Abstract
BACKGROUND
The approval of extended release injectable naltrexone (XR-NTX; Vivitrol®) has introduced a new option for treating opioid addiction, but studies are needed to identify its place within the spectrum of available therapies. The absence of physiological opioid dependence is a necessary and challenging first step for starting XR-NTX. Outpatient detoxification gives poor results and inpatient detoxification is either unavailable or too brief for the physiological effects of opioids to resolve. Here we present findings from an open label study that tested whether the transition from opioid addiction to XR-NTX can be safely and effectively performed in an outpatient setting using very low dose naltrexone and buprenorphine.
METHODS
Twenty treatment seeking opioid addicted individuals were given increasing doses of naltrexone starting at 0.25 mg with decreasing doses of buprenorphine starting at 4 mg during a 7-day outpatient XR-NTX induction procedure. Withdrawal discomfort, craving, drug use, and adverse events were assessed daily until the XR-NTX injection, then weekly over the next month.
RESULTS
Fourteen of the 20 participants received XR-NTX and 13 completed weekly assessments. Withdrawal, craving, and opioid or other drug use were significantly lower during induction and after XR-NTX administration compared with baseline, and no serious adverse events were recorded.
CONCLUSIONS
Outpatient transition to XR-NTX combining upward titration of very low dose naltrexone with downward titration of low dose buprenorphine was safe, well tolerated, and completed by most participants. Further studies with larger numbers of subjects are needed to see if this approach is useful for naltrexone induction.
Keywords: addiction, detoxification, pharmacotherapy, opioid agonist, opioid antagonist, minority recruitment
1. INTRODUCTION
Opioid use disorders have been among the fastest growing substance abuse problems in the U.S. (SAMHSA, 2013). Though the implementation of office-based treatment has seen a nine-fold increase in the proportion of patients being treated with buprenorphine, and the number of patients on methadone maintenance has also increased, only one in four opioid addicted individuals receive either of these treatments (SAMHSA, 2012, 2013). The reasons for this gap between treatment need and enrollment are complex and include resource limitations and attitudinal barriers (Oliva et al., 2011; Roman et al., 2011). Complicating the difficulty of closing the gap is that many addicted patients are not interested in treatment and others are interested but do not want opioid agonist maintenance despite the evidence that it is safe, effective, and has had a major role in reducing the spread of HIV (Metzger et al., 2010).
Naltrexone (NTX) offers a different approach but low interest and high dropout among patients that were treated with the oral formulation (Minozzi et al., 2011) led to the dismissal of NTX as a meaningful treatment in the minds of many clinicians and researchers (Adi et al., 2007; Mannelli et al., 2011). Concerns have also been expressed that NTX increases depression and anxiety and the risk for overdose death (Miotto et al., 1997; Ritter, 2002), however data from studies of oral and extended release naltrexone have shown that depression and anxiety actually decrease in patients that continue NTX (Krupitsky et al., 2012, 2004, 2006) and that there is no apparent increased risk of overdose death after treatment ends (Woody and Metzger, 2011).
The introduction of sustained release injectable NTX with the recommendation to be given every 4 weeks or once a month (XR-NTX; Vivitrol®; Vivitrol®, 2013), has attracted growing interest due to its advantages for improved adherence, however patients must be free of physiological opioid dependence before it is administered to avoid precipitating withdrawal, thus effective use is contingent on the management of opioid discontinuation (Mannelli et al., 2011). Among available interventions, outpatient detoxification has had very low success rates (Kleber, 2007) and though inpatient treatment is accessible to insured patients, the coverage often falls short of providing the 7 to 10 opioid-free days necessary to eliminate physiological dependence (Gonzalez and Brogden, 1988; Kleber, 2007) and avoid precipitated withdrawal with the first dose XR-NTX (Vivitrol®, 2013). These issues may lessen the interest of patients and physicians, and are a barrier to initiating XR-NTX treatment.
In an attempt to find an approach that improves this situation, we studied the feasibility of using low doses of NTX with low doses of buprenorphine/naloxone (BUP). This idea emerged when we were detoxifying patients on an inpatient unit and found that very low dose NTX combined with a methadone dose taper reduced withdrawal severity (Mannelli et al., 2003, 2009) and that the naltrexone dose could be titrated upward to the full oral dose without precipitating clinically significant withdrawal (Mannelli et al., 2003). Here we present the results of an open-label, office-based investigation to explore the transition from opioid dependence to the first XR-NTX injection using low and increasing doses of naltrexone combined with low and decreasing doses of buprenorphine.
2. METHODS
2.1 Study design
The objective of this open-label, uncontrolled, flexible dose study (ClinicalTrials.gov NCT01690546) was to evaluate the feasibility of very low dose NTX/BUP outpatient transfer from drug use to XR-NTX injection in opioid dependent patients. The underlying question was whether a NTX/BUP induction regimen is safe and tolerable for the transition. The treatment consisted of 7-day detoxification/induction, followed by XR-NTX injection and 4-week follow-up. Visits were scheduled on Days 1 through 9, then weekly through week 4. The primary endpoint was induction onto XR-NTX; secondary endpoints included attendance at study visits, opioid withdrawal and craving measured by rating scales, opioid and other drug use measured by urine drug testing and self-report, treatment satisfaction measured by a questionnaire, and safety measured by observed and reported adverse events.
2.2 Subjects
Recruitment occurred between October, 2012 and June, 2013 among individuals 18–65 years of age that were seeking treatment for opioid addiction at the Duke Addictions Program-Duke University Medical Center in Durham, NC. Seventy to eighty of them expressed interest in the study and 21 subjects were contacted by the research staff for purpose of screening. Participants met the Diagnostic and Statistical Manual of Mental Disorders 4th edition criteria for opioid dependence with physiological dependence (APA, 2000) based on the Structured Clinical Interview of DSM-IV (SCID) (First et al., 1994), and confirmed by urine drug testing. Exclusion criteria were inability to give informed consent, regular use of opioids for chronic pain or medical illness, daily use of methadone or buprenorphine, history of hypersensitivity to NTX, naloxone, buprenorphine or XR-NTX components, severe or unstable psychiatric or medical conditions requiring treatment, pregnancy or breastfeeding, failure to use adequate contraceptive methods, liver enzyme function tests greater than two times normal, or current dependence on substances other than opioids (excluding nicotine).
The PI of the study (PM) holds a research Investigational New Drug application to administer very low dose NTX (IND 113962). The institutional review board of Duke University approved the study and all subjects provided voluntary oral and written informed consent. Screening assessment comprised medical history, physical examination, ECG, routine clinical laboratory tests, including pregnancy test, saliva and urine drug testing.
2.3 Procedures
Participants were asked to abstain from opioids for 24 hours before starting treatment. Upon arrival at the study site they were tested for drug use and assessed for vital signs and symptoms of withdrawal, then received NTX (Day 1–7), BUP (Day 1–3), XR-NTX injection (Day 8), and ancillary medications as needed; details are below. Assessments were repeated an hour after administration of the medications and before leaving the clinic. Individuals were asked to remain at the study site for up to 4 hours. Participants were offered weekly sessions of individual drug counseling adapted to opioid addiction (Mercer and Woody, 2004), staff performed take-home medication reconciliation, querying volunteers about the occurrence of withdrawal discomfort and adverse events, and clinicians examined the injection site 1 day after XR-NTX administration and then weekly for 4 weeks. At study completion, participants received a physical examination, ECG, hematology/blood chemistry assessments, and were offered referral to continuing care. Treatment was provided at no expense and participants were compensated for time needed to complete assessments and travel.
2.4 Drugs and Dosing Schedules
NTX was administered daily in split doses with the intent to progressively reduce the level of physiological opioid dependence without precipitating clinically significant withdrawal. The range of doses was based on experience using it on inpatient units with patients tapering off methadone (Mannelli et al., 2003, 2009) or buprenorphine (Eissenberg et al., 1996; Johnson, 2001; Kosten et al., 1990; Umbricht et al., 1999). The BUP dose reduction schedule was based on available literature (Mannelli et al., 2012); dosing schedules are seen in Table 2.
Table 2.
Daily naltrexone (NTX) and buprenorphine/naloxone (BUP) dose schedule (N=20)
| Day | NTX Mean(SD) mg | Dose range mg | BUP mg |
|---|---|---|---|
| 1 | 0.43 (0.02) | 0.25–0.5 | 4 |
| 2 | 0.43 (0.082) | 0.25–0.5 | 2 |
| 3 | 0.83 (0.082) | 0.5–1 | 2 |
| 4 | 2.94 (1.81) | 2–6 | - |
| 5 | 5.30 (0.626) | 3–15 | - |
| 6 | 12.21 (6.03) | 5–35 | - |
| 7 | 31.30 (8.14) | 13–50 | - |
Non-opioid medications commonly used to help control withdrawal symptoms were offered at the study site and as take home doses according to clinical judgment in response to patient requests. They included ibuprofen (200–400 mg q4-6h); cyclobenzaprine (5–10 mg q6-8h) or acetaminophen (650–1000 mg q6-8h) for muscle aches; trazodone (50–100 mg qhs) or doxepin (50–100 mg qhs) for insomnia; lorazepam (1–2 mg q4-6h) or hydroxyzine (25–50 mg q4-6h) for anxiety/restlessness; promethazine (25 mg q8-12h) or loperamide (2 mg as needed, max 12mg/24h) for nausea, vomiting, or diarrhea; and clonidine (0.1–0.3 mg q4-6h) for symptoms of autonomic arousal (anxiety, sweating, rhinorrhea, lacrimation). Lactose-free capsules containing naltrexone hydrochloride USP powder 0,25mg, 1mg, and 5mg, were prepared at the Compounding Pharmacy at Duke University Medical Center; buprenorphine-naloxone sublingual films and ancillary medications were purchased at the Duke University Medical Center Outpatient Pharmacy.
Alkermes provided XR-NTX in the FDA-approved dose of 380 mg. It is administered via deep intramuscular injection and consists of microspheres composed of medical grade poly-(d,l-lactide-co-glycolide) that gradually release naltrexone. In patients with opioid dependence, XR-NTX treatment has been associated with significantly greater opioid abstinence, higher treatment retention, and a more marked reduction in opioid craving compared with placebo (Krupitsky et al., 2011). All medications were stored at the study site following federal and state regulations.
2.5 Measures
The Addiction Severity Index (ASI; McLellan et al., 1992) documented patient demographics and drug-related problems at admission and at week 4. It is a widely used instrument that provides severity profiles in seven domains (medical, employment, alcohol, drugs, family/social, legal and psychiatric) that are commonly affected by problematic substance use and summarizes them with composite scores ranging from 0 (no problem) to 1 (extreme severity). Opioid withdrawal symptoms were assessed by subjects using the Subjective Opiate Withdrawal Scale (SOWS; Handelsman et al., 1987) and by a physician or nurse using the Clinical Opiate Withdrawal Scale (COWS; Wesson and Ling, 2003). Opioid craving was measured by asking subjects to rate their desire for opioids on a visual analog scale from 0 to 100, with 0 = no craving and 100 = maximum craving. Drug use was assessed by urine toxicology testing for opioids, methadone, oxycodone, buprenorphine, cocaine, amphetamine, methamphetamine, tetrahydrocannabinol, benzodiazepines, barbiturates, propoxyphene, phencyclidine, and tricyclic antidepressants (Instant Technologies, iCup®); a saliva strip for alcohol (Devon Medical); and self-reports using the Timeline Followback method (Sobell and Sobell, 1992). Patient satisfaction was assessed by asking them to rate on a scale from 1–5 (highest=1, lowest=5): 1) Level of satisfaction with treatment; 2) How this treatment compared with previous withdrawal treatments; and 3) How much treatment helped.
2.6 Safety
These included reported and observed adverse events assessed at each study visit, vital signs (heart rate, blood pressure, skin temperature and respiratory rate) performed three or more times daily during the 4-hour sessions, physical exams, laboratory parameters (cell blood count, complete metabolic panel and pregnancy test), and ECG performed at screen and the last study visit.
2.7 Data analysis
Descriptive statistics (mean and SD, number, percent and percent changes from baseline) were calculated for demographic and clinical features, and efficacy measures. Baseline withdrawal and craving scores were compared with mean daily treatment scores. SOWS, COWS, craving and ASI scores were compared using paired Student’s t-tests (two-tailed; alpha = 0.05). Drug use comparisons with baseline were analyzed using the McNemar’s test. Number and percent of subjects experiencing adverse events or with clinically significant laboratory, physical exam, or vital sign findings were recorded and summarized.
3. RESULTS
3.1 Subjects
Twenty of the 21 opioid addicted individuals that were screened were included in the study; the subject that was not included was deemed unable to understand study procedures. As shown in Table 1, participants were primarily young to middle aged African American men with polydrug and tobacco use in addition to their opioid addiction. In the 30 days before study enrollment, 11 (60%) used heroin, 5 (25%) used ‘street’ methadone, and 8 (40%) used prescription opioids. Based on opioid consumption, the majority of participants had severe physiological dependence (75%), defined as using more than 6 bags of heroin per day or an equivalent amount of other opioid substances (Sigmon et al., 2012). Participants had not used opioid drugs for an average of 15 hours (range 12–31) when treatment started and received the study medications in the following days provided no opioid use was reported since the previous visit.
Table 1.
Baseline and drug use characteristics of subjects enrolled in the study (n= 20)
|
|
|
|---|---|
| % or mean(SD) | |
| DEMOGRAPHICS | |
| Age in years | 38.2 (8.42) |
| Age Range | 23–62 |
| African American | 75 |
| Caucasian | 25 |
| Non-Hispanic | 90 |
| Male | 75 |
| Married or cohabitant | 30 |
| Years of education | 7.8 (5.1) |
| Unemployed | 60 |
|
| |
| ADDICTION SEVERITY INDEX (ASI) SCORE | |
| Alcohol | 0.10 (0.14) |
| Drug | 0.66 (0.24) |
| Psychiatric | 0.30 (0.19) |
| Medical | 0.28 (0.28) |
| Family/Social | 0.35 (0.14) |
| Employment | 0.40 (0.23) |
| Legal | 0.06 (0.24) |
|
| |
| YEARS OF OPIOID USE | 9.7 (11.2) |
|
| |
| NUMBER OF OPIOID DETOXIFICATION | 2.8 (1.3) |
|
| |
| DRUG USE IN THE LAST MONTH | |
| Opioid/intravenous | 100/40 |
| Alcohol | 20 |
| Cocaine | 40 |
| Cannabis | 65 |
| Tobacco | 85 |
| More than one substance (excluding tobacco) | 80 |
|
| |
| Level of physiological opioid dependence* | |
| Severe | 75 |
| Moderate | 25 |
|
| |
| Hours since last opioid use | 14.7 (10.3) |
|
|
|
Equivalence: Severe > 6 bags/day of heroin, Moderate= 3–6 bags/day of heroin (Sigmon et al 2012)
3.2 Treatment retention and outcome
Fifteen of 20 patients successfully concluded the induction phase (Day 7) and 14 received XR-NTX (Day 8). Of the 6 remaining, one completed induction and was excluded from injection because we were unable to determine the status of his HCV disease, 3 were discontinued due to poor compliance with study assessments and observation time on Days 6, 7, and 8; and 2 were lost to follow-up on Days 6 and 7. Thirteen completed follow-up after the XR-NTX injection. Of the 160 possible visits up to Day 8, patients receiving the injection (n= 14) completed all the visits (112), the other participants (n= 6) attended 36 visits and missed 2 on Day 6, 4 on Day 7, and 6 on Day 8. Participants received the study medications at all the visits they completed. Of the potential 70 follow-up visits among XR-NTX treated patients (Day 9 and week 1–4), 13 participants completed 65, 1 attended 1 visit (Day 9).
A significant improvement was detected at the end of the study in ASI composite score (n=12) for drug use (t = 6.22, p=0.0001), family/social problems (t = 3.263, p= 0.004), psychiatric problems (t = 2.44, p= 0.02), and medical problems (t = 2.15, p= 0.03).
3.3 Opioid withdrawal and craving
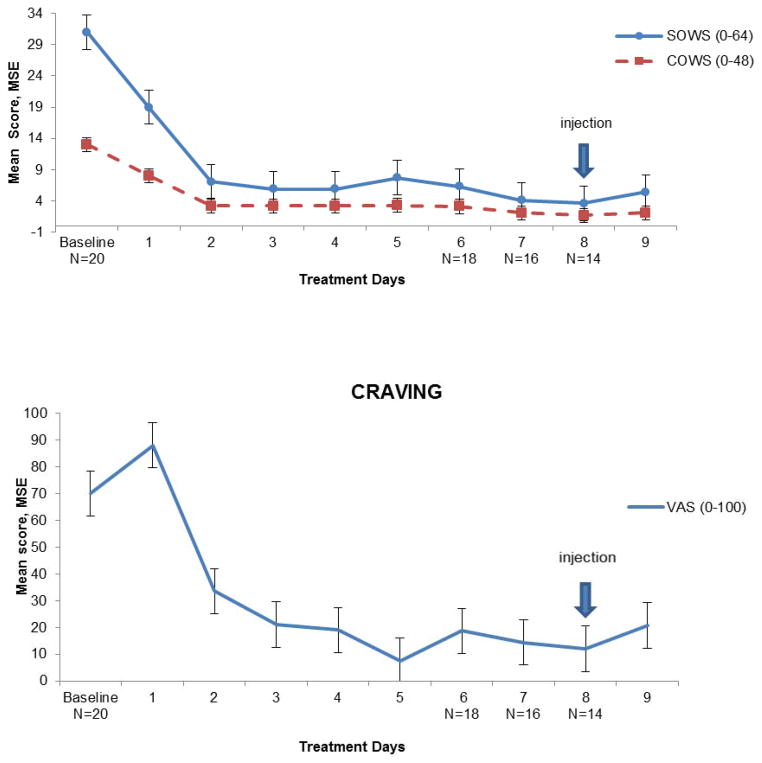
There was a significant decrease of subjective and clinician’s-rated withdrawal and craving scores from Day 1 (n=20; SOWS: t = 4.3, p= 0.00001; COWS: t = 5.6, p= 0.00001), and Day 2 (n=20; craving: t = 4.4, p= 0.002) over the course of the study (Figure 1). In particular, scores were significantly lower than baseline on the injection day (n=14; SOWS t = 5.9, p= 0.0001; COWS: t = 8.4, p= 0.0001; and craving: t = 7.7, p= 0.0001); on the day after XR-NTX administration (n=14; SOWS: t = 5.0, p= 0.001; COWS: t = 6.9, p= 0.001; craving: t = 5.5, p= 0.001), and 1 week after that (n=13; SOWS: t = 4.8, p= 0.002; COWS: t = 6.7, p= 0.001; craving: t = 7.8, p= 0.0001). Week 2–4 withdrawal and craving scores were non-significantly lower than those at week 1 (data not shown).
Figure 1.
Mean opioid withdrawal and craving scores during induction and after naltrexone extended release administration (Days 1–9), using SOWS (Subjective Opioid Withdrawal Scale), COWS (Clinical Opioid Withdrawal Scale) and VAS (Visual Analog Scale) for craving. Time point scores are the results of the mean score of each day of treatment, error bars represent +/−1 SEM. Number of participants is reported on the X-axis.
The most commonly prescribed medications were trazodone [37%, 58.1(6.7) mg)], cyclobenzaprine [28%, 10.9(5.6) mg], lorazepam [26%, 4.9(1.3) mg], and hydroxyzine [26%, 38.7(12.5) mg]. No ancillary medications were needed beyond week 1 after the injection.
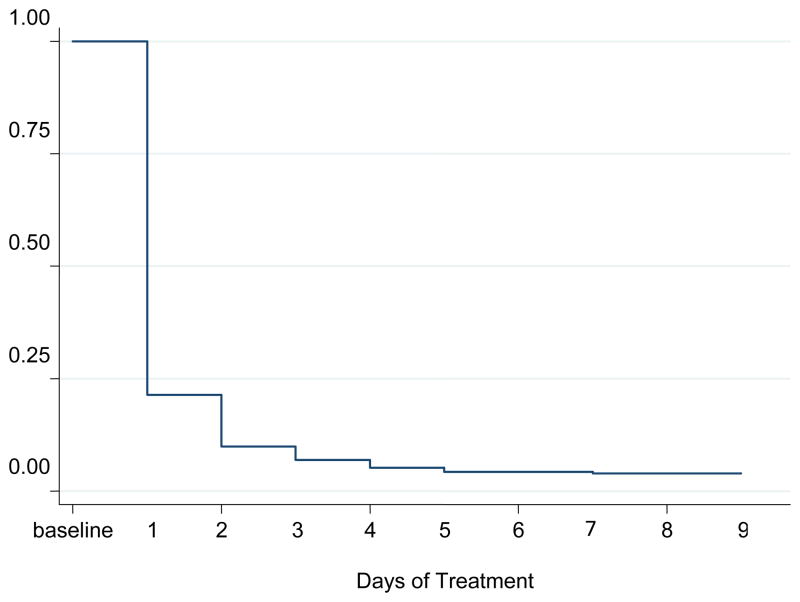
3.4 Drug use
All subjects had a positive opioid urine test at baseline with marked reduction by the time of NTX induction (Figure 2). The proportion of opioid-positive samples (excluding buprenorphine) was 23.8% on the second day of treatment and 14.1% on the injection day. Confirmed use of other drugs was also lower by Day 8 compared with pre-treatment (36% vs. 67%, χ2 (1) = 7.34; p= 0.001). In the 4 weeks following XR-NTX administration, 21.2% of weekly urine tests were positive for opioids and 33.3% positive for other drug use (the latter compared with baseline: χ2 (1) = 7.41; p= 0.001), with no evidence of relapse to addiction. In-treatment alcohol and tobacco use were non-significantly different from baseline (data not shown).
Figure 2.
In-treatment proportion of opioid positive urine samples (Day 1–9, N=20)
3.5 Safety and treatment satisfaction
Safety data were available for all 20 subjects and no serious adverse events occurred. All subjects reported mild symptoms of opioid withdrawal at different times, similar to those documented on their withdrawal assessments. Two described worsening withdrawal for a few hours after leaving the study site on Days 6 and 7, respectively. This event was reported the following day and did not require specific treatment or trigger opioid use, as confirmed by urine drug test. There were no adverse events associated with XR-NTX administration or unexpected injection site reactions. No subjects prematurely discontinued treatment because of an adverse event. Overall, there were no clinically significant shifts in hematology, chemistry, or urinalysis values over time. No clinically significant changes in vital signs or in physical examination results were recorded. Participants receiving the injection (N=14) were completely (N=13) or partly (N=1) satisfied with treatment and considered the medications to have helped a lot (57.1%) or quite a bit (42.9%). Compared with past opioid discontinuation attempts, 78.6% reported minimal or some withdrawal discomfort and 21.4% judged the severity of withdrawal to be about average.
4. DISCUSSION
These findings suggest that two thirds or more of opioid addicted individuals who are interested in XR-NTX treatment may be able to be detoxified and started on XR-NTX in an outpatient setting. This conclusion is based on moderately high treatment retention, decrease in withdrawal scores, low proportion of adverse events, and high degree of patient satisfaction. If replicated in other clinical samples, a XR-NTX transfer rate of 60–70% with a 30-day proportion of 80% negative opioid urine tests would compare well against results of induction and early retention onto oral NTX (10%–40%), (Mannelli et al., 2012; Tucker et al., 2004), or buprenorphine and methadone (Kakko et al., 2007; Mannelli et al., 2012).
Rates of other drug use and ASI composite scores (Table 1) were comparable with those in other studies of opioid addicted individuals at time of treatment entry (Back et al., 2011). Of interest was the high retention among subjects with multiple drug use, most of whom were African Americans. Also of note was that in the 4 weeks after injection, urine test results found that not only use of opioids but also use of other drugs was reduced, a finding consistent with the degree of improvement seen in ASI scores. Among others, significant improvement in ASI-rated psychiatric symptoms shows that that similar problems did not affect the course of treatment in this group of patients. However, symptoms such as anxiety and depression were not assessed in the early stages of the protocol and should be monitored in future studies in conjunction with the risk of overdose. Of particular interest was that combining up to 1 mg NTX per day with a low dose of BUP, and the administration of 2 to 6 mg of NTX 24 hours after last BUP dose (Table 2) were followed by clinically insignificant changes in withdrawal, no increase in drug use, and no drop-out. Study limitations include the small sample size, self-selected nature of participants, and open label design without a control group. Patients were compensated for their participation and received individualized protocols of ancillary medications. These common procedures may have affected the results, though compensation facilitated data collection but did not influence buprenorphine treatment outcome in one study (Wilcox et al., 2012), and the use of adjuvant drugs did not modify the results of another buprenorphine detoxification trial (Hillhouse et al., 2011).
In conclusion, this pilot study suggests that very low, gradually increasing doses of naltrexone, combined with low and decreasing doses of buprenorphine-naloxone, can result in successful outpatient induction onto XR-NTX among opioid addicted individuals that are interested in XR-NTX. If replicated in double-blind, placebo controlled investigations with adequate number of participants, this method may be a way to safely and effectively make the transition from opioid use to antagonist treatment in outpatient settings, and could be especially important for patients that do not have access to inpatient services.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adi Y, Juarez-Garcia A, Wang D, Jowett S, Frew E, Day E, Bayliss S, Roberts T, Burls A. Oral naltrexone as a treatment for relapse prevention in formerly opioid-dependent drug users: a systematic review and economic evaluation. Health Technol Assess. 2007;11:iii–iv. 1–85. doi: 10.3310/hta11060. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders. 4. American Psychiatric Association; Washington, DC: 2000. text rev. [Google Scholar]
- Back SE, Payne RL, Wahlquist AH, Carter RE, Stroud Z, Haynes L, Hillhouse M, Brady KT, Ling W. Comparative profiles of men and women with opioid dependence: results from a national multisite effectiveness trial. Am J Drug Alcohol Abuse. 2011;37:313–323. doi: 10.3109/00952990.2011.596982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg T, Greenwald M, Johnson R, Liebson I, Bigelow G, Stitzer M. Buprenorphine’s physical dependence potential: antagonist-precipitated withdrawal in humans. J Pharm Exp Thera. 1996;276:449–459. [PubMed] [Google Scholar]
- First M, Spitzer RMG, Williams J. Structured Clinical Interview for Axis I DSM-IV Disorders--Patient Edition. Washington, DC: 1994. [Google Scholar]
- Gonzalez J, Brogden R. Naltrexone: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the management of opioid dependence. Drugs. 1988;35:192–213. doi: 10.2165/00003495-198835030-00002. [DOI] [PubMed] [Google Scholar]
- Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293–308. doi: 10.3109/00952998709001515. [DOI] [PubMed] [Google Scholar]
- Hillhouse M, Canamar CP, Doraimani G, Thomas C, Hasson A, Ling W. Participant characteristics and buprenorphine dose. Am J Drug Alcohol Abuse. 2011;37:453–459. doi: 10.3109/00952990.2011.596974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RE. Buprenorphine: clinical use from maintenance to special populations. Res Clin Forums. 2001;23:25–41. [Google Scholar]
- Kakko J, Gronbladh L, Svanborg KD, von Wachenfeldt J, Ruck C, Rawlings B, Nilsson LH, Heilig M. A stepped care strategy using buprenorphine and methadone versus conventional methadone maintenance in heroin dependence: a randomized controlled trial. Am J Psychiatry. 2007;164:797–803. doi: 10.1176/ajp.2007.164.5.797. [DOI] [PubMed] [Google Scholar]
- Kleber HD. Pharmacologic treatments for opioid dependence: detoxification and maintenance options. Dialogues Clin Neurosci. 2007;9:455–470. doi: 10.31887/DCNS.2007.9.2/hkleber. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Krystal JH, Charney DS, Price LH, Morgan CH, Kleber HD. Opioid antagonist challenges in buprenorphine maintained patients. Drug Alcohol Depend. 1990;25:73–78. doi: 10.1016/0376-8716(90)90144-4. [DOI] [PubMed] [Google Scholar]
- Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence. Lancet. 2011;378:665. doi: 10.1016/S0140-6736(11)61331-7. author reply 666. [DOI] [PubMed] [Google Scholar]
- Krupitsky E, Zvartau E, Blokhina E, Verbitskaya E, Wahlgren V, Tsoy-Podosenin M, Bushara N, Burakov A, Masalov D, Romanova T, Tyurina A, Palatkin V, Slavina T, Pecoraro A, Woody GE. Randomized trial of long-acting sustained-release naltrexone implant vs oral naltrexone or placebo for preventing relapse to opioid dependence. Arch Gen Psychiatry. 2012;69:973–981. doi: 10.1001/archgenpsychiatry.2012.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupitsky EM, Zvartau EE, Masalov DV, Tsoi MV, Burakov AM, Egorova VY, Didenko TY, Romanova TN, Ivanova EB, Bespalov AY, Verbitskaya EV, Neznanov NG, Grinenko AY, O’Brien CP, Woody GE. Naltrexone for heroin dependence treatment in St. Petersburg, Russia. J Subst Abuse Treat. 2004;26:285–294. doi: 10.1016/j.jsat.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Krupitsky EM, Zvartau EE, Masalov DV, Tsoy MV, Burakov AM, Egorova VY, Didenko TY, Romanova TN, Ivanova EB, Bespalov AY, Verbitskaya EV, Neznanov NG, Grinenko AY, O’Brien CP, Woody GE. Naltrexone with or without fluoxetine for preventing relapse to heroin addiction in St. Petersburg, Russia. J Subst Abuse Treat. 2006;31:319–328. doi: 10.1016/j.jsat.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Mannelli P, Gottheil E, Buonanno A, De Risio S. Use of very low-dose naltrexone during opiate detoxification. J Addict Dis. 2003;22:63–70. doi: 10.1300/J069v22n02_05. [DOI] [PubMed] [Google Scholar]
- Mannelli P, Patkar AA, Peindl K, Gorelick DA, Wu LT, Gottheil E. Very low dose naltrexone addition in opioid detoxification: a randomized, controlled trial. Addict Biol. 2009;14:204–213. doi: 10.1111/j.1369-1600.2008.00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannelli P, Peindl KS, Lee T, Bhatia KS, Wu LT. Buprenorphine-mediated transition from opioid agonist to antagonist treatment: state of the art and new perspectives. Curr Drug Abuse Rev. 2012;5:52–63. doi: 10.2174/1874473711205010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannelli P, Peindl KS, Wu LT. Pharmacological enhancement of naltrexone treatment for opioid dependence: a review. Subst Abuse Rehab. 2011;2011:113–123. doi: 10.2147/SAR.S15853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Mercer D, Woody G. Community-Friendly Individual Drug Counseling. Center for Psychotherapy Research; Philadelphia, PA: 2004. [Google Scholar]
- Metzger DS, Woody GE, O’Brien CP. Drug treatment as HIV prevention: a research update. J Acquire Immune Defic Syndr. 2010;55(Suppl 1):S32–36. doi: 10.1097/QAI.0b013e3181f9c10b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst Rev. 2011:CD001333. doi: 10.1002/14651858.CD001333.pub2. [DOI] [PubMed] [Google Scholar]
- Miotto K, McCann MJ, Rawson RA, Frosch D, Ling W. Overdose, suicide attempts and death among a cohort of naltrexone-treated opioid addicts. Drug Alcohol Depend. 1997;45:131–134. doi: 10.1016/s0376-8716(97)01348-3. [DOI] [PubMed] [Google Scholar]
- Oliva EM, Maisel NC, Gordon AJ, Harris AH. Barriers to use of pharmacotherapy for addiction disorders and how to overcome them. Curr Psych Rep. 2011;13:374–381. doi: 10.1007/s11920-011-0222-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter AJ. Naltrexone in the treatment of heroin dependence: relationship with depression and risk of overdose. Aust N Z J Psychiatry. 2002;36:224–228. doi: 10.1046/j.1440-1614.2002.01012.x. [DOI] [PubMed] [Google Scholar]
- Roman PM, Abraham AJ, Knudsen HK. Using medication-assisted treatment for substance use disorders: evidence of barriers and facilitators of implementation. Addict Behav. 2011;36:584–589. doi: 10.1016/j.addbeh.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSA. Data on Substance Abuse Treatment Facilities (SMA) 12–4730. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2012. National Survey of Substance Abuse Treatment Services (N-SSATS): 2011. [Google Scholar]
- SAMHSA. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings (SMA) 13–4795) Substance Abuse and Mental Health Services Administration; Rockville, MD: 2013. [Google Scholar]
- Sigmon SC, Bisaga A, Nunes EV, O’Connor PG, Kosten T, Woody G. Opioid detoxification and naltrexone induction strategies: recommendations for clinical practice. Am J Drug Alcohol Abuse. 2012;38:187–199. doi: 10.3109/00952990.2011.653426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing selfreported alcohol consumption. In: Litten RZ, Allen J, editors. Measuring Alcohol Consumption: Psychosocial and Biological Methods. Humana Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Tucker T, Ritter A, Maher C, Jackson H. Naltrexone maintenance for heroin dependence: uptake, attrition and retention. Drug Alcohol Rev. 2004;23:299–309. doi: 10.1080/09595230412331289464. [DOI] [PubMed] [Google Scholar]
- Umbricht A, Montoya ID, Hoover DR, Demuth KL, Chiang CT, Preston KL. Naltrexone shortened opioid detoxification with buprenorphine. Drug Alcohol Depend. 1999;56:181–190. doi: 10.1016/s0376-8716(99)00033-2. [DOI] [PubMed] [Google Scholar]
- Vivitrol®. [accessed 12/18/13];Vivitrol® US prescribing information. 2013 From web page: http://www.vivitrol.com/hcptreating/treating.
- Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS) J Psychoactive Drugs. 2003;35:253–259. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]
- Wilcox CE, Bogenschutz MP, Nakazawa M, Woody GE. Compensation effects on clinical trial data collection in opioid-dependent young adults. Am J Drug Alcohol Abuse. 2012;38:81–86. doi: 10.3109/00952990.2011.600393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody GE, Metzger DS. Injectable extended-release naltrexone for opioid dependence. Lancet. 2011;378:664–665. doi: 10.1016/S0140-6736(11)61330-5. [DOI] [PubMed] [Google Scholar]