Abstract
Often experimentalists require a quantitative assessment of the levels of heterologously expressed proteins to best interpret changed Ca2+ signaling patterns. Here, we detail a rapid and convenient western blotting method for individual Xenopus oocytes. The method exploits recently introduced rapid blotting systems, commercially available from Invitrogen (iBlot) or Bio-Rad (Trans-Blot Turbo). The key advantage is speed: from live cell to transferred membrane in <1 h. Therefore, oocytes can be conveniently processed for western blotting to assess relative expression levels, even after a long day of Ca2+ imaging experiments.
MATERIALS
It is essential that you consult the appropriate Material Safety Data Sheets and your institution’s Environmental Health and Safety Office for proper handling of equipment and hazardous materials used in this protocol.
RECIPE: Please see the end of this article for recipes indicated by <R>. Additional recipes can be found online at http://cshprotocols.cshlp.org/site/recipes.
Reagents
Bradford protein assay kit
Electrophoresis gels, precast (e.g., Mini-PROTEAN TGX gel [Bio-Rad] or NuPAGE Bis-Tris gel [Invitrogen])
-
Homogenization solution (HS)
Prepare a 10× (200 mM) stock solution of HEPES and store at 4°C. Prepare a 1× working stock (20 mM HEPES, pH 7.3) by diluting with autoclaved Milli-Q water. Add protease inhibitors (and phosphatase inhibitor, if needed) immediately before use. Nonfat instant milk powder
Primary and secondary antibodies
Running buffer (e.g., 10× Tris/glycine/SDS [Bio-Rad] or NuPAGE MOPS SDS running buffer [Invitrogen])
Sample buffer containing a reducing agent (e.g., Laemmli sample buffer [Bio-Rad] and 2-mercapto-ethanol [Sigma] or NuPAGE LDS sample buffer and NuPAGE sample reducing agent [Invitrogen])
TBST for western blotting <R>
-
Xenopus oocytes in modified Barth’s solution (MBS)
For isolation and defolliculation procedures, see Nuclear Microinjection to Assess How Heterologously Expressed Proteins Impact Ca2+ Signals in Xenopus Oocytes (Lin-Moshier and Marchant 2013a).
Equipment
Benchtop microcentrifuge
Cut-off micropipette tips (see Step 2)
Gel electrophoresis equipment (e.g., Mini-PROTEAN Tetra Cell [Bio-Rad] or XCell SureLock Mini-Cell [Invitrogen])
Fluorescent imaging system (e.g., LI-COR Infrared Imaging System)
Hybridization bags (RPI)
Impulse sealer
Microcentrifuge tubes
Orbital shaker
Platform rocker
Semi-dry transfer pack and rapid transfer system (e.g., Trans-Blot Turbo [Bio-Rad] or iBlot [Invitrogen])
METHOD
Separate each oocyte for analysis into a small microcentrifuge tube. Carefully remove excess MBS by pipetting.
Prepare a fresh solution of 1× HS supplemented with protease inhibitors (and phosphatase inhibitor, if necessary). Add 20 μL of this solution to every tube. Homogenize each sample by pipetting up and down several times using a cut-off micropipette tip.
-
Remove the abundant yolk platelets by spinning in a benchtop microcentrifuge at 800g for 5 min at room temperature. Transfer the supernatant into a clean, labeled microcentrifuge tube.
At this stage, samples can be frozen and stored at −80°C until processing. -
Use 5 μL of the supernatant to perform a Bradford protein assay.
Determination of the protein concentration permits quantitative comparison between samples when necessary. -
Mix the remaining supernatant (~15 μL) with sample buffer containing a reducing agent (1× final concentration). Boil for 5 min. Chill on ice for 5 min.
This sample will be used for electrophoresis. Open the precast gel packs. Rinse the gel cassettes with distilled water and remove the tape on the bottom of the gel. Place the electrode assembly in the electrophoresis tank and fill with running buffer (1×) to a higher level than the gel wells. Remove the comb. Rinse the wells with running buffer to remove salt precipitates and gel debris. Before loading individual wells, vortex and briefly spin the samples. Carefully load the samples and a protein ladder. Start electrophoresis (150 V). Stop when the dye front runs out of the gel.
Assemble the semi-dry transfer device by placing the transfer pack on the rapid transfer apparatus following the manufacturer’s instructions. Remove a gel cassette from the electrode assembly and rinse with distilled water. Crack open the cassette with a spatula and immediately place the gel on top of the blotting membrane (nitrocellulose/PVDF) on the bottom part of the transfer pack. Place the upper part of the transfer pack onto the gel. Remove any air bubbles with a gel roller. Close the apparatus and start the transfer at 25 V (use Program 1 for iBlot or the Mini-TGX protocol for Trans-Blot Turbo). Transfer takes 3–6 min. Disassemble the transfer pack and remove the membrane.
Block the membrane by incubating in 10% nonfat milk in TBST on an orbital shaker at ~55 rpm for 1 h at room temperature. Rinse the membrane three times in TBST and leave it in TBST while preparing the primary antibody.
Dilute the primary antibody in 5% nonfat milk in TBST (typically 1:200–1:1000). Place the membrane in a hybridization bag and seal on three sides with an impulse sealer. Carefully add the primary antibody solution onto the protein side of the membrane and remove all bubbles before sealing the fourth side. Place the hybridization bag on a platform rocker and incubate for 1 h at room temperature or overnight at 4°C.
Remove the membrane from the hybridization bag and wash three times in TBST for 10 min each. Remove the TBST and incubate the membrane in secondary antibody diluted in 5% nonfat milk in TBST (typically 1:5000) on an orbital shaker in the dark for 1 h at room temperature. Wash the membrane three times in TBST for 10 min each. Detect immunoreactivity using a LI-COR Infrared Imaging System (see Fig. 1) or by a standard enhanced chemiluminescence protocol.
FIGURE 1.
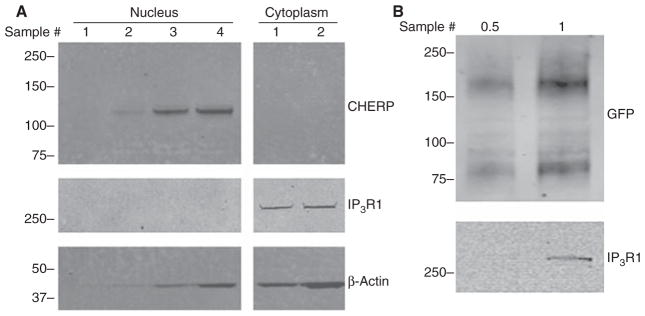
Regional immunoblotting in a Xenopus oocyte. (A) Detection of endogenous proteins in Xenopus oocyte nuclei and cytoplasm. Transferred nuclear (left) and cytoplasmic fractions (right) were probed for Ca2+ homeostasis endoplasmic reticulum protein (CHERP) (top), Xenopus type 1 InsP3 receptor (IP3R1) (middle), and β-actin (bottom). Intact nuclei were isolated by hand from individual oocytes and the remaining cytoplasm was pooled from the indicated number of cells. Western transfer was performed using iBlot. (B) Top panel: Detection of a heterologously expressed, GFP-tagged Ca2+ channel (TPC2-GFP) in a sample prepared from a half (0.5) or a whole (1) Xenopus oocyte. Bottom panel: Blot reprobed for IP3R1 expression. Western transfer was performed using Trans-Blot Turbo. For both blots, secondary antibodies were detected with a LI-COR Infrared Imaging System.
DISCUSSION
In many animal models, running a western blot from oocytes/eggs can be challenging. To detect relatively scarce proteins in mouse oocytes, hundreds of oocytes may be needed for a single lane immunodetection, and a single mouse contains only tens of oocytes (Gangeswaran and Jones 1997). For certain intracellular Ca2+ channel isoforms, extracts from as many as 5000 cells have been needed for immunoprecipitations and western blotting analyses (Ayabe et al. 1995). This concern is obviously less acute in the Xenopus system given the large quantity of protein in a single oocyte (125 μg), and the availability of oocytes (~15% mass of a frog). Immunodetection of expressed gene products in Xenopus oocytes is easily performed at the single cell and/or regional level (Fig. 1) and the importance of such single cell correlations of expression/activity are well evidenced relative to population analyses (Ferrell and Machleder 1998). The recent introduction of rapid western transfer equipment has streamlined procedures for analyzing oocytes used for functional assays (e.g., Ca2+ imaging). Different commercial systems are available, for example iBlot and Trans-Blot Turbo, and both have proved suitable for this purpose, although selection of compatible consumables is important. Both these systems are convenient, easy to use, and show single-cell sensitivity for detecting the transfer of large, endogenous proteins (e.g., Xenopus type 1 InsP3 receptor). The procedure is faster with the Trans-Blot Turbo system (time for electrophoresis and transfer is twice as short) and is more amenable to higher throughput (twice the number of gels can be processed simultaneously) such that the entire procedure is complete in ~1 h.
RELATED INFORMATION
For a general discussion of the utilization of the Xenopus oocyte as a model for investigating Ca2+-permeable channels and transporters, see The Xenopus Oocyte: A Single-Cell Model for Studying Ca2+ Signaling (Lin-Moshier and Marchant 2013b).
RECIPE
TBST for Western Blotting
| Reagent | Final concentration (1×) |
|---|---|
| NaCl | 137 mM |
| KCl | 2.7 mM |
| Tris base | 19 mM |
Prepare a 20× stock of TBS by combining appropriate amounts of the ingredients listed above. Autoclave and store at room temperature. Prepare a working solution of TBS (1×, pH 7.4) by diluting the 20× stock in Milli-Q water. For TBST, add 1 mL of Tween 20 per liter of TBS. Stir constantly until homogenous.
Acknowledgments
This work supported by the National Institutes of Health (GM088790).
References
- Ayabe T, Kopf GS, Schultz RM. Regulation of mouse egg activation: Presence of ryanodine receptors and effects of microinjected ryanodine and cyclic ADP ribose on uninseminated and inseminated eggs. Development. 1995;121:2233–2244. doi: 10.1242/dev.121.7.2233. [DOI] [PubMed] [Google Scholar]
- Ferrell JE, Jr, Machleder EM. The biochemical basis of an all-or-none cell fate switch in Xenopus oocytes. Science. 1998;280:895–898. doi: 10.1126/science.280.5365.895. [DOI] [PubMed] [Google Scholar]
- Gangeswaran R, Jones KT. Unique protein kinase C profile in mouse oocytes: lack of calcium-dependent conventional isoforms suggested by rtPCR and Western blotting. FEBS Lett. 1997;412:309–312. doi: 10.1016/s0014-5793(97)00782-5. [DOI] [PubMed] [Google Scholar]
- Lin-Moshier Y, Marchant JS. Nuclear microinjection to assess how heterologously expressed proteins impact Ca2+ signals in Xenopus oocytes. Cold Spring Harb Protoc. 2013a doi: 10.1101/pdb.prot072785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin-Moshier Y, Marchant JS. The Xenopus oocyte: A single-cell model for studying Ca2+ signaling. Cold Spring Harb Protoc. 2013b doi: 10.1101/pdb.top066308. [DOI] [PMC free article] [PubMed] [Google Scholar]


