of blood pressure in the nerve root compression group were the same as those seen in the control group (D). Intravenous injection of lipo-PGE1 also resulted in marked increase of blood flow in the normal nerve roots (B), but caused minimal enhancement of blood flow at the sites of nerve root compression exhibiting Wallerian degeneration (E). Histological examination revealed no degeneration of the nerve fibers in the control group (C). However, marked Wallerian degeneration was seen in the nerve root compression group (D). The relative number of small diameter axons increased in the compression group at 3 wk after operation compared with the control group (HE stain, original magnification X 50).
Figure 11.
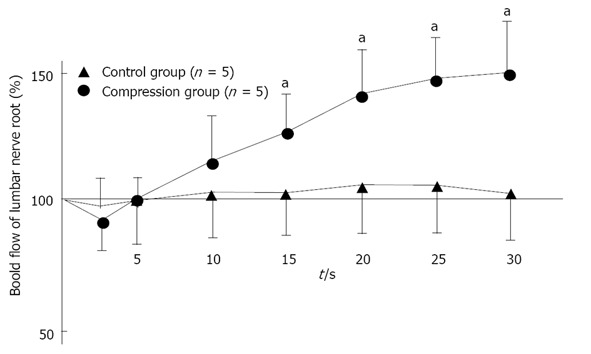
Changes in intraradicular blood flow after intravenous injection of lipo-prostaglandin E1. In normal (control) nerve roots (n = 5), intraradicular blood flow fell immediately after intravenous injection of lipo-Prostaglandin E1 (PGE1), but gradually increased (aP < 0.05). After 30 min, the mean blood flow was 49% higher than before lipo-PGE1 injection. However, the changes of intraradicular blood flow in the nerve root compression group (n = 5) were not as marked as in the control group, with the mean value after 30 min being only 5% higher than pretreatment value. Reproduced with permission from Kobayashi et al[81].
EFFECT OF PGE1 ON PATIENTS WITH NIC
PGE1 has been used in the field of orthopedic surgery for the treatment of cervical and lumbar diseases. The results of numerous studies have also suggested that PGE1 is likely to be useful for LCS, but its position has not been firmly established among the treatments available[66-75]. It was reported that PGE1 was effective for 57%-87% of patients with LCS accompanied by NIC (Table 2), but was ineffective in many patients with severe neurological deficits such as muscle weakness. Since it can be assumed that the impaired intraradicular blood flow is improved by the drug in patients whose NIC responds to administration of PGE1, this agent can be used for screening and staging of the disease. Yone et al[82] have reported that when lipo-PGE1 was injected intravenously in 11 LCS patients during surgery, symptoms were alleviated in 6 patients with dilation of vessels running along the cauda equina, but not in 5 patients with no change of their vessels. Dezawa et al[83] observed the movement of red blood cells in blood vessels running along the cauda equina by myelofiber scope in 8 LCS patients with NIC under local anesthesia, and reported that blood flow increased in 3 patients, stopped in 1, became retrograde in 2, and was unchanged in 2. In LCS patients, myelography and MRI often reveal a redundant nerve root. Suzuki et al[84] pathologically investigated the cauda equina at autopsy and reported that the cause of nerve root redundancy was Wallerian degeneration.
Table 2.
Effect of lipo-prostaglandin E1 on patients with neurogenic intermittent claudication in lumbar canal stenosis
| Author | Year | Drag | Dose | Period | Effective rate to NIC |
| Takakura[66] | 1986 | PGE1 | 40-60 μg/d | 14-28 consecutive days | 79.5% (39/49 cases) |
| Miura et al[67] | 1992 | Lipo-PGE1 | 10 μg/d | 10 d | 57.1% (8/14 cases) |
| Toribatake et al[68] | 1993 | Lipo-PGE1 | 10 μg/d | 10 consecutive days | 75.0% (12/16 cases) |
| Kurihara et al[69] | 1996 | Limaprost | 3 μg/d | 42 consecutive days | 27.3% (21/77 cases) |
| 15 μg/d | 47.8% (33/69 cases) | ||||
| Murakami et al[70] | 1997 | Lipo-PGE1 | 10 μg/d | 10 consecutive days | 77.5% (31/40 cases) |
| 5 μg/d | 87.0% (40/46 cases) | ||||
| Ono et al[71] | 1997 | Lipo-PGE1 | 10 μg/d | 14 consecutive days | 71.1% (27/38 cases) |
| 20 μg/d | 76.5% (26/34 cases) | ||||
| Miura et al[72] | 1997 | PGE1 | 120 μg/d | 14 consecutive days | 33.0% (5/12 cases) |
| Uratuji et al[73] | 2003 | Limaprost | 15 μg/d | 42 consecutive days | 59.3% (54/91 cases) |
| Harrison et al[74] | 2007 | Limaprost | 15 μg/d | 42 consecutive days | 50.7% (74/146 cases) |
| Nakanishi et al[75] | 2008 | PGE1 | 60 μg/d | 14 consecutive days | 71.4% (45/63 cases) |
Reproduced with permission from Kobayashi et al[81]. NIC: Neurogenic intermittent claudication; PGE1: Prostaglandin E1.
We treated LCS patients with PGE1 and compared their response with the magnetic resonance (MR) imaging. The subjects were 50 LCS patients with NIC (walking distance ≤ 300 m) and MR imaging evidence of central canal stenosis. They comprised 38 men and 12 women aged 75-95 years (mean: 81 years). Each patient received PGE1 intravenously at a dose of 10 μg/d for 14 d. After completing treatment, the MRI findings of 25 patients achieving relief from NIC (group I) and 25 patients without relief (group II) were retrospectively compared. In all patients, T1- and T2-weighted images were obtained. On T1-weighted images, the transverse area of the dural tube at the site of maximal canal stenosis was measured using a digitizer. The presence or absence of redundant nerve roots was also identified on T2-weighted images.
The transverse area of the dural tube at the site of maximal canal stenosis on MR images was 50.8-88.6 mm2 (mean: 69.8 ± 15.2 mm2) in group I and 35.8-59.8 mm2 (mean: 50.6 ± 10.3 mm2) in group II (Figure 12A). Redundant nerve roots were observed in 14 (56%) of the 25 patients from group II, being located proximal to the site of maximal stenosis, but were not seen in group I(Figure 12B). Intraradicular edema was observed in 21 (84%) of the 25 patients from group II, being located proximal to the site of maximal stenosis, but was only seen in 2 (8%) patients from group I(Figure 6). These results indicate that patients who have a transverse dural area ≤ 60 mm2 and redundant nerve roots (suggesting Wallerian degeneration) may achieve little relief of NIC with PGE1 therapy. From the findings of these clinical studies, it has been postulated that the severity of nerve degeneration is an important determinant of the response to PGE1. It is considered that LCS patients who are unresponsive to PGE1 have nerve root degeneration that progresses to the irreversible stage, so that decompression by laminectomy or other methods cannot be expected to provide immediate marked improvement of neurological deficits such as sensory disorders and muscle weakness. In other words, it seems that the outcome of decompression surgery may be predicted from the response to PGE1, but further studies are required to confirm this possibility.
Figure 12.

Magnetic resonance imaging of lumbar canal stenosis patients with neurogenic intermittent claudication. A: Cross-sectional area of dural sac (T1-w) at the site of maximal canal stenosis; B: Magnetic resonance imaging of redundunt nerve root (T2-w).
ACKNOWLEDGMENTS
The submitted manuscript does not contain information about medical devices or drugs. No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article. I would like to thank Dr. Kenichi Takeno of the Orthopaedic Surgery, Faculty of Medical Sciences, The University of Fukui, Fukui, Japan Dr. Katuhiko Hayakawa and Mr. Takashi Nakane of the Aiko Orthopaedic Clinic, Aichi, Japan, and Dr. Yoshihiko Suzuki of Suzuki Orthopaedic Clinic, Gifu, for their technical assistance.
Footnotes
Supported by Grant-in Aid from the Ministry of Education, Science and Culture of Japan, No, 25460719
P- Reviewers: Alimehmeti R, Bludovsky D, Serhan H, Vugt A S- Editor: Song XX L- Editor: O’Neill M E- Editor: Lu YJ
References
- 1.Martinelli TA, Wiesel SW. Epidemiology of spinal stenosis. Instr Course Lect. 1992;41:179–181. [PubMed] [Google Scholar]
- 2.Johnsson KE. Lumbar spinal stenosis. A retrospective study of 163 cases in southern Sweden. Acta Orthop Scand. 1995;66:403–405. doi: 10.3109/17453679508995574. [DOI] [PubMed] [Google Scholar]
- 3.Verbiest H. Pathomorphological aspects of developmental lumbar stenosis. Orthop Clin North Am. 1975;6:177–196. [PubMed] [Google Scholar]
- 4.Schatzker J, Pennal GF. Spinal stenosis, a cause of cauda equina compression. J Bone Joint Surg Br. 1968;50:606–618. [PubMed] [Google Scholar]
- 5.Kirkaldy-Willis WH, Paine KW, Cauchoix J, McIvor G. Lumbar canal stenosis. Clin Orthop. 1974;99:30–50. doi: 10.1097/00003086-197403000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Sumita M. Beitrage zur Lehre vonder Chondrodystrophia foetalis (Kaufmann) und osteogenesis imperfecta (Vrolik) mit besonderer Beruck sichtigung der anatomischen und klinischen Differential Diagnose. Dtsch Z Chir. 1910;107:1–110. [Google Scholar]
- 7.Sarpyener MA. Congenital structure of the spinal canal. J Bone Joint Surg. 1945;27:70–79. [PubMed] [Google Scholar]
- 8.Verbiest H. Sur certaines formes rares de compression de la queu de cheval. In: Hommage a Clovis Vincent., editor. Paris: Maloine; 1949. pp. 16–174. [Google Scholar]
- 9.Verbiest H. Primaire stenose van het lumbale wervelkanaal bij olwassenen. Ned T Geneesk. 1950;94:2415–2433. [PubMed] [Google Scholar]
- 10.Verbiest H. A radicular syndrome from developmental narrowing of the lumbar vertebral canal. J Bone Joint Surg. 1954;36B:230–237. doi: 10.1302/0301-620X.36B2.230. [DOI] [PubMed] [Google Scholar]
- 11.Verbiest H. Further experiences on the pathological influence of a developmental narrowness of the bony lumbar vertebral canal. J Bone Joint Surg. 1955;37B:576–583. doi: 10.1302/0301-620X.37B4.576. [DOI] [PubMed] [Google Scholar]
- 12.Arnoldi CC, Brodsky AE, Cauchoix J, Crock HV, Dommisse GF, Edgar MA, Gargano FP, Jacobson RE, Kirkaldy-Willis WH, Kurihara A, et al. Lumbar spinal stenosis and nerve root entrapment syndromes. Definition and classification. Clin Orthop Relat Res. 1976;115:4–5. [PubMed] [Google Scholar]
- 13.Adamkiewicz A. Die Blutgefässe des menschlichen Rückenmarkes. I. Die Geffäse der Rükenmarkssubstanz. Sitzungsber. Akad.d.Wiss, In Wien. Math.-Naturwiss Kl. 1881;84:469–502. [Google Scholar]
- 14.Adamkiewicz A. Die Blutgefässe des menschlichen Rückenmarkes. II. Die Geffäse der Rükenmarksoberfläche. Ibid. 1882;85:101–130. [Google Scholar]
- 15.Kadyi H. Über die Blutgefässe des Menchlichen Rükenmarkes. Anat Anz. 1886;1:304–314. [Google Scholar]
- 16.Kadyi H. Über die Blutgefässe des Menchlichen Rückenmarkes. Nach einer im XV. Bande der Denkschriften d.math.-naturw.Cl.d.Akad.d.Wissensch, in Krakau erschienenen Monographie, aus dem Polischen übersetzt vom Verfasser. Lemberg: Gubrynowicz and Schmidt; 1889. [Google Scholar]
- 17.Bolton B. The blood supply of the human spinal cord. J Neurol Psychiat. 1939;2:137–148. doi: 10.1136/jnnp.2.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suh TH, Alexander L. Vascular system of the human spinal cord. Arch Neurol. Psychiat. 1939;41:659–677. [Google Scholar]
- 19.Woolam DHM, Millen JW. The arterial supply of the spinal cord and its significance. J Neurol Neurosurg Psyehiat. 1955;18:97–102. doi: 10.1136/jnnp.18.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassler O. Blood supply of human spinal cord. A microangioraphic study. Arch Neurol. 1966;15:302–307. doi: 10.1001/archneur.1966.00470150080013. [DOI] [PubMed] [Google Scholar]
- 21.Gillilan LA. Veins of the spinal cord. Anatomic details; suggested clinical applications. Neurology. 1970;20:860–868. doi: 10.1212/wnl.20.9.860. [DOI] [PubMed] [Google Scholar]
- 22.Lazorthes G, Gouaze A, Zadeh JO, Santini JJ, Lazorthes Y, Burdin P. Arterial vascularization of the spinal cord. Recent studies of the anastomotic substitution pathways. J Neurosurg. 1971;35:253–262. doi: 10.3171/jns.1971.35.3.0253. [DOI] [PubMed] [Google Scholar]
- 23.Dommisse GF. Arteries and vein of the human spinal cord from birth. New York: Churchill Livingstone; 1975. [Google Scholar]
- 24.Crock HV, Yoshizawa H. The blood supply of the vertebral column and spinal cord in man. New York: Springer-Verlag; 1977. [Google Scholar]
- 25.Corbin JL. Anatome et pathologie arterielles de la molle. Paris: Masson et Cie; 1961. [Google Scholar]
- 26.Parke WW, Gammell K, Rothman RH. Arterial vascularization of the cauda equina. J Bone Joint Surg. 1981;63A:53–62. [PubMed] [Google Scholar]
- 27.Crock HV, Yamagishi M, Crock MC. The conus medullaris and cauda equina in man. New York: Springer-verlag; 1986. [Google Scholar]
- 28.Kobayashi S, Yoshizawa H, Nakai S. Experimental study on the dynamics of lumbosacral nerve root circulation. Spine (Phila Pa 1976) 2000;25:298–305. doi: 10.1097/00007632-200002010-00007. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi S, Yoshizawa H, Hachiya Y, Ukai T, Morita T. Volvo award winner in basic science. Vasogenic edema induced by compression injury to the spinal nerve root: Distribution of intravenously injected protein tracers and gadolinium-enhanced magnetic resonance imaging. Spine. 1993;18:1410–1424. [PubMed] [Google Scholar]
- 30.Olmarker K, Rydevik B, Holm S. Edema formation in spinal nerve roots induced by experimental, graded compression. An experimental study on the pig cauda equina with special reference to differences in effects between rapid and slow onset of compression. Spine (Phila Pa 1976) 1989;14:569–573. [PubMed] [Google Scholar]
- 31.Blau JN, Logue V. The natural history of intermittent claudication of the cauda equina: an unusual syndrome resulting from central protrusion of a lumbar intervertebral disc. Lancet. 1961;20:1081–1086. [Google Scholar]
- 32.Evans JG. Neurogenic intermittent claudication. Br Med J. 1964;2:985–987. doi: 10.1136/bmj.2.5415.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ehni G. Significance of the small lumbar canal: Cauda equina compression syndrome due to spondylosis. J Neurosurg. 1969;31:490–494. doi: 10.3171/jns.1969.31.5.0490. [DOI] [PubMed] [Google Scholar]
- 34.Yamada H, Oya M, Okada T, Shiozawa Z. Intermittent cauda equina compression due to narrow spinal canal. J Neurosurg. 1972;37:83–88. doi: 10.3171/jns.1972.37.1.0083. [DOI] [PubMed] [Google Scholar]
- 35.Kavanaugh GJ, Svien HJ, Holman CB, Johnson RM. “Pseudoclaudication” syndrome produced by compression of the cauda equina. JAMA. 1968;206:2477–2481. [PubMed] [Google Scholar]
- 36.Verbiest H. Neurogenic intermittent claudication. Amsterdam: North Holland Publishing Co; New York: American Elsevier; 1976. pp. 749–753. [Google Scholar]
- 37.Kobayashi S, Yoshizawa H, Yamada S. Pathology of lumbar nerve root compression. Part 1: Intraradicular inflammatory changes induced by mechanical compression. J Orthop Res. 2004;22:170–179. doi: 10.1016/S0736-0266(03)00131-1. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi S, Kokubo Y, Uchida K, Yayama T, Takeno K, Negoro K, Nakajima H, Baba H, Yoshizawa H. Effect of lumbar nerve root compression on primary sensory neurons and their central branches: changes in the nociceptive neuropeptides substance P and somatostatin. Spine (Phila Pa 1976) 2005;30:276–282. doi: 10.1097/01.brs.0000152377.72468.f4. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi S, Sasaki S, Shimada S, Kaneyasu M, Mizukami Y, Kitade I, Ogawa M, Kawahara H, Baba H, Yoshizawa H. Changes of calcitonin gene-related peptide in primary sensory neurons and their central branch after nerve root compression of the dog. Arch Phys Med Rehabil. 2005;86:527–533. doi: 10.1016/j.apmr.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi S, Uchida K, Yayama T, Takeno K, Miyazaki T, Shimada S, Kubota M, Nomura E, Meir A, Baba H. Motor neuron involvement in experimental lumbar nerve root compression: a light and electron microscopic study. Spine (Phila Pa 1976) 2007;32:627–634. doi: 10.1097/01.brs.0000257559.84494.15. [DOI] [PubMed] [Google Scholar]
- 41.Cooper RG, Freemont AJ, Hoyland JA, Jenkins JP, West CG, Illingworth KJ, Jayson MI. Herniated intervertebral disc-associated periradicular fibrosis and vascular abnormalities occur without inflammatory cell infiltration. Spine (Phila Pa 1976) 1995;20:591–598. doi: 10.1097/00007632-199503010-00016. [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi S, Takeno K, Miyazaki T, Kubota M, Shimada S, Yayama T, Uchida K, Normura E, Mwaka E, Baba H. Effects of arterial ischemia and venous congestion on the lumbar nerve root in dogs. J Orthop Res. 2008;26:1533–1540. doi: 10.1002/jor.20696. [DOI] [PubMed] [Google Scholar]
- 43.Kobayashi S, Mwaka ES, Meir A, Uchida K, Kokubo Y, Takeno K, Miyazaki T, Nakajima H, Kubota M, Shimada S, et al. Changes in blood flow, oxygen tension, action potentials, and vascular permeability induced by arterial ischemia or venous congestion on the lumbar dorsal root ganglia in dogs. J Neurotrauma. 2009;26:1167–1175. doi: 10.1089/neu.2008.0837. [DOI] [PubMed] [Google Scholar]
- 44.Usubiaga JE, Moya F, Usubiaga LE. Effect of thoracic and abdominal pressure changes on the epidural space pressure. Br J Anaesth. 1967;39:612–618. doi: 10.1093/bja/39.8.612. [DOI] [PubMed] [Google Scholar]
- 45.Heddle RW, Guiler ER. Epidural space manometry and multiple ligation in the investigation of posterior vena cava occlusion in the rat. J Anat. 1971;110:491. [PubMed] [Google Scholar]
- 46.Kobayashi S, Uchida K, Takeno K, Baba H, Suzuki Y, Hayakawa K, Yoshizawa H. Imaging of cauda equina edema in lumbar canal stenosis by using gadolinium-enhanced MR imaging: experimental constriction injury. AJNR Am J Neuroradiol. 2006;27:346–353. [PMC free article] [PubMed] [Google Scholar]
- 47.Jinkins JR. Gd-DTPA enhanced MR of the lumbar spinal canal in patients with claudication. J Comput Assist Tomogr. 1993;17:555–562. doi: 10.1097/00004728-199307000-00007. [DOI] [PubMed] [Google Scholar]
- 48.Jinkins JR. MR of enhancing nerve roots in the unoperated lumbosacral spine. AJNR Am J Neuroradiol. 1993;14:193–202. [PMC free article] [PubMed] [Google Scholar]
- 49.Jinkins R. Magnetic resonance imaging of benign nerve root enhancement in the unoperated and postoperative lumbosacral spine. Neuroimag Clin N Am. 1993;3:525–541. [Google Scholar]
- 50.Yoshizawa H, Kobayashi S, Morita T. Chronic nerve root compression. Pathophysiologic mechanism of nerve root dysfunction. Spine (Phila Pa 1976. ) 1995;20:397–407. doi: 10.1097/00007632-199502001-00001. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi K, Kagechika K, Takino T, Matsui T, Miyazaki T, Shima I. Changes in epidural pressure during walking in patients with lumbar spinal stenosis. Spine (Phila Pa 1976) 1995;20:2746–2749. doi: 10.1097/00007632-199512150-00017. [DOI] [PubMed] [Google Scholar]
- 52.Ikawa M, Atsuta Y, Tsunekawa H. Ectopic firing due to artificial venous stasis in rat lumbar spinal canal stenosis model: a possible pathogenesis of neurogenic intermittent claudication. Spine (Phila Pa 1976) 2005;30:2393–2397. doi: 10.1097/01.brs.0000184718.56122.90. [DOI] [PubMed] [Google Scholar]
- 53.Rydevik BL, Myers RR, Powell HC. Pressure increase in the dorsal root ganglion following mechanical compression. Closed compartment syndrome in nerve roots. Spine (Phila Pa 1976) 1989;14:574–576. doi: 10.1097/00007632-198906000-00004. [DOI] [PubMed] [Google Scholar]
- 54.Myers RR. The neuropathology of nerve injury and pain. In: Weinstein J, editor. Low back pain. A scientific and clinical overview. Rosemont, IL: American Academy of Orthpaedic Surgerons; 1997. pp. 247–264. [Google Scholar]
- 55.Kobayashi S, Shizu N, Suzuki Y, Asai T, Yoshizawa H. Changes in nerve root motion and intraradicular blood flow during an intraoperative straight-leg-raising test. Spine (Phila Pa 1976) 2003;28:1427–1434. doi: 10.1097/01.BRS.0000067087.94398.35. [DOI] [PubMed] [Google Scholar]
- 56.Kobayashi S, Suzuki Y, Asai T, Yoshizawa H. Changes in nerve root motion and intraradicular blood flow during intraoperative femoral nerve stretch test. Report of four cases. J Neurosurg. 2003;99:298–305. doi: 10.3171/spi.2003.99.3.0298. [DOI] [PubMed] [Google Scholar]
- 57.Howe JF, Loeser JD, Calvin WH. Mechanosensitivity of dorsal root ganglia and chronically injured axons: a physiological basis for the radicular pain of nerve root compression. Pain. 1977;3:25–41. doi: 10.1016/0304-3959(77)90033-1. [DOI] [PubMed] [Google Scholar]
- 58.Calvin WH. Some design features of axons and how neuralgias may defeat them; advances in pain research and therapy. Pain. 1979;3:297–309. [Google Scholar]
- 59.Clifford PC, Martin MF, Sheddon EJ, Kirby JD, Baird RN, Dieppe PA. Treatment of vasospastic disease with prostaglandin E1. Br Med J. 1980;281:1031–1034. doi: 10.1136/bmj.281.6247.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Itoh Y, Yasui T, Kakizawa H, Makino M, Fujiwara K, Kato T, Imamura S, Yamamoto K, Hishida H, Nakai A, et al. The therapeutic effect of lipo PGE1 on diabetic neuropathy-changes in endothelin and various angiopathic factors. Prostaglandins Other Lipid Mediat. 2001;66:221–234. doi: 10.1016/s0090-6980(01)00165-4. [DOI] [PubMed] [Google Scholar]
- 61.Strong CG, Bohr DF. Effects of prostaglandins E1, E2, A1, and F1-alpha on isolated vascular smooth muscle. Am J Physiol. 1967;213:725–733. doi: 10.1152/ajplegacy.1967.213.3.725. [DOI] [PubMed] [Google Scholar]
- 62.Greenberg RA, Sparks HV. Prostaglandins and consecutive vascular segments of the canine hindlimb. Am J Physiol. 1969;216:567–571. doi: 10.1152/ajplegacy.1969.216.3.567. [DOI] [PubMed] [Google Scholar]
- 63.Makita S, Nakamura M, Ohhira A, Itoh S, Hiramori K. Effects of prostaglandin E1 infusion on limb hemodynamics and vasodilatory response in patients with arteriosclerosis obliterans. Cardiovasc Drugs Ther. 1997;11:441–448. doi: 10.1023/a:1007793321780. [DOI] [PubMed] [Google Scholar]
- 64.Stewart MA, Sherman WR, Kurien MM, Moonsammy GI, Wisgerhof M. Polyol accumulations in nervous tissue of rats with experimental diabetes and galactosaemia. J Neurochem. 1967;14:1057–1066. doi: 10.1111/j.1471-4159.1967.tb09516.x. [DOI] [PubMed] [Google Scholar]
- 65.Gabbay KH, O’Sullivan JB. The sorbitol pathway. Enzyme localization and content in normal and diabetic nerve and cord. Diabetes. 1968;17:239–243. doi: 10.2337/diab.17.5.239. [DOI] [PubMed] [Google Scholar]
- 66.Takakura Y. Clinical effect of prostaglandin on spinal canal stenosis. Gendai iryo. 1986;18:673–679. [Google Scholar]
- 67.Miura Y, Yamaura I. The clinical response of Lipo-PGE1 to compressive cervical myelopathy and compressive lesion of cauda equina and nerve root. Igaku yakugaku. 1992;28:925, 935. [Google Scholar]
- 68.Toribatake Y, Takahashi K, Kawahara N, Tomita K. Effect of Lipo PGE1 on neurogenic intermittent claudication. Sinyaku Rinsho. 1993;42:158, 166. [Google Scholar]
- 69.Kurihara A, Kataoka O, Sugawara S, Sano S, Shirai Y. Clinical benefit of OP-1206a-CD on lumbar spinal canal stenosis. Multi-center comparative double-blind clinical study. Rinsyo Iyaku. 1996;12:511, 529. [Google Scholar]
- 70.Murakami M, Takahashi K, Sekikawa T, Yasuhara K, Yamagata M, Moriya H. Effects of intravenous lipoprostaglandin E1 on neurogenic intermittent claudication. J Spinal Disord. 1997;10:499–504. [PubMed] [Google Scholar]
- 71.Ono K, Miura Y, Tsuji H, Kataoka O. Clinical assessment of PGE1 formulation for lumbar spinal canal stenosis. Sekitui Sekizui. 1997;10:517, 524. [Google Scholar]
- 72.Miura J, Kurihara A, Sya T, Uratsuj M, Sakamoto T. Drip infusion therapy with prostaglandin E1 for lumbar canal stenosis. J Lumbar Spine Disord. 1997;3:55, 59. [Google Scholar]
- 73.Uratsuji M, Kurihara A, Ooi T. Conservative treatment of lumbar spinal stenosis with prostaglandin E1 derivative results of a double-blind, multi-center trial. International Society for the Study of the Lumbar Spine (ISSLS), 23rd Annual Meeting. Burlington: 1996. p. Abstract 84. [Google Scholar]
- 74.Harrison TS, Plosker GL. Limaprost. Drugs. 2007;67:109, 118. doi: 10.2165/00003495-200767010-00010. [DOI] [PubMed] [Google Scholar]
- 75.Nakanishi K, Tanaka M, Misawa H, Takigawa T, Ozaki T. Midterm results of prostaglandin E1 treatment in patients with lumbar spinal canal stenosis accompanied by intermittent claudication. Spine (Phila Pa 1976) 2008;33:1465–1469. doi: 10.1097/BRS.0b013e3181753c1e. [DOI] [PubMed] [Google Scholar]
- 76.Konno S, Kayama S, Olmarker K, Kikuchi S. Effects of OP-1206 (prostaglandin E1) on nerve-conduction velocity in the dog cauda equina subjected to acute experimental compression. J Spinal Disord. 1996;9:103–106. [PubMed] [Google Scholar]
- 77.Nakai K, Takenobu Y, Eguchi K, Takimizu H, Honjo K, Akimaru S, Maegawa H, Marsala M, Katsube N. The effects of OP-1206 alpha-CD on walking dysfunction in the rat neuropathic intermittent claudication model. Anesth Analg. 2002;94:1537–1541, table of contents. doi: 10.1097/00000539-200206000-00030. [DOI] [PubMed] [Google Scholar]
- 78.Nakai K, Takenobu Y, Takimizu H, Akimaru S, Ito H, Maegawa H, Marsala M, Katsube N. Effects of orally administered OP-1206 alpha-CD with loxoprofen-Na on walking dysfunction in the rat neuropathic intermittent claudication model. Prostaglandins Leukot Essent Fatty Acids. 2003;69:269–273. doi: 10.1016/s0952-3278(03)00109-1. [DOI] [PubMed] [Google Scholar]
- 79.Sekiguchi M, Konno S, Kikuchi S. Effects on improvement of blood flow in the chronically compressed cauda equina: comparison between a selective prostaglandin E receptor (EP4) agonist and a prostaglandin E1 derivate. Spine (Phila Pa 1976) 2006;31:869–872. doi: 10.1097/01.brs.0000209256.96186.a7. [DOI] [PubMed] [Google Scholar]
- 80.Shirasaka M, Takayama B, Sekiguchi M, Konno S, Kikuchi S. Vasodilative effects of prostaglandin E1 derivate on arteries of nerve roots in a canine model of a chronically compressed cauda equina. BMC Musculoskelet Disord. 2008;9:41. doi: 10.1186/1471-2474-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kobayashi S, Baba H, Takeno K, Shimada S, Kubota M, Yayama T, Miyazaki T, Uchida K, Suzuki Y. Blood flow analysis of compressed nerve root after intravenous injection of lipo-prostaglandin E1. J Orthop Res. 2009;27:1252–1257. doi: 10.1002/jor.20881. [DOI] [PubMed] [Google Scholar]
- 82.Yone K, Sakou T, Kawauchi Y. The effect of Lipo prostaglandin E1 on cauda equina blood flow in patients with lumbar spinal canal stenosis: myeloscopic observation. Spinal Cord. 1999;37:269–274. doi: 10.1038/sj.sc.3100780. [DOI] [PubMed] [Google Scholar]
- 83.Dezawa A, Kitahara H. The orthopaedic study with the ultrathin fiberoptic spinal endoscope. Byoutaiseiri. 1992;11:703–708. [Google Scholar]
- 84.Suzuki K, Ishida Y, Ohmori K, Sakai H, Hashizume Y. Redundant nerve roots of the cauda equina: clinical aspects and consideration of pathogenesis. Neurosurgery. 1989;24:521–528. doi: 10.1227/00006123-198904000-00006. [DOI] [PubMed] [Google Scholar]


