Abstract
In this paper review we describe benefits and disadvantages of the established methods of cartilage regeneration that seem to have a better long-term effectiveness. We illustrated the anatomical aspect of the knee joint cartilage, the current state of cartilage tissue engineering, through mesenchymal stem cells and biomaterials, and in conclusion we provide a short overview on the rehabilitation after articular cartilage repair procedures. Adult articular cartilage has low capacity to repair itself, and thus even minor injuries may lead to progressive damage and osteoarthritic joint degeneration, resulting in significant pain and disability. Numerous efforts have been made to develop tissue-engineered grafts or patches to repair focal chondral and osteochondral defects, and to date several researchers aim to implement clinical application of cell-based therapies for cartilage repair. A literature review was conducted on PubMed, Scopus and Google Scholar using appropriate keywords, examining the current literature on the well-known tissue engineering methods for the treatment of knee osteoarthritis.
Keywords: Cartilage, Repair, Mesenchymal stem cells, Scaffolds, Tissue engineering, Osteoarthritis
Core tip: In this paper review we describe benefits and disadvantages of the established methods of cartilage regeneration that seem to have a better long-term effectiveness. We illustrated the anatomical aspect of the knee joint cartilage, the current state of cartilage tissue engineering through mesenchymal stem cells and biomaterials and in conclusion we provided a short overview on the rehabilitation after articular cartilage repair procedures.
INTRODUCTION
The knee is one of the largest and most complex joints in our body. It plays an essential role in movement related to carrying the body weight in horizontal (running and walking) and vertical (jumping) directions[1]. The knee joint consists of two articulations, one between the femur and tibia, and one between the femur and patella[1]. The knee is a mobile angular ginglymus or troclear, which permits flexion and extension as well as a slight medial and lateral rotation[2]. The joint is bathed in synovial fluid, which is contained inside the synovial membrane called the joint capsule. Ligaments join the knee bones and tendons connect the knee bones to the leg muscles, providing stability to the knee. Since in humans the knee supports nearly the whole weight of the body, it is vulnerable to both acute injury and chronic development of osteoarthritis. Two C-shaped pieces of cartilage called the medial and lateral menisci lie between the articular surfaces of the femur and tibia[3-5]. The menisci are shock absorbers of the load and make concordant the articular surfaces between the femoral condyles and the tibial plateau[3-5]. During flexion the menisci slide forward, during extension slide back[2]. The menisci are divided into outer rim, inner rim and core[3-5]. The inner rim is the most delicate part, because it is not vascularized. The lateral meniscus has the form of an almost complete circle and adheres to the two cruciates[3-5]. The medial meniscus has the form of a half moon and is more extensive than lateral, with its extremities adhering to anterior and posterior intercondylar areas. Between the two menisci, the medial meniscus is more subject to trauma, because it is less mobile than the lateral for the presence of the semimembranosus tendon, but also because usually we tend to have a slight valgus during gait[3-5]. Numerous bursae, or fluid-filled sacs, are located between the bones and tendons. This anatomical structure helps to reduce the friction between the bones during movement, for helping the knee to move smoothly. The joint capsule of the knee is strengthened by different ligaments, important for the stability of the joint, they are: the patellar ligament or patellar tendon, the lateral and medial retinaculum of the patella, the medial and lateral alar ligaments, the medial and lateral collateral ligaments (preventing the femur from sliding side to side), the popliteal ligaments and the anterior and posterior cruciate ligaments.
Articular cartilage is a form of hyaline cartilage that covers the articulating surfaces of long bones and sesamoid bones within synovial joints[6,7], and in the growth plate of the metaphysis, the zone between diaphysis and epiphysis[8,9]. Cartilage is a porous, viscoelastic composite that relies on a complex interaction and organization of its constituents to provide the resilient load-bearing, energy-dissipating lubrication and frictional properties[6,7]. The impressive load-bearing capacity of this tissue reflects in part the intrinsic matrix toughness and turgidity, as the ability of the tissue to swell is opposed by the internal structure. The degradation, loss, or breakdown of this unique relationship between the collagenous matrix and heavily hydrated charge-carrying proteoglycans caused by trauma or chronic and progressive degenerative joint disease (e.g., osteoarthritis or rheumatoid arthritis) has great functional, biomechanical, clinical, and social implications[10]. Knee osteoarthritis (Figures 1, 2) is the most common type of osteoarthritis[10]. Early diagnosis and treatment may help to manage its symptoms. Deterioration of articular cartilage is the main problem associated with knee osteoarthritis. The condition can be caused by: previous knee injury like fractures, ligament tears and meniscal injury or repetitive strain on the knee which can affect alignment, obesity, and genetics which make some people more likely to develop knee osteoarthritis[11]. Medical history, physical examination, and X-rays are used to diagnose knee osteoarthritis. The evidence of joint space narrowing on X-rays is crucial for the diagnosis and rules out other causes of knee pain[12]. If more detailed imaging is needed, an MRI may be ordered[12]. Arthroscopic knee surgery is another way to view the condition of the knee[12]. Knee osteoarthritis typically develops gradually over a period of years. The primary symptoms include: pain (mild, moderate, or severe), stiffness, limited range of motion in the knee, localized swelling. Knee osteoarthritis pain is usually worse following activity, especially overuse of the affected knee[10-13]. Stiffness can worsen after sitting for prolonged periods of time. As knee osteoarthritis progresses, symptoms generally become more severe. Then pain can become continuous rather than only when weight-bearing. The consequence in many cases is an inability to work and often the substitution of the diseased joint with an artificial implant becomes inevitable[6,7]. Joint replacement also called knee arthroplasty has had a major impact on the management of OA. After injury, articular cartilage is unable to naturally restore itself back to a functional tissue, and, because of this, a widely studied alternative to avoid the knee replacement surgery for osteoarthritis is tissue engineering[11-13].
Figure 1.
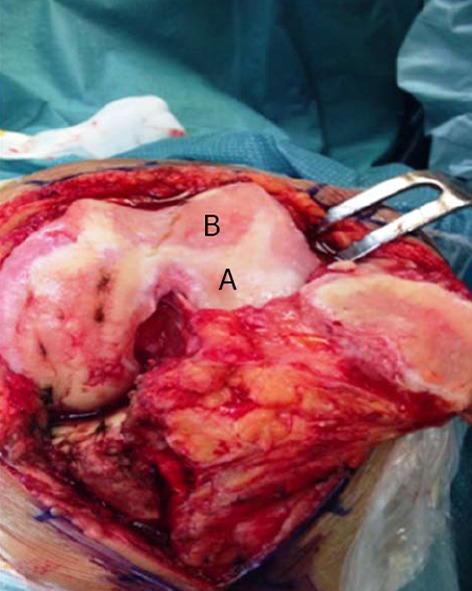
Macroscopic signs of osteoarthritis knee hyaline cartilage. A: Healthy cartilage; B: Osteoarthritis cartilage.
Figure 2.
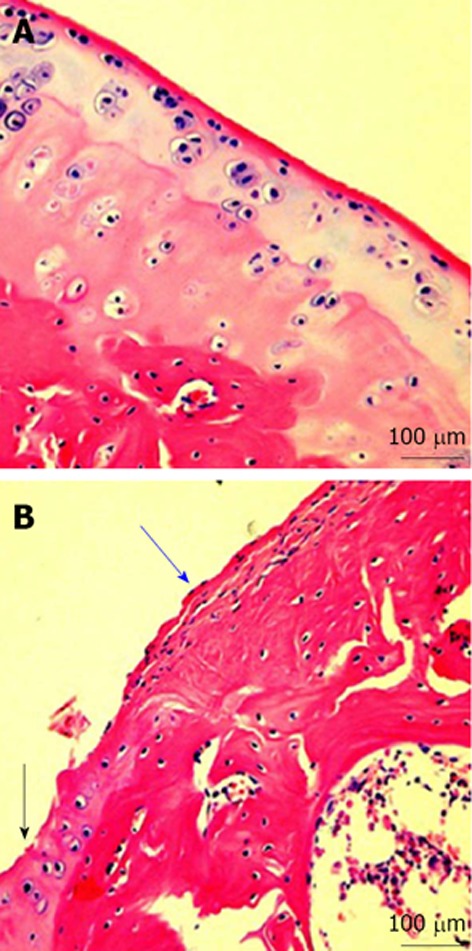
Microscopic signs. A: Microscopic signs of healthy knee hyaline cartilage. The histological (HE staining) analysis of cartilage from normal donor, showed a preserved morphological structure with no sign of cartilage degradation. Moreover, the surface of healthy hyaline cartilage appears white, shiny, elastic and firm. Magnification x 20; Scale bars: 100 μm; B: Microscopic signs of osteoarthritis (OA) knee hyaline cartilage. The histological (HE staining) analysis of cartilage from OA donor. The donor demonstrated joint swelling and oedema, horizontal cleavage tears or flaps, the surface becomes dull and irregular and had minimal healing capacity. Magnification x 20; Scale bars: 100 μm. Moderate OA cartilage (black arrow), the structural alterations included a reduction of cartilage thickness of the superficial and the middle zones. The structure of the collagen network is damaged, which leads to reduced thickness of the cartilage. The chondrocytes are unable to maintain their repair activity with subsequent loss of the cartilage tissue. Severe OA cartilage (blue arrow), demonstrated deep surface clefts, disappearance of cells from the tangential zone, cloning, and a lack of cells in the intermediate and radial zone, which are not arranged in columns. The tidemark is no longer intact and the subchondral bone shows fibrillation.
TISSUE ENGINEERING
Tissue engineering (Figure 3), is the use of a combination of cells, biochemical and physio-chemical factors, engineering and biomaterials to improve or replace biological functions[14-16]. While it was once categorized as a sub-field of biomaterials, having grown in scope and importance it can be considered as a field in its own right. While most definitions of tissue engineering cover a broad range of applications, in practice the term is closely associated with applications that repair or replace portions of or whole tissues (i.e., bone, cartilage, blood vessels, skin, muscle, nerve etc.)[14-16]. Often, the tissues involved require certain mechanical and structural properties for proper functioning. The term regenerative medicine is often used synonymously with tissue engineering, although those involved in regenerative medicine place more emphasis on the use of stem cells to produce tissues[14-16]. Tissue engineering of natural cartilage tissue has become an attractive new area of research. For this reason, we discuss briefly the most widely used techniques in the treatment of cartilage lesions to solve the problem of the management of cartilage defects. In recent years, surgeons and researchers have been working hard to elaborate surgical cartilage repair interventions for patients who suffer from articular cartilage damage. They provide pain relief, helping patients to return to their original lifestyle (regaining mobility, going back to work and even practicing sports again), while at the same time slowing down the progression of damage or considerably delaying joint replacement. Though these solutions do not perfectly restore cartilage, some of the latest technologies start to bring very promising results in repairing cartilage from traumatic injury or chondropathies. Although initially considered a tissue with a simple structure, reproducing the finely balanced structural interactions has proven to be difficult. Tissue engineering is able to create live tissue to replace, repair or strengthen harmed tissue. It is based on cell and genetic therapy and offers some of the most promising strategies of tissue repair, including articular cartilage repair. Although it has concentrated on finding therapies for focal lesions, it has now developed sufficiently to begin considering the challenge of finding novel solutions for the extensive joint damage seen in osteoarthritis.
Figure 3.
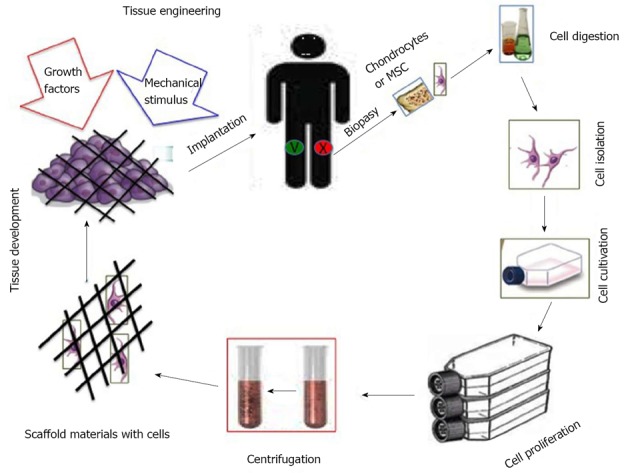
Graphic representation of the cartilage tissue engineering. MSC: Mesenchymal stem cell.
At the present time, a variety of clinical methods is available for repairing a chondral defect: marrow stimulation, autologous chondrocyte implantation (ACI), and most recently, next-generation ACI involving scaffolds or cell-seeded scaffolds, microfracture, osteoarticular transfer system (OATS) or mosaicplasty, penetration of the subchondral bone, osteochondral plug transplantation and matrix-induced autologous chondrocyte implantation (MACI)[6,7]. The cartilage repair procedure seeks to restore the surface of an articular joint’s hyaline cartilage and to replace the defect with an optimal repair tissue, mechanically stable, in order to prevent further degeneration. Today almost none of the mentioned procedures prove capable of generating hyaline cartilage and the clinical outcome needs to be further improved. ACI procedures take place in three stages. First, chondrocytes are extracted arthroscopically from the patient’s healthy articular cartilage that is located in a nonload-bearing area of either the intercondylar notch or the superior ridge of the femoral condyles. Then these extracted cells are transferred to an “in vitro” environment in specialized laboratories where they grow and replicate, for approximately four to six weeks, until their population has increased to a sufficient amount. Finally, the patient undergoes a second surgery where the “in vitro” chondrocytes are applied to the damaged area. In this procedure, chondrocytes are injected and applied to the damaged area in combination with either a membrane or a matrix structure. These transplanted cells grow in their new environment, forming new articular cartilage[6,7]. Increasing the source of cells for artificial repair of cartilage defects is becoming a problem[6,7]. The limited supply of cartilage, as a source of chondrocytes, requires a phase of expansion in monolayer culture. Chondrocyte differentiation and the maintenance of function require both transient and long-lasting control through humoral factors, particularly under stress, repair and regeneration in vivo or in vitro. To date, humoral factors from all major classes of molecules are known to contribute: ions (calcium), steroids (estrogens), terpenoids (retinoic acid), peptides (PTHRP, PTH, insulin, FGFs) and complex proteins (IGF-1, BMPs)[17]. BMP-4, a stimulator of chondrogenesis, both in vitro and in vivo, is a potential therapeutic agent for cartilage regeneration. BMP-4 delivery can improve the healing process of an articular cartilage defect by stimulating the synthesis of the cartilage matrix constituents: type II collagen and aggrecan. BMP-4 has also been shown to suppress chondrogenic hypertrophy and maintain regenerated cartilage. Use of an appropriate carrier for BMP-4 is crucial for successful reconstruction of cartilage defects[18].
Chondrocyte expansion is complicated by the fact that monolayer-cultured chondrocytes de-differentiate, lose their characteristic phenotype and synthesize type I (typical of fibrocartilage) rather than type II collagen (typical of hyaline cartilage)[8]. Osteochondral plug transplantation, or ostechondral autograft transfer system (OATS), usually applied for mid-sized defects[19], immediately recovers the joint surface. Small sized articular lesions are commonly addressed arthroscopically by penetration of the underlying subchondral bone[20-22] to promote a fibrous scar within the defect by invasion of adult mesenchymal stem cells. However, the reparative tissue does not withstand repetitive mechanical forces because of its poor quality, consisting mainly of collagen type I, and clinical outcome deteriorates over time[23,24]. This has led to investigation into the use of mesenchymal stem cells (MSCs). MSCs (Figure 4) can be relatively easily harvested and the procedures using them are less invasive or destructive than articular cartilage harvesting procedures.
Figure 4.
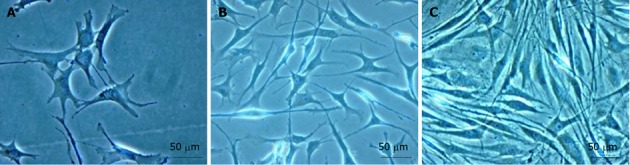
Mesenchymal stem cells development. A: First day of culture; B: Third day of culture; C: One week of culture. Magnification x 40; Scale bars: 50 μm.
The inherent ability of MSCs to self-renew opens the possibility that cell expansion may be achievable post-implantation[25]. The differentiation of MSCs into different cell types, in this case to produce cartilage tissue, is reliant on the local microenvironment, and growth factors, extracellular matrix and mechanical forces[25,26]. MSCs are easily available from bone marrow, synovial membrane, adipose tissue[27,28], etc., so then, we can get a variable number of cells from a different tissue[29,30]. MSCs show a high proliferation and differentiation potential, although coming from different tissue, and have an uneven chondrogenic differentiation capacity probably related to the special cytokines, growth factor and induction molecules composition of the medium[31,32].
Marrow stimulating techniques attempt to solve articular cartilage damage through an arthroscopic procedure. Firstly, damaged cartilage is drilled or punched until the underlying bone is exposed. By doing this, the subchondral bone is perforated to generate a blood clot within the defect. Studies have shown that marrow stimulation techniques often have insufficiently filled the chondral defect and the repair material is often fibrocartilage (which is not as good mechanically as hyaline cartilage)[6,7,33]. The blood clot takes about 8 wk to become fibrous tissue and it takes 4 mo to become fibrocartilage. This has implications for the rehabilitation[2]. Further on, it is common that only 1 or 2 years after the surgery symptoms start to return as the fibrocartilage wears away, forcing the patient to reengage in articular cartilage repair. This is not always the case and microfracture surgery is therefore considered to be an intermediate step. An evolution of the microfracture technique is the implantation of a collagen membrane onto the site of the microfracture to protect and stabilize the blood clot and to enhance the chondrogenic differentiation of the MSCs[6,7]. One of the cons of chondrocyte transplantation is the dedifferentiation process that these cells suffer when they are treated in vitro and the limited ability to redifferentiate them[34]. On the contrary, MSCs are very stable and they do not suffer this dedifferentiation process and have a high differentiation capacity[35]. Beside the characteristics of MSCs expounded above, these cells have self-renewal potential as well as multilineage differentiation potential[36,37], including chondrogenesis[25]. A defined medium for in vitro chondrogenesis of MSCs was first reported by Johnstone et al[25] in 1998, who used micromass culture with TGF-β and dexamethasone. To date, the micromass culture is widely used to evaluate chondrogenic potential of MSCs “in vitro”. However, this “in vitro” chondrogenesis does not imitate cartilage formation during development. During micromass culture, MSCs increase expressions of both collagen type II (chondrocytes marker) and X (hypertrophic chondrocytes marker)[25]. Other cytokines such as insulin like growth factor (IGF), bone morphogenetic protein (BMPs) and parathyroid hormone related peptide (PTHrP) had been tried for better differentiation of the cells, but it is still difficult to obtain “in vitro” MSC-based cartilage formation comparative to native cartilage tissue[25]. Those molecules may reach chondrocytes via free diffusion or may be bound to collagens or proteoglycans on extracellular matrix superstructures, becoming available after metabolic processing of collagens and/or proteoglycans. Depending on their position in the metabolic cascade controlling chondrocyte development and homeostasis, they may be used in tissue engineering and regenerative approaches towards cartilage repair by direct application, carrier-mediated release or genetic delivery[17].
BIOMATERIALS
Recently a huge expansion in biomaterial technologies, scaffolds, cell sources, and molecular and genetic manipulations took place to create functional tissue replacements to treat cartilage injuries or osteoarthritis[38-40]. A new generation of materials is being developed and it is influenced by the knowledge of the anatomical and structural complexity of articular cartilage. The increasing capacity to design and synthesize materials with molecular resolution that ranges across organizational levels is generating great excitement in the biomaterials community[25]. The combination of technological advances and an increased knowledge in the fields of molecular and cell biology are generating new biomaterial scaffolds with many desired properties[25]. In addition to being biocompatible and accommodating cell adhesion, proliferation, and matrix synthesis, an ideal biomaterial scaffold for cartilage regeneration can now be bioactive, biomimetic, biodegradable and bioresponsive, providing signaling with spatio-temporal control and response that is selective to defined stimuli. Scaffolds analogous to the natural three-dimensional extracellular matrix may provide important microenvironmental clues to cells. A wide array of materials has been used in various “in vitro” and “in vivo” studies for articular cartilage engineering (Tables 1-3). Scaffolds that are most often studied in cartilage tissue engineering include hydrogels made from poly(ethylene glycol) diacrylate (PEGDA)[7,11-13,41,42], collagen[43], fibrin[44,45], agarose, and synthetic peptides[46,47]; sponge-like scaffolds manufactured from materials such as collagen, polyglycolic acid, polylactic acid[48], and polyurethane[49]; materials with a naturally-occurring porous structure, such as coral, devitalized articular cartilage[50], and hyaluronan based scaffolds[51]. The three-dimensional scaffold provides the structural support for cell contact and matrix deposition prevents dedifferentiation of autologous chondrocytes even after long periods and promotes the expression of chondrocyte-specific markers[52]. Advantages of this procedure are a more uniform cell distribution, avoidance of periosteal harvest and implantation, and increased technical ease without the need for suturing to adjacent articular cartilage. These scaffold-less platforms develop a robust ECM framework of their own and permit long-term maintenance of phenotype, at least in long-term in vitro culture, and can improve biophysical properties by mechanical loading. Scaffold-free constructs using alginate as an intermediate step have also been produced[53] and subjected to mechanical loading[54]. The challenge with such scaffold-free systems is producing them in a cost-effective and timely manner for clinical use, especially with autologous cells. This is also true for scaffold-based systems, but they have biomechanical properties that are immediately functional “in vivo”, showing the ability to direct growth; further they can be designed to deliver relevant bioactive factors[25].
Table 1.
Natural and synthetic materials
| Natural and synthetic materials | Materials | Advantages |
| Natural | Natural Silk, collagen, gelatin, fibrinogen, hyaluronic acid, alginate | Biodegradable |
| Easily available | ||
| Bioactive, interact with cells | ||
| Synthetic | PEG, PGA, PMMA, PLGA | Facilitate restoration of structure of damaged tissues |
| Inert | ||
| Long shelf-life | ||
| Easily tailored for desired porosity and degradation time | ||
| Predictable and reproducible mechanical and physical properties |
PGA: Polyglycolic acid; PLGA: Poly (lactic-co-glycolic acid); PEG: Polyethylene glycol; PMMA: Polymethyl methacrylate.
Table 3.
Overview of advantages and disadvantages of various scaffolds
| Cells | Material | Results |
| Chondrocyctes | Poly(epsilon-caprolactone)-block-poly(L-lactide) | Applicable for cartilage tissue engineering |
| Rabbit marrow mesenchymal stem cells | Oligo(poly(ethylene glycol) fumarate) with encapsulated cells and gelatin microparticles loaded with TGF-β1 | Maintained viability of cells for 14 d |
| Differentiation of cells into chondrocyte-like cells | ||
| Chondrocytes | Gelatin microparticle aggregates, +/- TGF-β1 | Supported viability and function of chondrocytes |
| Applications in cartilage-engineering | ||
| Human adipose derived stem cells | Genipin-crosslinked cartilage derived matrix | Using genipin resulted in contraction free biomaterial. |
| Chondrogenesis | ||
| Human mesenchmal stem cells | Poly(epsilon-caprolactone) | Cell colonization, proliferation and osteogenic differentiation were related to the micro-architecture of the pore structure |
| Human chondrocytes | Blend of poly (lactic-co-glycolic acid) and polyvinyl alcohol | Supported cell adhesion and growth |
| After implantation, there was better bone in-growth and bone formation inside the scaffold. | ||
| Bone marrow stem cells | Polyglycolic acid, poly (lactic acid) | Cell infiltrated the scaffold |
| Good cellular compatibility | ||
| Applicable to repair craniomaxillofacial bone defects |
TGF: Transforming growth factor.
Table 2.
Overview of advantages and disadvantages of various scaffolds
| Scaffold | Advantages | Disadvantages |
| Porous scaffolds | High porosity | Use of highly toxic solvent |
| Interconnected structure | Low pore interconnectivity | |
| Simple and easy to manufacture | Difficulty in homogenous cell seeding post scaffold fabrication | |
| Highly porous scaffolds can have weak mechanical properties | ||
| Lack of control over scaffold thickness | ||
| Fibrous scaffolds | Fiber meshes and fiber bonding are simple techniques | Fiber meshes lack mechanical integrity |
| Large surface area-volume ratio | Fiber bonding lacks control over porosity and pore size | |
| High inter-fiber distances for nutrition and gas exchange | Small pore sizes produced during fabrication processes such as electrospinning limit cell infiltration and 3-D cellular integration with host tissue after implantation | |
| Hydrogels | Can form stable and highly ordered scaffolds using self assembly | |
| Tissue like flexibility | Higher cost | |
| Viscoelasticity | Non-adherent and usually need to be secured by a secondary dressing, for in-vivo testing | |
| Custom scaffolds (Computer-aided design technique) | Intestinal flow and diffusive transport | Natural polymer hydrogels like collagen gelatin, alginate and agarose may evoke inflammatory responses |
| Controlled matrix architecture: size, shape, interconnectivity, branching, geometry and orientation | Low resolution of current systems | |
| Can control pore and pore size | Selective polymeric materials can only be used | |
| Controlled mechanical properties and degradation kinetics | ||
| Microspheres | Reproducible architecture and compositional variations | |
| Used as cell carriers, when fabricated using biodegradable and non-toxic materials | Difficult to remove once injected or implanted | |
| Large surface area for cell attachment and growth | Unknown toxicity associated with microsphere/beads | |
| Native/Extracellular matrix scaffolds | Applicable for 3-D cell culture in a stirred suspension bioreactor Simulates the cell's natural microenvironment in terms of composition, bioactive signal and mechanical properties | Difficult to control degree of decellularization and retain all ECM |
| Non-uniform distribution of cells | ||
| Immunogenicity upon incomplete decellularization |
REHABILITATION
Mechanical stimuli are of crucial importance for the development and maintenance of articular cartilage[55]. Rehabilitation, following any articular cartilage repair procedure is crucial for the success of any articular cartilage resurfacing technique[2]. The rehabilitation is often long as it takes a long time for the cartilage cells to adapt and mature into repair tissue. Cartilage is a slow adapting substance, indeed where a muscle takes approximately 35 wk to fully adapt, cartilage only undergoes 75% adaptation in 2 years. If the rehabilitation period is too short, the cartilage repair might be put under too much stress, causing the repair to fail[2]. Over the years a variety of cartilage restorative procedures have been developed for athletes to address focal, full-thickness cartilaginous defects in the knee joint[56]. In most rehabilitation protocols, continuous passive motion or range of motion exercises are performed within the first day after injury or surgery. Ice, compression, elevation, weight-bearing activities, and electrical stimulation are also started immediately, and the intensity and repetition of these exercises increases as the rehabilitation program progresses. In addition, exercises to address the complimentary musculoskeletal system are also introduced, especially if distinct asymmetries are noted[57]. The type of mobilization exercises used depends on the injury. Experimental and clinical studies demonstrate that early, controlled mobilization is superior to immobilization for primary treatment of acute musculoskeletal soft-tissue injuries and postoperative management[58]. Early mobilization helped return the patients more quickly to physical activity, reduce persistent swelling, restore stability, restore range of motion, and improve patient satisfaction with the rehabilitation outcome[58]. Postoperative rehabilitation programs following articular cartilage repair procedures will vary greatly among patients and need to be individualized, based on the nature of the lesion, the unique characteristics of the patient, and the type and detail of each surgical procedure[59]. These programs are based on knowledge of the basic science, anatomy, and biomechanics of articular cartilage as well as the biological course of healing following surgery[59]. The goal is to restore full function in each patient as quickly as possible by facilitating a healing response without overloading the healing articular cartilage[2]. A patient, lesion, and sports-specific approach is required on the part of the trainer or physical therapist to gradually restore knee joint function and strength so that the athlete may be able to return to competitive play[56]. In this paper review we also take the opportunity to remind readers of the importance of a healthy lifestyle, including physical activity (mild exercise) and balanced diet such as Mediterranean Diet, in the medical therapy to prevent OA disease, in order to preserve the articular cartilage and then the entire joint[59].
CONCLUSION
In conclusion, the treatment of articular cartilage defects can be approached by different procedures in relation to cartilage lesions. Further “in vivo” and “in vitro” studies must be carried out in order to confirm their successful clinical outcomes.
ACKNOWLEDGMENTS
The authors would like to thank Prof. Iain Halliday for commenting and making corrections to the paper.
Footnotes
Supported by The Department of Bio-Medical Sciences, University of Catania
P- Reviewers: Fenichel I, Kongtawelert P, Regauer M, van den Bekerom MPJ S- Editor: Ma YJ L- Editor: A E- Editor: Lu YJ
References
- 1.Blackburn TA, Craig E. Knee anatomy: a brief review. Phys Ther. 1980;60:1556–1560. doi: 10.1093/ptj/60.12.1556. [DOI] [PubMed] [Google Scholar]
- 2.Bennell KL, Hinman RS. A review of the clinical evidence for exercise in osteoarthritis of the hip and knee. J Sci Med Sport. 2011;14:4–9. doi: 10.1016/j.jsams.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Musumeci G, Carnazza ML, Leonardi R, Loreto C. Expression of β-defensin-4 in “an in vivo and ex vivo model” of human osteoarthritic knee meniscus. Knee Surg Sports Traumatol Arthrosc. 2012;20:216–222. doi: 10.1007/s00167-011-1630-x. [DOI] [PubMed] [Google Scholar]
- 4.Musumeci G, Loreto C, Carnazza ML, Cardile V, Leonardi R. Acute injury affects lubricin expression in knee menisci: an immunohistochemical study. Ann Anat. 2013;195:151–158. doi: 10.1016/j.aanat.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Musumeci G, Leonardi R, Carnazza ML, Cardile V, Pichler K, Weinberg AM, Loreto C. Aquaporin 1 (AQP1) expression in experimentally induced osteoarthritic knee menisci: an in vivo and in vitro study. Tissue Cell. 2013;45:145–152. doi: 10.1016/j.tice.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Musumeci G, Loreto C, Castorina S, Imbesi R, Leonardi R, Castrogiovanni P. Current Concepts in the Treatment of Cartilage Damage. A Review. IJAE. 2013;118:189–203. [PubMed] [Google Scholar]
- 7.Musumeci G, Loreto C, Castorina S, Imbesi R, Leonardi R, Castrogiovanni P. New perspectives in the treatment of cartilage damage. Poly(ethylene glycol) diacrylate (PEGDA) scaffold. A review. IJAE. 2013;118:204–210. [PubMed] [Google Scholar]
- 8.Pichler K, Musumeci G, Vielgut I, Martinelli E, Sadoghi P, Loreto C, Weinberg AM. Towards a better understanding of bone bridge formation in the growth plate - an immunohistochemical approach. Connect Tissue Res. 2013;54:408–415. doi: 10.3109/03008207.2013.828715. [DOI] [PubMed] [Google Scholar]
- 9.Musumeci G, Castrogiovanni P, Loreto C, Castorina S, Pichler K, Weinberg AM. Post-traumatic caspase-3 expression in the adjacent areas of growth plate injury site: a morphological study. Int J Mol Sci. 2013;14:15767–15784. doi: 10.3390/ijms140815767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Musumeci G, Loreto C, Carnazza ML, Martinez G. Characterization of apoptosis in articular cartilage derived from the knee joints of patients with osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2011;19:307–313. doi: 10.1007/s00167-010-1215-0. [DOI] [PubMed] [Google Scholar]
- 11.Musumeci G, Carnazza ML, Loreto C, Leonardi R, Loreto C. β-defensin-4 (HBD-4) is expressed in chondrocytes derived from normal and osteoarthritic cartilage encapsulated in PEGDA scaffold. Acta Histochem. 2012;114:805–812. doi: 10.1016/j.acthis.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Musumeci G, Loreto C, Carnazza ML, Coppolino F, Cardile V, Leonardi R. Lubricin is expressed in chondrocytes derived from osteoarthritic cartilage encapsulated in poly (ethylene glycol) diacrylate scaffold. Eur J Histochem. 2011;55:e31. doi: 10.4081/ejh.2011.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Musumeci G, Loreto C, Carnazza ML, Strehin I, Elisseeff J. OA cartilage derived chondrocytes encapsulated in poly(ethylene glycol) diacrylate (PEGDA) for the evaluation of cartilage restoration and apoptosis in an in vitro model. Histol Histopathol. 2011;26:1265–1278. doi: 10.14670/HH-26.1265. [DOI] [PubMed] [Google Scholar]
- 14.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 15.Langer R, Vacanti JP. Artificial organs. Sci Am. 1995;273:130–133. [PubMed] [Google Scholar]
- 16.Vacanti JP, Langer R. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet. 1999;354 Suppl 1:SI32–SI34. doi: 10.1016/s0140-6736(99)90247-7. [DOI] [PubMed] [Google Scholar]
- 17.Gaissmaier C, Koh JL, Weise K. Growth and differentiation factors for cartilage healing and repair. Injury. 2008;39 Suppl 1:S88–S96. doi: 10.1016/j.injury.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 18.Miljkovic ND, Cooper GM, Marra KG. Chondrogenesis, bone morphogenetic protein-4 and mesenchymal stem cells. Osteoarthritis Cartilage. 2008;16:1121–1130. doi: 10.1016/j.joca.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Benz K, Breit S, Lukoschek M, Mau H, Richter W. Molecular analysis of expansion, differentiation, and growth factor treatment of human chondrocytes identifies differentiation markers and growth-related genes. Biochem Biophys Res Commun. 2002;293:284–292. doi: 10.1016/S0006-291X(02)00223-1. [DOI] [PubMed] [Google Scholar]
- 20.Nejadnik H, Hui JH, Feng Choong EP, Tai BC, Lee EH. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med. 2010;38:1110–1116. doi: 10.1177/0363546509359067. [DOI] [PubMed] [Google Scholar]
- 21.Becerra J, Andrades JA, Guerado E, Zamora-Navas P, López-Puertas JM, Reddi AH. Articular cartilage: structure and regeneration. Tissue Eng Part B Rev. 2010;16:617–627. doi: 10.1089/ten.TEB.2010.0191. [DOI] [PubMed] [Google Scholar]
- 22.Becerra J, Santos-Ruiz L, Andrades JA, Marí-Beffa M. The stem cell niche should be a key issue for cell therapy in regenerative medicine. Stem Cell Rev. 2011;7:248–255. doi: 10.1007/s12015-010-9195-5. [DOI] [PubMed] [Google Scholar]
- 23.Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19:477–484. doi: 10.1053/jars.2003.50112. [DOI] [PubMed] [Google Scholar]
- 24.Basad E, Ishaque B, Bachmann G, Stürz H, Steinmeyer J. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg Sports Traumatol Arthrosc. 2010;18:519–527. doi: 10.1007/s00167-009-1028-1. [DOI] [PubMed] [Google Scholar]
- 25.Johnstone B, Alini M, Cucchiarini M, Dodge GR, Eglin D, Guilak F, Madry H, Mata A, Mauck RL, Semino CE, et al. Tissue engineering for articular cartilage repair--the state of the art. Eur Cell Mater. 2013;25:248–267. doi: 10.22203/ecm.v025a18. [DOI] [PubMed] [Google Scholar]
- 26.Miyanishi K, Trindade MC, Lindsey DP, Beaupré GS, Carter DR, Goodman SB, Schurman DJ, Smith RL. Effects of hydrostatic pressure and transforming growth factor-beta 3 on adult human mesenchymal stem cell chondrogenesis in vitro. Tissue Eng. 2006;12:1419–1428. doi: 10.1089/ten.2006.12.1419. [DOI] [PubMed] [Google Scholar]
- 27.Guilak F, Estes BT, Diekman BO, Moutos FT, Gimble JM. 2010 Nicolas Andry Award: Multipotent adult stem cells from adipose tissue for musculoskeletal tissue engineering. Clin Orthop Relat Res. 2010;468:2530–2540. doi: 10.1007/s11999-010-1410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Musumeci G, Lo Furno D, Loreto C, Giuffrida R, Caggia S, Leonardi R, Cardile V. Mesenchymal stem cells from adipose tissue which have been differentiated into chondrocytes in three-dimensional culture express lubricin. Exp Biol Med (Maywood) 2011;236:1333–1341. doi: 10.1258/ebm.2011.011183. [DOI] [PubMed] [Google Scholar]
- 29.Winter A, Breit S, Parsch D, Benz K, Steck E, Hauner H, Weber RM, Ewerbeck V, Richter W. Cartilage-like gene expression in differentiated human stem cell spheroids: a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 2003;48:418–429. doi: 10.1002/art.10767. [DOI] [PubMed] [Google Scholar]
- 30.Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432–463. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 31.Hennig T, Lorenz H, Thiel A, Goetzke K, Dickhut A, Geiger F, Richter W. Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFbeta receptor and BMP profile and is overcome by BMP-6. J Cell Physiol. 2007;211:682–691. doi: 10.1002/jcp.20977. [DOI] [PubMed] [Google Scholar]
- 32.Claros S, Rodríguez-Losada N, Cruz E, Guerado E, Becerra J, Andrades JA. Characterization of adult stem/progenitor cell populations from bone marrow in a three-dimensional collagen gel culture system. Cell Transplant. 2012;21:2021–2032. doi: 10.3727/096368912X636939. [DOI] [PubMed] [Google Scholar]
- 33.Kreuz PC, Steinwachs MR, Erggelet C, Krause SJ, Konrad G, Uhl M, Südkamp N. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14:1119–1125. doi: 10.1016/j.joca.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Chung C, Burdick JA. Engineering cartilage tissue. Adv Drug Deliv Rev. 2008;60:243–262. doi: 10.1016/j.addr.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung C, Mesa J, Randolph MA, Yaremchuk M, Burdick JA. Influence of gel properties on neocartilage formation by auricular chondrocytes photoencapsulated in hyaluronic acid networks. J Biomed Mater Res A. 2006;77:518–525. doi: 10.1002/jbm.a.30660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song L, Baksh D, Tuan RS. Mesenchymal stem cell-based cartilage tissue engineering: cells, scaffold and biology. Cytotherapy. 2004;6:596–601. doi: 10.1080/14653240410005276-1. [DOI] [PubMed] [Google Scholar]
- 37.Elisseeff J, Anseth K, Sims D, McIntosh W, Randolph M, Langer R. Transdermal photopolymerization for minimally invasive implantation. Proc Natl Acad Sci USA. 1999;96:3104–3107. doi: 10.1073/pnas.96.6.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elisseeff J, McIntosh W, Anseth K, Riley S, Ragan P, Langer R. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. J Biomed Mater Res. 2000;51:164–171. doi: 10.1002/(sici)1097-4636(200008)51:2<164::aid-jbm4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 39.Dorotka R, Windberger U, Macfelda K, Bindreiter U, Toma C, Nehrer S. Repair of articular cartilage defects treated by microfracture and a three-dimensional collagen matrix. Biomaterials. 2005;26:3617–3629. doi: 10.1016/j.biomaterials.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 40.Ameer GA, Mahmood TA, Langer R. A biodegradable composite scaffold for cell transplantation. J Orthop Res. 2002;20:16–19. doi: 10.1016/S0736-0266(01)00074-2. [DOI] [PubMed] [Google Scholar]
- 41.Peretti GM, Randolph MA, Villa MT, Buragas MS, Yaremchuk MJ. Cell-based tissue-engineered allogeneic implant for cartilage repair. Tissue Eng. 2000;6:567–576. doi: 10.1089/107632700750022206. [DOI] [PubMed] [Google Scholar]
- 42.Kisiday J, Jin M, Kurz B, Hung H, Semino C, Zhang S, Grodzinsky AJ. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc Natl Acad Sci USA. 2002;99:9996–10001. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kisiday JD, Jin M, DiMicco MA, Kurz B, Grodzinsky AJ. Effects of dynamic compressive loading on chondrocyte biosynthesis in self-assembling peptide scaffolds. J Biomech. 2004;37:595–604. doi: 10.1016/j.jbiomech.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Grande DA, Halberstadt C, Naughton G, Schwartz R, Manji R. Evaluation of matrix scaffolds for tissue engineering of articular cartilage grafts. J Biomed Mater Res. 1997;34:211–220. doi: 10.1002/(sici)1097-4636(199702)34:2<211::aid-jbm10>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 45.Grad S, Lee CR, Gorna K, Gogolewski S, Wimmer MA, Alini M. Surface motion upregulates superficial zone protein and hyaluronan production in chondrocyte-seeded three-dimensional scaffolds. Tissue Eng. 2005;11:249–256. doi: 10.1089/ten.2005.11.249. [DOI] [PubMed] [Google Scholar]
- 46.Hangody L, Vásárhelyi G, Hangody LR, Sükösd Z, Tibay G, Bartha L, Bodó G. Autologous osteochondral grafting--technique and long-term results. Injury. 2008;39 Suppl 1:S32–S39. doi: 10.1016/j.injury.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 47.Kang JY, Chung CW, Sung JH, Park BS, Choi JY, Lee SJ, Choi BC, Shim CK, Chung SJ, Kim DD. Novel porous matrix of hyaluronic acid for the three-dimensional culture of chondrocytes. Int J Pharm. 2009;369:114–120. doi: 10.1016/j.ijpharm.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 48.Filová E, Jelínek F, Handl M, Lytvynets A, Rampichová M, Varga F, Cinátl J, Soukup T, Trc T, Amler E. Novel composite hyaluronan/type I collagen/fibrin scaffold enhances repair of osteochondral defect in rabbit knee. J Biomed Mater Res B Appl Biomater. 2008;87:415–424. doi: 10.1002/jbm.b.31119. [DOI] [PubMed] [Google Scholar]
- 49.Masuda K, Sah RL, Hejna MJ, Thonar EJ. A novel two-step method for the formation of tissue-engineered cartilage by mature bovine chondrocytes: the alginate-recovered-chondrocyte (ARC) method. J Orthop Res. 2003;21:139–148. doi: 10.1016/S0736-0266(02)00109-2. [DOI] [PubMed] [Google Scholar]
- 50.Stoddart MJ, Ettinger L, Häuselmann HJ. Enhanced matrix synthesis in de novo, scaffold free cartilage-like tissue subjected to compression and shear. Biotechnol Bioeng. 2006;95:1043–1051. doi: 10.1002/bit.21052. [DOI] [PubMed] [Google Scholar]
- 51.Grad S, Eglin D, Alini M, Stoddart MJ. Physical stimulation of chondrogenic cells in vitro: a review. Clin Orthop Relat Res. 2011;469:2764–2772. doi: 10.1007/s11999-011-1819-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nho SJ, Pensak MJ, Seigerman DA, Cole BJ. Rehabilitation after autologous chondrocyte implantation in athletes. Clin Sports Med. 2010;29:267–282, viii. doi: 10.1016/j.csm.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 53.Kerkhoffs GM, Rowe BH, Assendelft WJ, Kelly KD, Struijs PA, van Dijk CN. Immobilisation for acute ankle sprain. A systematic review. Arch Orthop Trauma Surg. 2001;121:462–471. doi: 10.1007/s004020100283. [DOI] [PubMed] [Google Scholar]
- 54.Andrews JR, Harrelson GL, Wilk KE. Physical rehabilitation of the injured athlete, 2nd ed. Philadelphia, PA: W.B. Saunders; 1998. [Google Scholar]
- 55.Reinold MM, Wilk KE, Macrina LC, Dugas JR, Cain EL. Current concepts in the rehabilitation following articular cartilage repair procedures in the knee. J Orthop Sports Phys Ther. 2006;36:774–794. doi: 10.2519/jospt.2006.2228. [DOI] [PubMed] [Google Scholar]
- 56.Musumeci G, Loreto C, Leonardi R, Castorina S, Giunta S, Carnazza ML, Trovato FM, Pichler K, Weinberg AM. The effects of physical activity on apoptosis and lubricin expression in articular cartilage in rats with glucocorticoid-induced osteoporosis. J Bone Miner Metab. 2013;31:274–284. doi: 10.1007/s00774-012-0414-9. [DOI] [PubMed] [Google Scholar]
- 57.Pichler K, Loreto C, Leonardi R, Reuber T, Weinberg AM, Musumeci G. RANKL is downregulated in bone cells by physical activity (treadmill and vibration stimulation training) in rat with glucocorticoid-induced osteoporosis. Histol Histopathol. 2013;28:1185–1196. doi: 10.14670/HH-28.1185. [DOI] [PubMed] [Google Scholar]
- 58.Musumeci G, Maria Trovato F, Imbesi R, Castrogiovanni P. Effects of dietary extra-virgin olive oil on oxidative stress resulting from exhaustive exercise in rat skeletal muscle: A morphological study. Acta Histochem. 2014;116:61–69. doi: 10.1016/j.acthis.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 59.Musumeci G, Trovato FM, Pichler K, Weinberg AM, Loreto C, Castrogiovanni P. Extra-virgin olive oil diet and mild physical activity prevent cartilage degeneration in an osteoarthritis model. An “in vivo” and “in vitro” study on lubricin expression. J Nutr Biochem. 2013;24:2064–2075. doi: 10.1016/j.jnutbio.2013.07.007. [DOI] [PubMed] [Google Scholar]


