Abstract
The NKG2D cell receptor and its ligands have attracted considerable interest as a potential strategy to attack tumor cells. NKG2D ligands are expressed on most types of tumors, and they demonstrate relative selectivity of ligand expression on tumor cells compared with healthy cells. Several different variants of NKG2D-based chimeric antigen receptors (CAR) have been developed and extensive in vivo mechanistic studies performed demonstrating that cytotoxicity and cytokines are important for the efficacy NKG2D CAR adoptive T cell therapy. NKG2D CARs target tumor cells and they also target immunosuppressive cells within the tumor microenvironment. Under certain conditions, NKG2D ligand expression can be found on non-tumor tissue, so potential off-tumor toxicity remain. In this article, we review the use of NKG2D as a basis for CAR targeting of tumors.
Keywords: chimeric antigen receptor, immunotherapy, NK cells, T cells, cytokines, toxicity, immunosuppression, adoptive cell therapy
Introduction
Effector Cells of the immune system have the potential to attack and eradicate cancer cells in patients, but successful cellular therapy requires a sufficient number of tumor-specific effector cells in vivo. One approach to achieve this is through the use of chimeric antigen receptors (CAR) that combine specific antigen recognition with signaling capability. CARs can be expressed in lymphocytes, using viral transduction or mRNA transfection, to create a large number of antigen-specific cells that upon binding to antigen-expressing cells mediate cytotoxicity and cytokine production. Enrichment and cell expansion techniques produce >109 T cells specific for a targeted molecule. A CARs' specificity is often based on antibody scFv regions or TCR binding domains, but NK cell receptors have also been used. These ligand binding domains are combined using recombinant DNA technology with extracellular, transmembrane, and signaling domains from other cell proteins, including CD8, CD28, 4-1BB, or OX40. A primary signaling domain is included from CD3ζ or FcRγ, which induces lymphocyte activation when the CAR binds to its specific ligand 1,2. NK cells have the ability to recognize different types of tumor cells through various cell surface receptors. The ligands recognized by NK cell receptors are found on a different tumor types, providing attractive candidates for tumor-targeting. The NK cell receptor NKG2D and its ligands have attracted considerable interest as a potential strategy to attack tumor cells. NKG2D ligands are expressed on most types of tumor cells, and they demonstrate relative selectivity of ligand expression on tumor cells compared with healthy cells 3. In this article, we review the use of NKG2D as a basis for CAR targeting of tumors.
NKG2D and its Ligands
There are six to eight NKG2D ligands in humans and mice, with significant differences between ligands within a species. NKG2D ligands in non-human primates are quite different from those in humans, although the extent of cross-reactivity of NKG2D and its ligands among species is not known. Since NKG2D ligands are expressed on various types of tumor cells and immunosuppressive cells (e.g. Tregs and myeloid-derived suppressor cells (MDSCs) within tumor microenvironments, these ligands provide attractive targets for cancer therapy. In fact, most human tumor cells express NKG2D ligands, including: carcinomas (ovarian, bladder, breast, lung, liver, colon, kidney, prostate, melanoma, Ewing's sarcoma, glioma, and neuroblastoma), various leukemias (AML, CML, CLL), lymphomas, and multiple myeloma 3. NKG2D ligands can be induced at sites of chronic inflammation, transiently after some infections, and following local irradiation (See Spear, et al. 3) for a more detailed review of NKG2D targeting). Thus, NKG2D-based CARs target a large number of tumor types, independent of MHC expression.
A variety of immune cells express NKG2D receptors, including NK cells, NKT cells, γδ T cells, CD8+ T cells, and a subset of CD4+ T cells 4,5. There are differences between species' expression of the NKG2D receptor. For example, human CD8+ T cells constitutively express NKG2D whereas only activated murine CD8+ T cells express NKG2D. NKG2D signals differently in NK cells than in T cells. NK cells use either Dap12 (via Syk) or Dap10 (via PI3-kinase) to signal via NKG2D, while T cells only use Dap10 6. Triggering through NKG2D leads to both cytotoxicity and cytokine release from NK cells. However, Dap10 provides a co-stimulation signal to T cells rather than a primary activation signal.
NKG2D CARs
NKG2D-based CAR therapy was first reported in 2005, and these results demonstrated that an NKG2D CAR was effective in murine models and against human tumor cells 7,8. A series of studies from our research group established that NKG2D CAR therapy is effective against a number of tumor types, including multiple myeloma, ovarian carcinoma, and lymphoma in vitro and in vivo 9-12. In addition, detailed molecular mechanisms have been elucidated demonstrating that cellular cytotoxicity, cytokine production, and the host immune response are critical for the efficacy of this NKG2D CAR therapy 9,13-16. An additional benefit was that the NKG2D CAR not only recognized tumor cells, but the CAR also recognized NKG2D ligands expressed on immunosuppressive cells, such as MDSCs and Tregs, and endothelial cells within the tumor microenvironment 13,17,18. In the past two years, three additional laboratories have used NKG2D based CARs directed against human tumor cells. (Figure 1). In these studies, NKG2D CAR T cells killed tumor cells and produced numerous cytokines in response to human cell lines derived from Ewings sarcoma, ovarian carcinoma, prostate carcinoma, osteosarcoma and a variety of other tumor cell lines. Co-stimulation was provided by Dap10, 4-1BB, or CD28 19-21.
Figure 1. NKG2D based CARs.
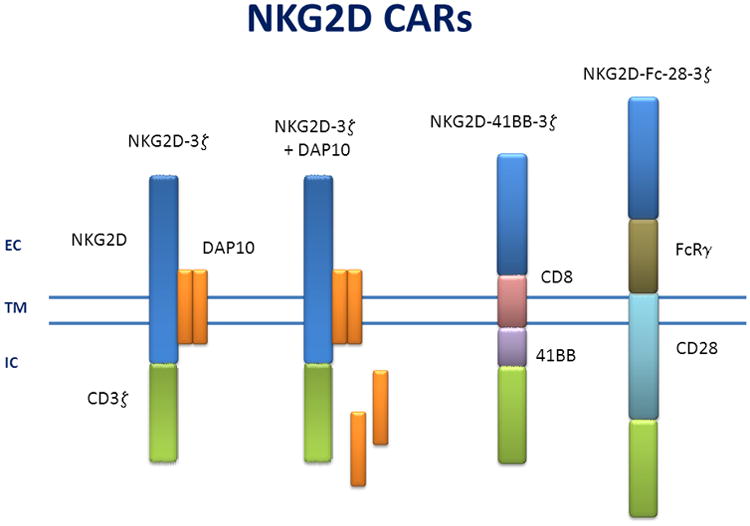
The different variations of NKG2D based CARs are shown. These CARs are believed to associate as dimers at the cell surface. EC: extra-cellular; TM: transmembrane; IC: intra-cellular. NKG2D-3z (7,8), NKG2D-3z +DAP10 (19), NKG2D-41BB-3z (21), NKG2D-Fc-28-3z (20).
The full length NKG2D is used in two CAR designs and associates with Dap10 (Figure 1). These CAR designs result in no foreign extracellular domain that can be recognized by the host immune system, in contrast to CARs that involve scFv, TCR, or other fused extracellular proteins. The cytoplasmic portion of CD3z is attached in a reverse orientation because NKG2D is a type II protein. The association with DAP10, which occurs with endogenous NKG2D, provides a co-stimulation signal via PI3-kinase and AKT that promotes Th1-type cytokines and inhibits the production of Th2 cytokines in CD8+ T cells 22. Effector cells that express these DAP10 associating CARs produce IFNγ, GM-CSF, various chemokines, and occasionally TNFα, but minimal amounts of IL-5, IL-9, IL-10, or other cytokines 9,10,19. The construct used by Chang et al. was expressed in activated NK cells rather than T cells. Even though NK cells normally express NKG2D, these CAR-expressing NK cells demonstrated greater cytotoxicity and produced more cytokines, including IL-13, than mock-transduced NK cells. NK cell function is regulated by positive and negative signals through a variety of activating and inhibitory receptors, respectively 6. Expression of a CAR in NK cells greatly enhanced NK cells' activity beyond the endogenous NKG2D receptor alone 19.
Two additional NKG2D CAR designs have utilized CD28 or 4-1BB signaling platforms. Because NKG2D is a type II protein, while the other proteins in these constructs are type I proteins, the ligand binding portion of NKG2D (aa 81/82 - 216) was connected to a transmembrane portion of the platform in a reverse orientation that maintained the ligand binding function yet allowed expression as a type I protein 20,21. T cells expressing these CARs produced IFN-γ or TNFα upon stimulation with tumor cell lines or primary ovarian cancer specimens. The CAR effector cells were highly cytotoxic, and cytotoxicity was observed using both CD4+ T cells and CD8+ T cells in vitro. Analysis of the cytokines produced by these T cells has not been published, so it is possible that these NKG2D CARs produce different cytokines, such as IL-2 or IL-5, compared to the DAP10 associated NKG2D CARs based on the function of CD28 and 4-1BB signaling domains. These cytokines have been observed from T cells using CARs with these signaling domains 23,24. Cytokines appear to be a key mechanism used by NKG2D CAR T cells to change the tumor microenvironment and to induce host anti-tumor immunity. Thus, it would be interesting to determine how these different NKG2D CAR designs eliminate tumors in vivo and whether differential cytokine production yields unique outcomes in various tumor models and in human cancer.
CD19 CARs that utilize CD28 or 4-1BB costimulatory domains expand greatly in vivo. It would be expected that NKG2D CARs that use similar signaling domains will have similar cell expansion in vivo. This massive T cell expansion can result in a large percentage of T cells in the blood expressing the CAR 25. In contrast, the NKG2D CAR that associates with DAP10 does not survive long in vivo in animal studies 12. CAR down-regulation has been reported, and endogenous NKG2D may be down-regulated in the presence of cytokines or soluble ligands 26-28. However, NKG2D CAR inhibition does not occur under physiological concentrations of soluble recombinant ligands or patient sera 8,20. Membrane bound ligands down-regulated NKG2D on NK cells, but a NKG2D CAR was not down-regulated when it was expressed under the control of a lentiviral promoter but only when the CAR was expressed using mRNA in T cells 20. Thus viral transduction of a NKG2D CAR may not be readily inhibited by exposure to soluble ligands or tumor cells that demonstrate high expression of its ligands.
Potential Toxicity Associated with NKG2D – based CARs
NKG2D based CARs have the potential to recognize approximately 90% of human tumor types, but these ligands are also induced under a variety of physiological circumstances which raises concerns about “on-target off-tumor” toxicity. The normal physiologic expression of NKG2D ligands in humans is unknown. Acute exposure to certain microbial components (e.g. LPS) may induce transient ligand expression, although some of these results are based on experimental systems that may not reflect human tissue physiology. Chronic inflammation, such as observed in the joints of patients with rheumatoid arthritis, is associated with expression of NKG2D ligands on synoviocytes 29. Activation of DNA repair mechanisms involving ATM/ATR repair pathways induce ligand expression 30. Similar mechanisms are likely responsible for the observation that most tumor cells and other cells within the tumor microenvironment express NKG2D ligands. Malignant cells in patients express varying amounts of ligands. For example, tumor cells in patients with advanced cancer demonstrate different amounts of ligand expression compared with patients with limited stages of cancer, or compared with normal individuals 31,32. Thus treatments that target NKG2D ligands will need to be used with caution until the extent of ligand expression on normal tissue cells is known. However, large numbers of activated lymphocytes (>109 cells), which express NKG2D and can recognize NKG2D ligand expressing cells (e.g. NKT cells, gd T cells, NK cells), have been infused into patients with little toxicity 33-36.
CAR therapies have been developed as cell transplants, and results support the concept that the longer CAR T lymphocytes survive in vivo, the better the clinical outcome 2,37. CARs based on CD28 or 4-1BB co-stimulation domains result in cell expansion and long-term effector T cell survival in vivo, and this is believed to be one reason for the beneficial clinical outcomes reported for CD19-specific CAR therapy. However, NKG2D ligands can be induced on different cell types, so long-lived NKG2D CAR effector cells pose a risk for toxicity against non-tumor cells. CARs recognizing molecules that are not strictly limited to tumor cells can result in significant toxicity, suggesting that maintaining CAR T cells for a long period of time in vivo may not be always be an optimal approach to follow 38-40. Toxicity from CAR T cell therapy may be caused by different responses (e.g. cytotoxicity against healthy cells, cytokine storm), but these may be managed and potentially prevented 41. The NKG2D CAR based on the full-length NKG2D protein does not appear to induce long-term CAR T cell survival, which may be a valuable trait to avoid toxicity with these NKG2D CAR cells. Recent evidence indicated that patients demonstrated remarkable tumor regression within a few weeks following the infusion of CAR T cells, so it may be that long-term persistence of CAR-bearing cells is not required to demonstrate clinical benefits 24. Thus, it is possible that administering CAR T cells as “cellular drugs” rather than as cell transplants may be an effective cancer treatment approach for some targets, although this approach may require multiple cellular infusions to demonstrate maximal efficacy.
Why Not Just Use NK Cells?
If NKG2D can be used to target tumor cells and NK cells express high amounts of NKG2D that can trigger cytotoxicity, then why not just infuse NK cells? This is an excellent question without a clear answer. NK cells use NKG2D, among other receptors, to recognize and activate their effector functions in the presence of tumor cells, yet the infusion of a large number of activated NK cells into patients has failed to demonstrate robust clinical responses in many patients 34,42. The role and potential of NK cells in cancer therapy is beyond the scope of this review and is reviewed elsewhere 43. Clinical data have shown that a large number of NK cells can be given to patients with little toxicity, but the anti-tumor effects have been modest. NK cells express a number of inhibitory receptors that bind to MHC class I and other molecules, and NK cells are sensitive to a variety of inhibitory molecules found within the tumor microenvironment 6,44. NK cells may be a useful tool against selected tumor types, but NK cells have not yet provided consistent anti-tumor effects. New approaches that use cytokines to stimulate NK cells, such as IL-15 or IL-18, may result in better efficacy after NK cell infusion. In fact, NK cells' anti-tumor efficacy can be enhanced by transduction of an NKG2D CAR into NK cells 19.
Is It Possible To Induce NKG2D Ligands?
One possible way to improve targeting tumor cells through NKG2D would be to increase the expression of NKG2D ligands on malignant cells or at the tumor site. Localized irradiation induces expression of NKG2D ligands within tumors 45. In addition, several drugs increase NKG2D ligand expression on tumor cells 46-48. Bortezamib (a proteosome inhibitor) increases NKG2D ligand expression on myeloma cells. Histone deacetylease (HDAC) inhibitors increase ligand expression on tumor cells but not on normal blood cells 19. Thus, it is possible that treatment with several medications could be used to increase ligand expression on various tumor cell types, however the induction of ligands on normal cells must be avoided to prevent unwanted toxicity.
Conclusions
NKG2D-based CARs are a novel strategy to target different types of tumors, while inducing potent anti-tumor immunity in patients. The development of CARs based on full-length NKG2D or on the NKG2D ligand binding domain demonstrates the potential to target effector cells against ligand expressing tumor cells. The results indicate that cytotoxicity against tumor cells is important but that cytokines produced by NKG2D CAR T cells are also critical for complete tumor cell eradication and long-term survival in animal models. Unlike many targeting strategies, NKG2D targets tumor cells, immunosuppressive cells, and other cells in the microenvironment that support tumor survival and progression. This multi-pronged attack on the tumor microenvironment may be one reason that NKG2D targeting is efficacious against several different types of tumors and induces host anti-tumor immunity, even though these CAR T cells do not survive long-term in vivo. Because of the potential expression of these ligands on normal cells, there are concerns about potential “off-tumor, on-target” toxicity that must be addressed. There is great potential for NKG2D CARs to improve patient health. The initial clinical results will be vital to demonstrate that the benefits are sufficient and the potential risks manageable.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (CA130911, CA164178). The views in this paper reflect the authors' opinions and do not necessarily reflect the opinions of the National Institutes of Health.
Footnotes
Conflict of Interest Statement: NKG2D CAR technology has been developed by Dr. Sentman and it is licensed by Celdara Medical, LLC. Dr. Sentman and Celdara are developing the technology for clinical use, for which he receives compensation. These activities are in full compliance with the policies of Dartmouth College.
References
- 1.Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3:388–98. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.June CH. Principles of adoptive T cell cancer therapy. Journal of Clinical Investigation. 2007;117:1204–12. doi: 10.1172/JCI31446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spear P, Wu MR, Sentman ML, Sentman CL. NKG2D ligands as therapeutic targets. Cancer Immun. 2013;13:8. [PMC free article] [PubMed] [Google Scholar]
- 4.Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 2002;17:19–29. doi: 10.1016/s1074-7613(02)00333-3. [DOI] [PubMed] [Google Scholar]
- 5.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–90. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 6.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–74. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 7.Zhang T, Lemoi BA, Sentman CL. Chimeric NK-receptor-bearing T cells mediate antitumor immunotherapy. Blood. 2005;106:1544–51. doi: 10.1182/blood-2004-11-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang T, Barber A, Sentman CL. Generation of antitumor responses by genetic modification of primary human T cells with a chimeric NKG2D receptor. Cancer Research. 2006;66:5927–33. doi: 10.1158/0008-5472.CAN-06-0130. [DOI] [PubMed] [Google Scholar]
- 9.Zhang T, Barber A, Sentman CL. Chimeric NKG2D modified T cells inhibit systemic T-cell lymphoma growth in a manner involving multiple cytokines and cytotoxic pathways. Cancer Res. 2007;67:11029–36. doi: 10.1158/0008-5472.CAN-07-2251. [DOI] [PubMed] [Google Scholar]
- 10.Barber A, Zhang T, DeMars LR, Conejo-Garcia J, Roby KF, Sentman CL. Chimeric NKG2D receptor-bearing T cells as immunotherapy for ovarian cancer. Cancer Res. 2007;67:5003–8. doi: 10.1158/0008-5472.CAN-06-4047. [DOI] [PubMed] [Google Scholar]
- 11.Barber A, Zhang T, Megli CJ, Wu J, Meehan KR, Sentman CL. Chimeric NKG2D receptor-expressing T cells as an immunotherapy for multiple myeloma. Exp Hematol. 2008;36:1318–28. doi: 10.1016/j.exphem.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barber A, Meehan KR, Sentman CL. Treatment of multiple myeloma with adoptively transferred chimeric NKG2D receptor-expressing T cells. Gene Ther. 2011;18:509–16. doi: 10.1038/gt.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barber A, Rynda A, Sentman CL. Chimeric NKG2D expressing T cells eliminate immunosuppression and activate immunity within the ovarian tumor microenvironment. J Immunol. 2009;183:6939–47. doi: 10.4049/jimmunol.0902000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barber A, Sentman CL. Chimeric NKG2D T cells require both T cell- and host-derived cytokine secretion and perforin expression to increase tumor antigen presentation and systemic immunity. J Immunol. 2009;183:2365–72. doi: 10.4049/jimmunol.0900721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spear P, Barber A, Rynda-Apple A, Sentman CL. Chimeric Antigen Receptor T Cells Shape Myeloid Cell Function within the Tumor Microenvironment through IFN-gamma and GM-CSF. J Immunol. 2012;188:6389–98. doi: 10.4049/jimmunol.1103019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spear P, Barber A, CL S. Collaboration of chimeric antigen receptor (CAR) and host T cells for optimal elimination of established ovarian tumors. Oncoimmunology. 2013;2 doi: 10.4161/onci.23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang T, Sentman CL. Cancer immunotherapy using a bi-specific NK receptor- fusion protein that engages both T cells and tumor cells. Cancer Research. 2011;71:2066–76. doi: 10.1158/0008-5472.CAN-10-3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang T, Sentman CL. Mouse tumor vasculature expresses NKG2D ligands and can be targeted by chimeric NKG2D-modified T cells. J Immunol. 2013;190:2455–63. doi: 10.4049/jimmunol.1201314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang YH, Connolly J, Shimasaki N, Mimura K, Kono K, Campana D. A chimeric receptor with NKG2D specificity enhances natural killer cell activation and killing of tumor cells. Cancer Res. 2013;73:1777–86. doi: 10.1158/0008-5472.CAN-12-3558. [DOI] [PubMed] [Google Scholar]
- 20.Lehner M, Gotz G, Proff J, et al. Redirecting T Cells to Ewing's Sarcoma Family of Tumors by a Chimeric NKG2D Receptor Expressed by Lentiviral Transduction or mRNA Transfection. PLoS One. 2012;7:e31210. doi: 10.1371/journal.pone.0031210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song DG, Ye Q, Santoro S, Fang C, Best A, Powell DJ., Jr Chimeric NKG2D CAR-expressing T cell-mediated attack of human ovarian cancer is enhanced by histone deacetylase inhibition. Hum Gene Ther. 2013;24:295–305. doi: 10.1089/hum.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barber A, Sentman CL. NKG2D receptor regulates human effector T-cell cytokine production. Blood. 2011;117:6571–81. doi: 10.1182/blood-2011-01-329417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song H, Hur DY, Kim KE, et al. IL-2/IL-18 prevent the down-modulation of NKG2D by TGF-β in NK cells via the c-Jun N-terminal kinase (JNK) pathway. Cellular Immunology. 2006;242:39–45. doi: 10.1016/j.cellimm.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol. 2009;182:240–9. doi: 10.4049/jimmunol.182.1.240. [DOI] [PubMed] [Google Scholar]
- 28.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–8. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 29.Groh V, Bruhl A, El-Gabalawy H, Nelson JL, Spies T. Stimulation of T cell autoreactivity by anomalous expression of NKG2D and its MIC ligands in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2003;100:9452–7. doi: 10.1073/pnas.1632807100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–90. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holdenrieder S, Stieber P, Peterfi A, Nagel D, Steinle A, Salih HR. Soluble MICA in malignant diseases. Int J Cancer. 2006;118:684–7. doi: 10.1002/ijc.21382. [DOI] [PubMed] [Google Scholar]
- 32.Carbone E, Neri P, Mesuraca M, et al. HLA class I, NKG2D, and natural cytotoxicity receptors regulate multiple myeloma cell recognition by natural killer cells. Blood. 2005;105:251–8. doi: 10.1182/blood-2004-04-1422. [DOI] [PubMed] [Google Scholar]
- 33.Meehan KR, Talebian L, Tosteson TD, et al. Adoptive cellular therapy using cells enriched for NKG2D+CD3+CD8+T cells after autologous transplantation for myeloma. Biol Blood Marrow Transplant. 2013;19:129–37. doi: 10.1016/j.bbmt.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–7. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 35.Abe Y, Muto M, Nieda M, et al. Clinical and immunological evaluation of zoledronate-activated Vgamma9gammadelta T-cell-based immunotherapy for patients with multiple myeloma. Exp Hematol. 2009;37:956–68. doi: 10.1016/j.exphem.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Nakajima J, Murakawa T, Fukami T, et al. A phase I study of adoptive immunotherapy for recurrent non-small-cell lung cancer patients with autologous gammadelta T cells. Eur J Cardiothorac Surg. 2010;37:1191–7. doi: 10.1016/j.ejcts.2009.11.051. [DOI] [PubMed] [Google Scholar]
- 37.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–93. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morgan RA, Chinnasamy N, Abate-Daga D, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother. 2013;36:133–51. doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case Report of a Serious Adverse Event Following the Administration of T Cells Transduced With a Chimeric Antigen Receptor Recognizing ERBB2. Molecular Therapy. 2010;18:843–51. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Linette GP, Stadtmauer EA, Maus MV, et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013;122:863–71. doi: 10.1182/blood-2013-03-490565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sentman CL. Challenges of creating effective chimeric antigen receptors for cancer therapy. Immunotherapy. 2013;5:783–5. doi: 10.2217/imt.13.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA. Adoptive Transfer of Autologous Natural Killer Cells Leads to High Levels of Circulating Natural Killer Cells but Does Not Mediate Tumor Regression. Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-11-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol. 2012;12:239–52. doi: 10.1038/nri3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar V, McNerney ME. A new self: MHC-class-I-independent natural-killer-cell self-tolerance. Nat Rev Immunol. 2005;5:363–74. doi: 10.1038/nri1603. [DOI] [PubMed] [Google Scholar]
- 45.Ruocco MG, Pilones KA, Kawashima N, et al. Suppressing T cell motility induced by anti-CTLA-4 monotherapy improves antitumor effects. J Clin Invest. 2012;122:3718–30. doi: 10.1172/JCI61931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skov S. Cancer Cells Become Susceptible to Natural Killer Cell Killing after Exposure to Histone Deacetylase Inhibitors Due to Glycogen Synthase Kinase-3-Dependent Expression of MHC Class I-Related Chain A and B. Cancer Research. 2005;65:11136–45. doi: 10.1158/0008-5472.CAN-05-0599. [DOI] [PubMed] [Google Scholar]
- 47.Vales-Gomez M, Chisholm SE, Cassady-Cain RL, Roda-Navarro P, Reyburn HT. Selective induction of expression of a ligand for the NKG2D receptor by proteasome inhibitors. Cancer Res. 2008;68:1546–54. doi: 10.1158/0008-5472.CAN-07-2973. [DOI] [PubMed] [Google Scholar]
- 48.Armeanu S, Bitzer M, Lauer UM, et al. Natural killer cell-mediated lysis of hepatoma cells via specific induction of NKG2D ligands by the histone deacetylase inhibitor sodium valproate. Cancer Res. 2005;65:6321–9. doi: 10.1158/0008-5472.CAN-04-4252. [DOI] [PubMed] [Google Scholar]


