Abstract
The neuronal ceroid lipofuscinoses, a family of neurodegenerative lysosomal storage disorders, represent the most common cause of pediatric-onset neurodegeneration. The infantile form has a devastatingly early onset and one of the fastest progressing disease courses. Despite decades of research, the molecular mechanisms driving neuronal loss in infantile neuronal ceroid lipofuscinosis remain unknown. We have previously shown that NMDA-type glutamate receptors in the Ppt1−/− mouse model of this disease exhibit a hyperfunctional phenotype and postulate that aberrant glutamatergic activity may contribute to neural pathology in both the mouse model and human patients. To test this hypothesis, we treated Ppt1−/− mice with the NMDA receptor antagonist memantine and assessed their response to the drug using an accelerating rotarod. At 20 mg/kg, memantine treatment induced a delayed but notable improvement in Ppt1−/− mice. Much remains to be assessed before moving to patient trials, but these results suggest memantine has potential as a treatment.
Keywords: Batten disease, infantile neuronal ceroid lipofuscinosis, memantine, NMDA receptor, rotarod
Introduction
The neuronal ceroid lipofuscinoses are the most common cause of pediatric neurodegeneration worldwide.1 There are 9 different disease variants caused by mutations in 13 identified genes. The clinical presentations of these variants are all very similar, varying primarily in age of onset and speed of progression. Common symptoms include retinal blindness, neurocognitive decline, seizures of increasing severity, and, eventually, premature death. Severe atrophy of defined brain regions and ubiquitous accumulation of autofluorescent storage material are classic pathological hallmarks of these diseases.2
Of the neuronal ceroid lipofuscinoses, infantile neuronal ceroid lipofuscinosis is one of the most devastating variants. Afflicted children are delivered and develop normally until 6 to 18 months of age when retardation of mental development is noted. Shortly after the commencement of mental decline, motor deterioration sets in, as illustrated by ataxia and muscular hypotonia. Seizures manifest around the age of 2, and children succumb to a vegetative state during the third year of life. Death generally results around the age of 10, following a persistent vegetative state.3–4 The only existing treatment options for infantile neuronal ceroid lipofuscinosis or any of the other neuronal ceroid lipofuscinoses are purely palliative.
Infantile neuronal ceroid lipofuscinosis is caused by mutations in the CLN1 gene that encodes palmitoyl protein thioesterase 1 (PPT1),5 an enzyme known to remove palmitate modifications from proteins in vitro.6 Targeted deletion of the murine homolog of this gene has resulted in the creation of a mouse model of infantile neuronal ceroid lipofuscinosis, the Ppt1−/− mouse.7 Examination of the mouse has shown that it reliably recapitulates the human disease course on a pathological level.7–10 The mice also exhibit behavioral phenotypes, including a motor coordination deficit as measured by the rotarod task.10–11 The similarities between the ataxia noted in afflicted patients and the rotarod phenotype manifested in the Ppt1−/− mouse further support the use of this animal as a disease model.
Glutamate is the primary excitatory neurotransmitter used by the mammalian central nervous system,12 and dysfunction of the glutamatergic system has been linked to pathology in many different neurological diseases.13 Indeed, extensive research has shown that glutamatergic activity is disrupted in a number of the neuronal ceroid lipofuscinoses.10,14–23 Recent studies from our lab have further demonstrated this connection by showing that selectively targeting the function of glutamate receptors with pharmacological agents can improve the performance of the Cln3ex1–6 mouse model of juvenile neuronal ceroid lipofuscinosis on the rotarod.21,23–24
We have previously shown that cerebellar granule cells isolated from Ppt1−/− mice have an N-methyl-D-aspartate (NMDA) receptor hyperfunction phenotype.25 As NMDA receptor-mediated excitotoxicity may be driving cell death in the Ppt1−/− mouse, we exposed mice to the uncompetitive NMDA receptor antagonist, memantine, to determine whether attenuation of NMDA receptor activity can improve rotarod performance. As pathological studies have shown that neuronal loss begins relatively early in Ppt1−/− mice, we treated 3- and 5-month-old mice with a single dose of memantine and tested their motor coordination monthly until they reached 7 months of age. At each of those time points, mice were given either 10 mg/kg or 20 mg/kg of memantine; neither dose at either time point produced significant long-term effects. We also treated significantly older mice that had already begun to manifest considerable neurological deficits. No improvement was seen in response to the lower dose administered (10 mg/kg) at this later time point, but a higher dose (20 mg/kg) was found to have a delayed but notable effect on motor learning in the Ppt1−/− mouse.
Methods
Animals
Ppt1−/− mice7 were maintained on the C57BL/6J background. Sex-matched wild-type and Ppt1−/− mice obtained from our in-house breeding colony were used for this study.
Phenotypic Assessment by Rotarod
Mice were transported into the behavior suite, weighed, ear punched, and marked for identification using a permanent marker (King Size Sharpie; Newell Rubbermaid Office Products, Oak Brook, Illinois). Following this process, mice were allowed to acclimate to the ambient conditions of the behavioral suite for at least 20 minutes. During a training period, mice were placed on an accelerating rotarod (Columbus Instruments, Columbus, Ohio) set to accelerate from zero rpm to 40 over the course of 120 seconds. Animals were allowed to “practice” for 2 sets of 2 runs each, separated by a 15-minute rest. After the practice runs, mice rested for one hour and were then subjected to the “test” measurement of motor coordination containing 4 sets of 2 runs each, with a 15-minute rest between each set.
The latencies to fall from the rotarod were recorded for each mouse for each run. In the event that a mouse remained on the rod for the entire 120 seconds, the run was stopped at that point and the animal was given a score of 120. The arithmetic mean of all runs was calculated for each mouse and considered that animal’s latency to fall for each test set.
Starting at 4 weeks of age, mice were subjected to the described “test” protocol every 4 weeks until they reached 28 weeks of age. They were then run at 30 weeks and weekly thereafter until no longer able to complete the task.
Memantine Treatment
At either 3 or 5 months of age, wild-type and Ppt1−/− mice were subjected to the previously described rotarod protocol. Two and a half hours after the end of testing, mice received a single intraperitoneal injection of the NMDA receptor antagonist memantine (Tocris Cookson, Bristol, United Kingdom). The dose was either 10 mg/kg or 20 mg/kg with an injection volume of 10 mL/kg. Control mice were injected with the drug vehicle (sterile filtered physiological saline, 0.9% NaCl). Thirty minutes as well as 1, 4, 7, and 10 days following the injection, mice were again assessed via accelerating rotarod using the same test protocol of 4 sets of 2 runs each, separated by a 15-minute rest. After this initial testing, motor coordination in the mice was reexamined at monthly intervals; every 30 days, the mice were retested on the rotarod (4 sets of 2 runs each, with a 15-minute rest between each set) for 3 consecutive days to evaluate both motor coordination and capacity for motor learning.
Wild-type and Ppt1−/− mice were also treated at a more advanced stage of disease. Thirty-week-old mice were subjected to the above treatment protocol. Two and a half hours after the end of an initial rotarod testing, mice were injected with a single dose of memantine. The dose was either 10 mg/kg or 20 mg/kg with an injection volume of 10 mL/kg. Control mice were injected with the drug vehicle. Thirty minutes as well as 1, 4, 7, 10, 14, 17, and 20 days after the injection, mice were again assessed via the rotarod using the same test protocol of 4 sets of 2 runs each, separated by a 15-minute rest.
Statistical Analysis
One-way and two-way ANOVA were performed in GraphPad Prism 6.01 (GraphPad Software, San Diego, California).
Results
Ppt1−/− Mice Develop a Significant Motor Coordination Phenotype
The existence of a motor coordination deficit as measured by the rotarod task has been well established in the Ppt1−/− mouse.10–11 A prior study reported that the onset of this phenotype becomes visible at 3 months of age and significant at 5.10 As behavioral tests have been reported to be sensitive to user variation and lab location,26 we opted to confirm the age of onset of this motor coordination deficit. Figure 1 shows the relative latency to fall scores from the “Practice” runs as described in the Methods section. Due to low breeding yield at the time of this experiment, we were unable to use different groups of mice for the various time points. As the treatments were done with mice never before exposed to the rotarod task, it was determined that assessment of phenotypic onset would be best done with the training runs. However, with repeated test trials, motor learning also contributes to the performance of the mice. Although the mice had previously been exposed to the rotarod task, using these numbers from the training runs was the closest we could get to a true measure of motor coordination that was not significantly affected by motor learning. The motor coordination deficit became statistically significant at the same time point when the test runs were analyzed (data not shown).
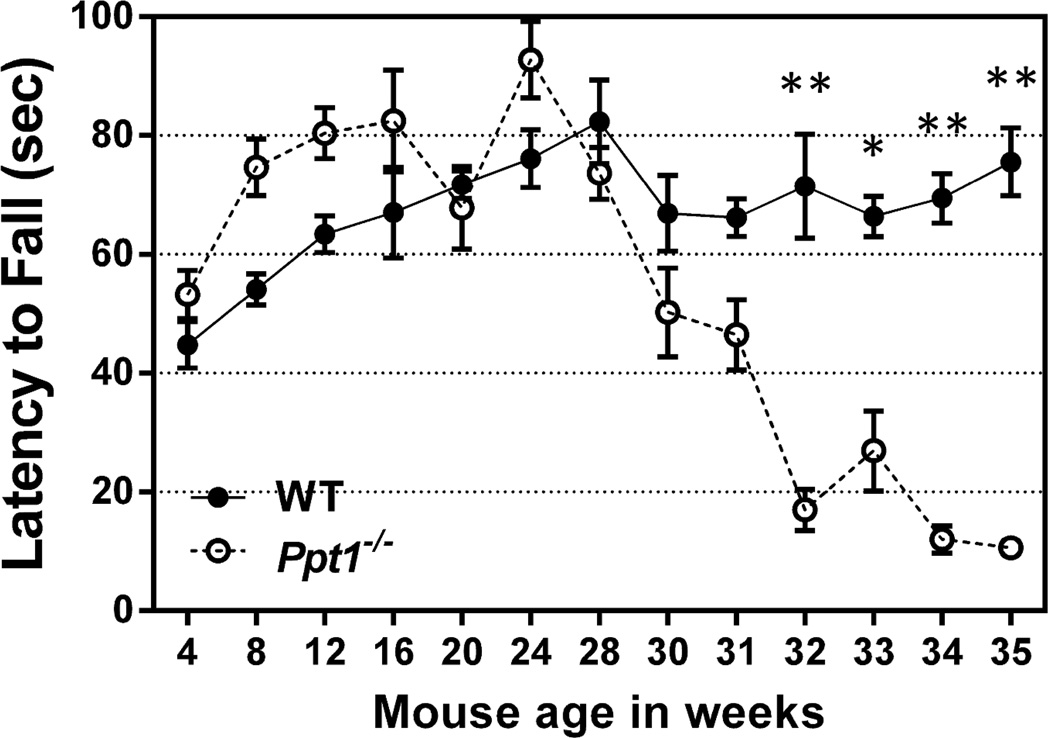
Figure 1.
Ppt1−/− mice develop a motor coordination deficit that becomes significant at 32 weeks.
An accelerating rotarod (0-40 rpm in 120 seconds) was used to measure the motor skills of Ppt1−/− and wild-type (WT) mice beginning at the age of 4 weeks. Data points represent mean ± S.E.M. of the time mice were able to stay on the rod. Two-way ANOVA with Bonferroni’s posttest for multiple comparisons was used to determine statistical significance (*P < 0.001, **P <0.0001). Five wild-type mice were assessed at all time points. The experiment started with 9 Ppt1−/− mice, but that number decreased over time due to mouse death (n = 9 through 30 weeks; 31 and 32 weeks, n = 8; 33 weeks, n = 7; 34 weeks, n = 4; 35 weeks, n = 3).
When the same Ppt1−/− mice were repeatedly exposed to the rotarod task, we found that their performance on the accelerating rotarod peaked at 24 weeks of age and declined steadily thereafter (Figure 1). Their latencies to fall became significantly lower than those of wild-type mice at the age of 32 weeks. The wild-type mice subjected to the same protocol as controls did not exhibit the age-dependent peak or decline, suggesting that the deterioration of the Ppt1−/− population was indeed due to disease progression and not external factors.
Treating Ppt1−/− Mice with Memantine Significantly Before Phenotypic Onset Has No Effect
Our previous results demonstrating an increase in NMDA receptor activity25 coupled with the extensive upregulation of microglial activation in Ppt1−/− brains reported by others8–9 suggest that memantine, a compound originally described as a NMDA receptor antagonist27 and more recently shown to decrease pathological microglial activation,28 has great potential as a treatment for infantile neuronal ceroid lipofuscinosis. As Ppt1−/− mice exhibit significant neural pathology before the onset of an observable motor coordination deficit9 and changes on the molecular level occur even earlier,10 we first treated mice at time points significantly before the age at which we observed a measureable rotarod phenotype.
When Ppt1−/− mice were given a single injection of memantine at 3 months of age, 4 months before the observed decline in motor coordination, they did not progress any differently than mice given an equivalent injection of physiological saline. A 10 mg/kg dose failed to produce any notable effect in younger mice at all, aside from a slight drop in performance in the Ppt1−/− population at the 30-minute time point (Figure 2). At a dose of 20 mg/kg, memantine has a slight negative effect on both genotypes immediately following injection, but that effect is neither statistically significant nor prolonged (Figure 3). The results of a single memantine dose administered to wild-type and Ppt1−/− mice at 5 months of age follow a similar pattern. Neither genotype is affected by the 10 mg/kg dose in a significant fashion (Figure 4). Increasing the dose to 20 mg/kg in the 5-month-old population results in an observable, but not statistically significant, drop in performance 30 minutes after injection in both wild-type and Ppt1−/− mice. The memantine- and vehicle-treated populations are indistinguishable aside from that single difference (Figure 5).
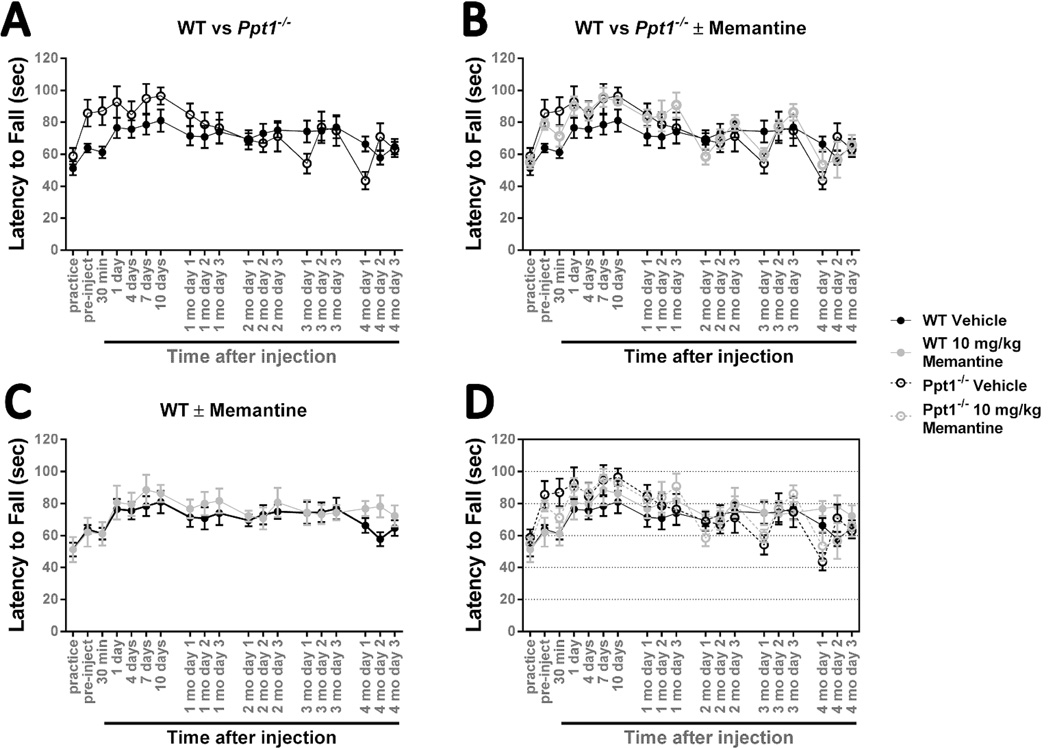
Figure 2.
Treating 3-month-old Ppt1−/− mice with 10 mg/kg memantine has no effect.
An accelerating rotarod (0-40 rpm in 120 seconds) was used to measure the motor skills of Ppt1−/− and wild-type (WT) mice. Mice were intraperitoneally injected with the NMDA receptor antagonist memantine (10 mg/kg) or a vehicle control (physiological saline) at 3 months of age and reevaluated on a monthly basis. (A) There is no significant difference between Ppt1−/− and wild-type mice when exposed to this testing paradigm. (B) Treatment with 10 mg/kg memantine at 3 months of age does not significantly affect Ppt1−/− performance. (C) Treatment with 10 mg/kg memantine at 3 months of age does not have a significant effect on wild-type mice. Panel D is an overlay of the previous data sets. Data points represent mean ± S.E.M. of the time mice were able to stay on the rod. Two-way ANOVA was used to compare vehicle treated wild-type mice to other conditions and to compare treated and untreated Ppt1−/− populations. Wild-type vehicle, n = 8; Ppt1−/− vehicle, n = 7; wild-type memantine, n = 7; Ppt1−/− memantine, n = 8.
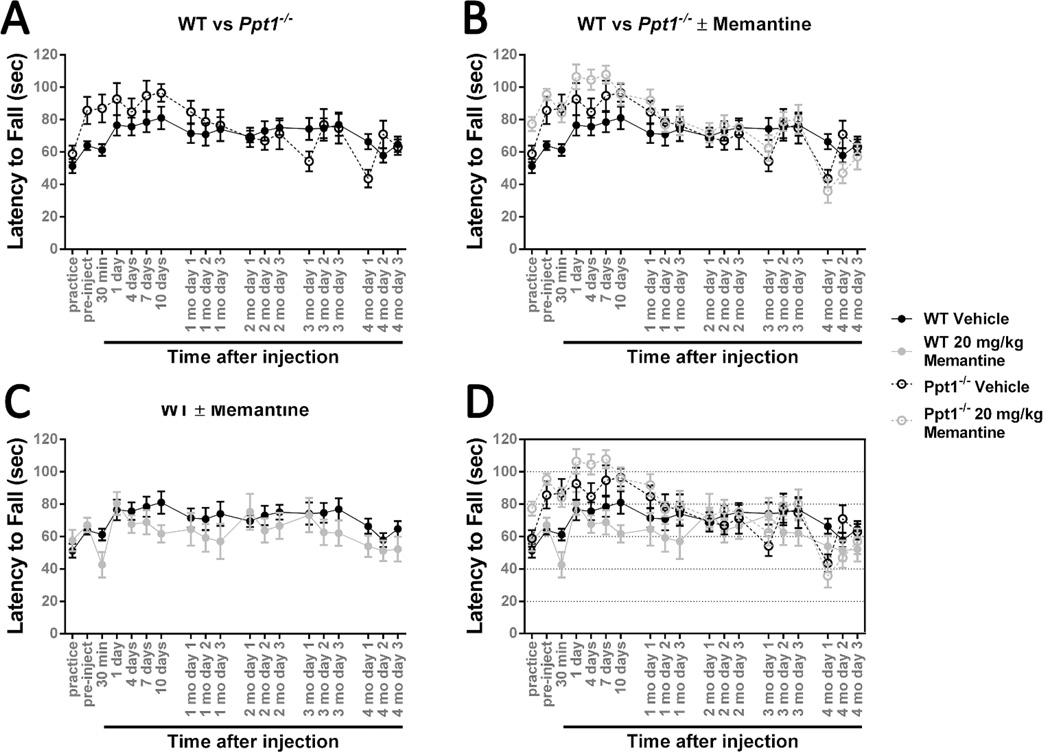
Figure 3.
Treating 3-month-old Ppt1−/− mice with 20 mg/kg memantine has no effect.
An accelerating rotarod (0-40 rpm in 120 seconds) was used to measure the motor skills of Ppt1−/− and wild-type (WT) mice. Mice were intraperitoneally injected with the NMDA receptor antagonist memantine (20 mg/kg) or a vehicle control (physiological saline) at 3 months of age and reevaluated on a monthly basis. (A) There is no significant difference between Ppt1−/− and wild-type mice when exposed to this testing paradigm. Vehicle-treated populations are the same as those shown in Figure 2. (B) Treatment with 20 mg/kg memantine at 3 months of age does not significantly affect Ppt1−/− performance. (C) Treatment with 20 mg/kg memantine at 3 months of age does not have a significant effect on wild-type mice. Panel D is an overlay of the previous data sets. Data points represent mean ± S.E.M. of the time mice were able to stay on the rod. Two-way ANOVA was used to compare vehicle-treated wild-type mice to other conditions and to compare treated and untreated Ppt1−/− populations. Wild-type vehicle, n = 8; Ppt1−/− vehicle, n = 7; wild-type memantine, n = 5; Ppt1−/− memantine, n = 5.
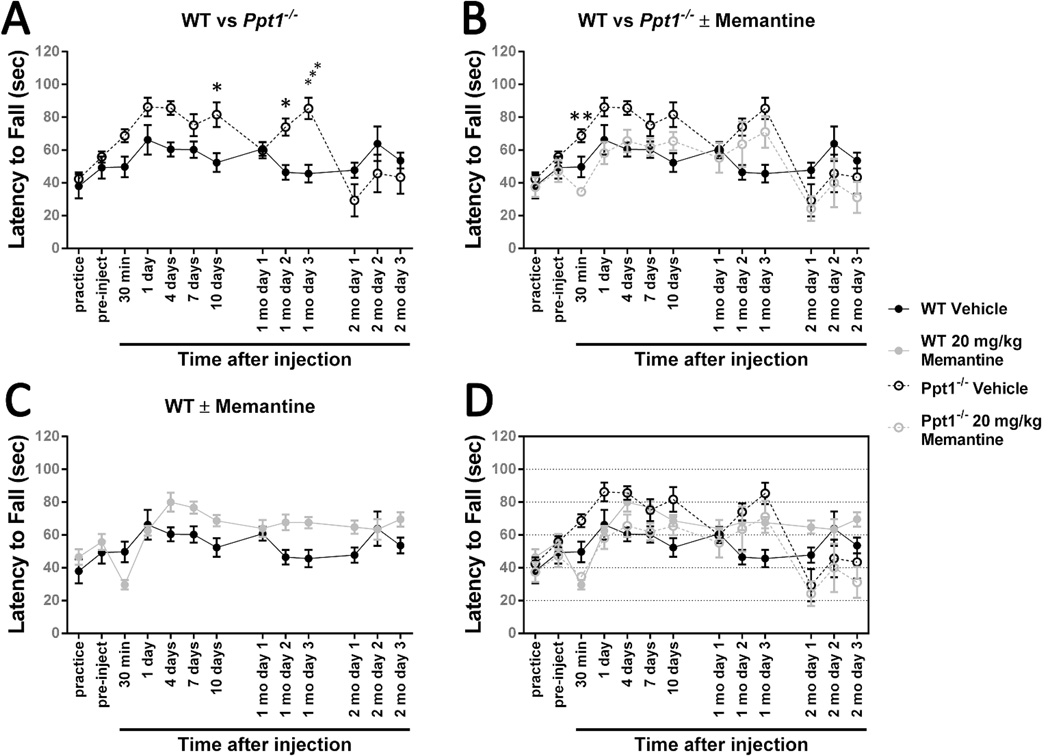
Figure 4.
Treating 5-month-old Ppt1−/− mice with 10 mg/kg memantine has no effect.
An accelerating rotarod (0-40 rpm in 120 seconds) was used to measure the motor skills of Ppt1−/− and wild-type (WT) mice. Mice were intraperitoneally injected with the NMDA receptor antagonist memantine (10 mg/kg) or a vehicle control (physiological saline) at 5 months of age and reevaluated on a monthly basis. (A) At 3 time points, Ppt1−/− mice performed significantly better than wild-type mice. (B) Treatment with 10 mg/kg memantine at 5 months of age does not significantly affect Ppt1−/− performance. (C) Treatment with 10 mg/kg memantine at 5 months of age does not have a significant effect on wild-type mice. Panel D is an overlay of the previous data sets. Data points represent mean ± S.E.M. of the time mice were able to stay on the rod. Two-way ANOVA with Sidak’s posttest for multiple comparisons was used to compare vehicle-treated wild-type mice to other conditions and to compare treated and untreated Ppt1−/− populations (*P < 0.05, ***P < 0.001). Wild-type vehicle, n = 6; Ppt1−/− vehicle, n = 8; wild-type memantine, n = 6; Ppt1−/− memantine, n = 7.
Figure 5.
Treating 5-month-old Ppt1−/− mice with 20 mg/kg memantine has a short-term negative effect.
An accelerating rotarod (0-40 rpm in 120 seconds) was used to measure the motor skills of Ppt1−/− and wild-type mice (WT). Mice were intraperitoneally injected with the NMDA receptor antagonist memantine (20 mg/kg) or a vehicle control (physiological saline) at 5 months of age and reevaluated on a monthly basis. (A) At 3 time points, Ppt1−/− mice performed significantly better than wild-type mice. Vehicle treated populations are the same as those shown in Figure 4. (B) Treatment with 20 mg/kg memantine at 5 months of age significantly impairs Ppt1−/− performance as compared with vehicle-treated controls 30 minutes after injection. (C) Treatment with 20 mg/kg memantine does not have a significant effect on 5-month-old wild-type mice. Panel D is an overlay of the previous data sets. Data points represent mean ± S.E.M. of the time mice were able to stay on the rod. Two-way ANOVA with Sidak’s posttest for multiple comparisons was used to compare vehicle-treated wild-type mice to other conditions and to compare treated and untreated Ppt1−/− populations (*P < 0.05, **P < 0.01, ***P < 0.001). Wildtype vehicle, n = 6; Ppt1−/− vehicle, n = 8; wild-type memantine, n = 7; Ppt1−/− memantine, n = 7.
Treating Ppt1−/− Mice with Memantine After Phenotypic Onset Has a Modest Beneficial Effect
As 30 weeks of age (7 months) is the point at which Griffey and colleagues11 did their assessment of phenotypic rescue and an age that falls between peak performance (24 weeks) and complete loss of ability (32 weeks) as assessed by our time course experiment (Figure 1), we chose to treat mice at that time point. Mice were trained on the rotarod, subjected to the pretreatment rotarod test, given one intraperitoneal injection of 10 mg/kg memantine, and then repeatedly assessed via rotarod at multiple points following injection. Ppt1−/− vehicle-treated mice performed significantly worse than the vehicle treated wild-type mice at all points examined (Figure 6A; P < 0.001). Vehicle-treated wild-type and Ppt1−/− mice are capable of motor learning as evidenced by the upward trend between the initial practice runs and the 4-day post-injection time points. Following that time period, both populations stop improving (Figure 6A).
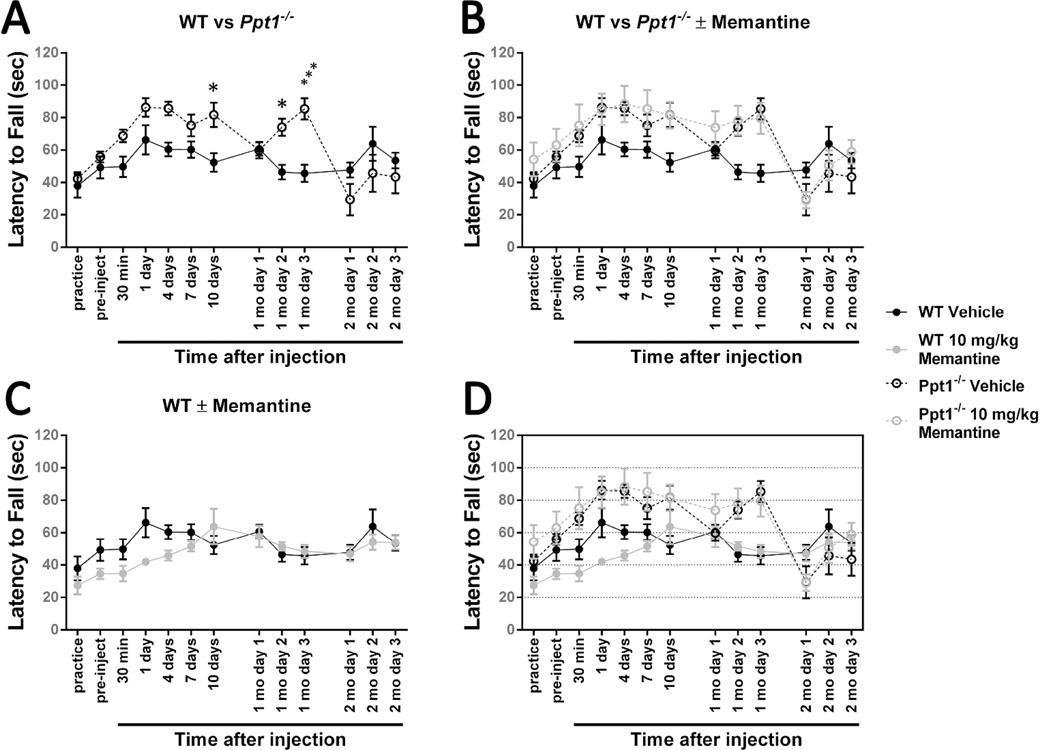
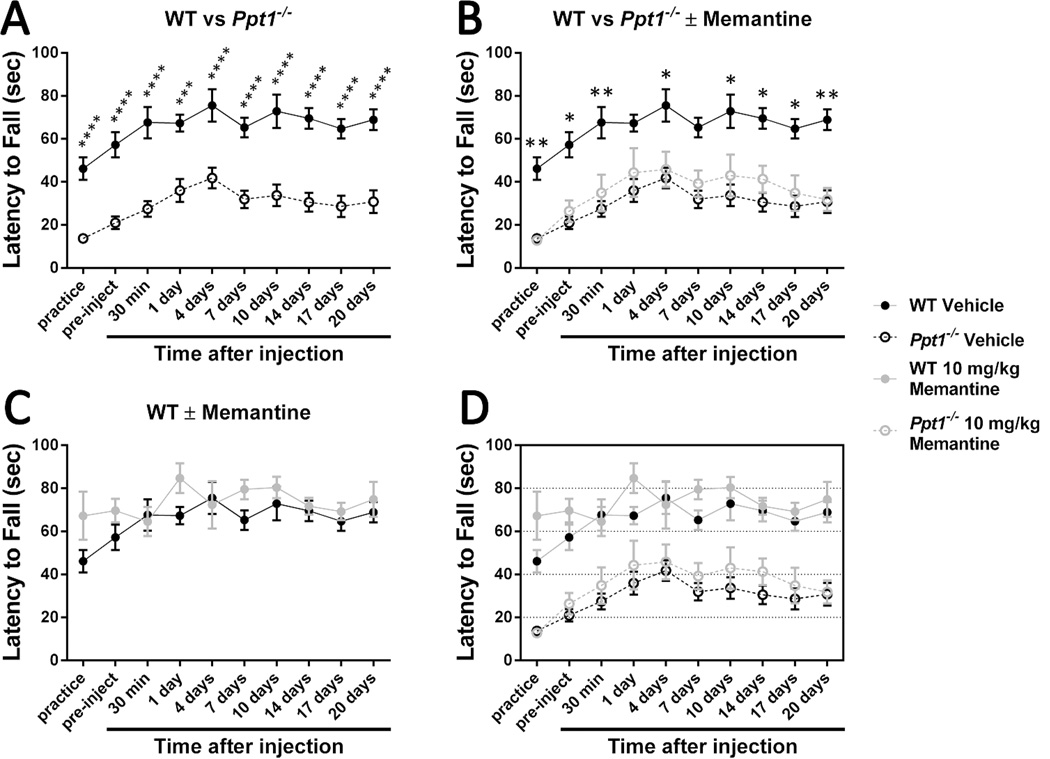
Figure 6.
Treating 30-week-old Ppt1−/− mice with 10 mg/kg memantine has no significant effect on rotarod performance.
An accelerating rotarod (0-40 rpm in 120 seconds) was used to measure the motor skills of 30-week-old Ppt1−/− and wild-type mice (WT). Mice were intraperitoneally injected with the NMDA receptor agonist memantine (10 mg/kg) or a vehicle control (physiological saline). (A) Ppt1−/− mice exhibit a motor coordination deficit at 30 weeks. (B) Treatment with 10 mg/kg memantine at 30 weeks of age does not significantly affect Ppt1−/− performance. (C) Treatment with 10 mg/kg memantine does not have a significant effect on 30-week-old wild-type mice. Panel D is an overlay of the previous data sets. Data points represent mean ± S.E.M. of the time mice were able to stay on the rod. Two-way ANOVA with Bonferroni’s posttest for multiple comparisons was used to compare vehicle-treated wild-type mice to other conditions and to compare treated and untreated Ppt1−/− populations (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). One-way ANOVA with Tukey’s posttest for multiple comparisons was used to compare the four latency to fall scores immediately following after drug injection. Wild-type vehicle, n = 10; Ppt1−/− vehicle, n = 10; WT memantine, n = 10; Ppt1−/− memantine, n = 11 (n = 10 for 10- and 14-day time points due to a death).
Wild-type mice had a slight negative reaction to the drug injection as evidenced by the lack of perceptible learning-driven improvement at the 30-minute time point. Following this relative slump, memantine-treated wild-type mice appear to perform better than their vehicle-treated counterparts one day after injection, but there are no statistically significant differences between the two populations (Figure 6C). Ppt1−/− mice treated with 10 mg/kg memantine never performed significantly better than their vehicle-treated counterparts, but the single dose did improve performance slightly such that the significant difference between Ppt1−/− and wild-type performance was eliminated at the 1- and 7-day post-injection time points (Figure 6B). It should be noted, however, that this improvement may be due to slight variation between the groups of mice as the treated group performed slightly better during the pre-injection trial.
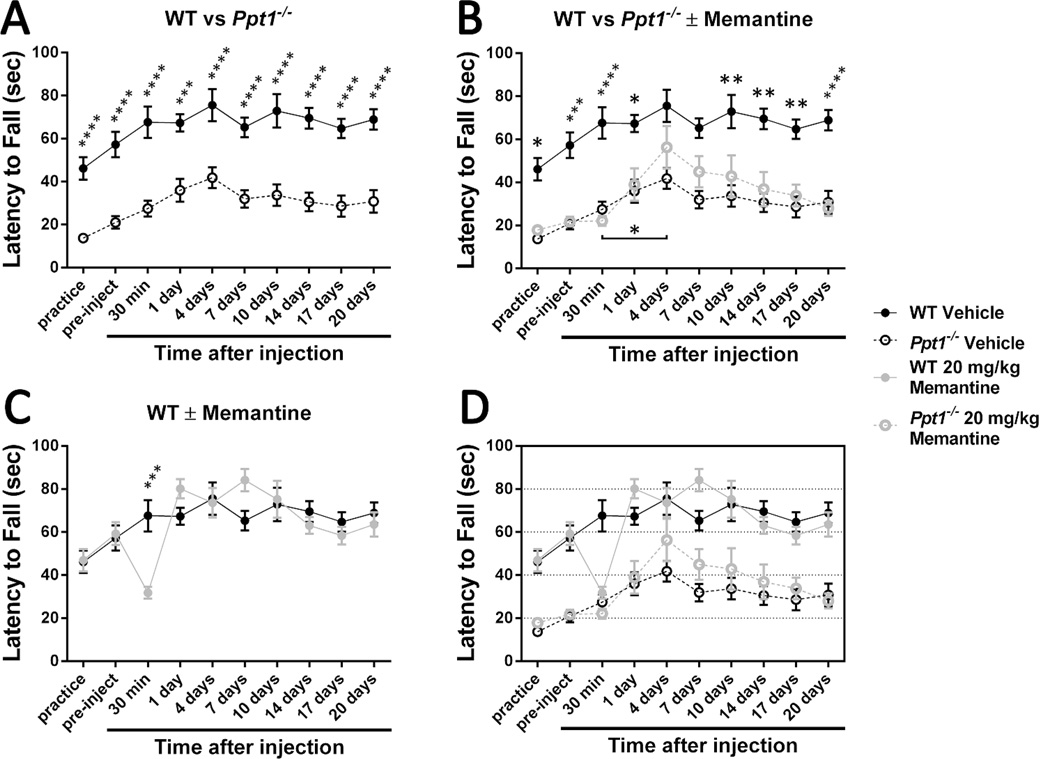
A higher memantine concentration had a significant effect on wild-type mice. Thirty minutes following the 20 mg/kg injection, mice were unsteady on their feet and generally uncoordinated. This adverse effect is quite visible in their rotarod performance (Figure 7C; P < 0.001). However, the mice recovered from the relative overdose and were unable to be differentiated from their vehicle-treated counterparts at subsequent time points. The effect of this higher dose on the Ppt1−/− population was also striking (Figure 7B). Treated Ppt1−/− mice were indistinguishable from vehicle-treated mice for the first day. The injection can be said to have slightly impaired learning at the 30-minute post-injection time point, but this is not significant. Although the memantine-treated Ppt1−/− mice never performed significantly better than the vehicle-treated controls, they did exhibit significant improvement between the 30-minute and 4-day post-injection time points (Figure 7B; P < 0.05). This enhanced learning resulted in the treated Ppt1−/− mice no longer being statistically significantly different from the wild-type at the 4- and 7-day time points. The decline in coordination visible after the performance peak at 4 days post-injection suggests that memantine stops having an effect on Ppt1−/− mice between 4 days and one week following administration.
Figure 7.
Treating 30-week-old Ppt1−/− mice with 20 mg/kg memantine has a moderately positive effect on rotarod performance and motor learning.
An accelerating rotarod (0-40 rpm in 120 seconds) was used to measure the motor skills of 30-week-old Ppt1−/− and wild-type (WT) mice. Mice were intraperitoneally injected with the NMDA receptor agonist memantine (20 mg/kg) or a vehicle control (physiological saline). (A) Ppt1−/− mice exhibit a motor coordination deficit at 30 weeks. Vehicle-treated populations are the same as those shown in Figure 6. (B) Treatment with 20 mg/kg memantine at 30 weeks of age improves motor learning in Ppt1−/− mice. (C) Treatment with 20 mg/kg memantine at 30 weeks of age has a temporary negative effect on wild-type mice that disappears within 24 hours. Panel D is an overlay of the previous data sets. Data points represent mean ± S.E.M. of the time mice were able to stay on the rod. Two-way ANOVA with Bonferroni’s posttest for multiple comparisons was used to compare vehicle-treated wild-type mice to other conditions and to compare treated and untreated Ppt1−/− populations (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). One-way ANOVA with Tukey’s posttest for multiple comparisons was used to compare the four latency to fall scores immediately following drug injection (*P < 0.05). Wild-type vehicle, n = 10; Ppt1−/− vehicle, n = 10; wild-type memantine, n = 10; Ppt1−/− memantine, n = 9.
Discussion
We have shown that a single injection of 20 mg/kg memantine at 30 weeks of age improved motor learning and, consequently, had a moderately positive effect on motor coordination of the Ppt1−/− mouse model of infantile neuronal ceroid lipofuscinosis. At this time point, disease progression is considerable, and significant cell loss and atrophy have already occurred in numerous brain regions.8–9 As a result, this experimental paradigm is a good model of the drug’s effectiveness after symptomatic onset, suggesting that memantine may provide hope for children who have already developed symptoms. Most therapeutic studies with the Ppt1−/− mouse treat the mice at birth or soon after and assess pathology at later points.11,29–34 Unfortunately, the vast majority of human patients are not definitively diagnosed until later on in disease progression, making perinatal treatment strategies impractical.
The molecular events driving this improvement in motor learning and the reason for its relative delay are currently unknown. As we have previously demonstrated abnormally enhanced NMDA receptor function in Ppt1−/− neurons,25 it is tempting to assert that the effects reported are due to memantine’s ion channel antagonism. Excitotoxic cell death mediated by overactivation of NMDA receptors has been implicated in numerous neurodegenerative disorders, including Alzheimer’s disease, amyotrophic lateral sclerosis, Huntington’s disease, and Parkinson’s disease;13 and memantine has been found to be neuroprotective in cases of moderate to severe Alzheimer’s disease as well as other neurodegenerative diseases.35–36 Recent evidence has also suggested that some portion of the neuroprotection afforded by memantine treatment may be due to drug-induced reduction of microglia-associated inflammation.28 As extensive gliosis is present in the brains of patients with infantile neuronal ceroid lipofuscinosis as well as in the brains of Ppt1−/− mice, it is also possible that a reduction in astrocytic and microglial activation drove the observed improvement.
However, it is important to note that, although memantine has a half-life of approximately 100 hours in humans, it is degraded much faster in rodents.27 This significantly shorter rodent half-life (3 to 5 hours) means that it is unlikely that memantine was still active in the brains of treated animals at the time of reported improvement. Temporary inhibition of NMDA receptors resulting in long-lasting alterations in glutamatergic behavior is not unheard of,37–38 and studies treating the Cln3ex1–6 mouse model of juvenile neuronal ceroid lipofuscinosis with memantine also report a delay in improvement of motor skills,24 so it is likely that the temporary inhibition of NMDA receptor activity or the decrease in glial activity initiated a cascade of longer-lasting changes.
The fact that memantine’s positive effects are delayed poses an interesting conundrum as far as patient treatment is concerned. Dosing schedules have the potential to be complex, and intermittently exposing children to high doses of memantine may have unforeseen negative effects. However, we did not examine the effects of chronic exposure to lower levels the drug. It is entirely possible that extended exposure to a lower, consistent dose may be equivalent to or perhaps more beneficial than our single high dose. In the event that overactivation of NMDA receptors is driving neuronal loss in the Ppt1−/− mouse and patients with infantile neuronal ceroid lipofuscinosis, it is also possible that chronically treating with a lower dose beginning at an earlier time point may slow or even halt neuronal loss and disease progression.
In addition to the beneficial effects of memantine, our results also suggest there may be a benefit to repeated exposure to different tasks. Although no pathology has been done on the mice involved in this study to examine whether being repeatedly subjected to the rotarod task is neuroprotective, comparison of latency to fall scores from our different treatment groups suggest it may be. The Ppt1−/− animals used to determine phenotypic onset have a mean latency to fall of 50.22 seconds at the 30-week time point, and that is achieved without the benefit of a training or practice round. Alternatively, the Ppt1−/− animals dosed with memantine at 30 weeks and not previously exposed to the rotarod have a mean latency to fall of 20.99 seconds following a number of practice runs designed to acclimate them to the task. A similar trend can be seen in animals treated at 3 and 5 months of age and then evaluated at the age of 30 weeks. Ppt1−/− mice injected at 3 months of age had a mean latency to fall of 43.59 seconds on the first day of testing, which then increased to 70.90 and 63.00 seconds on the second and third days of testing, respectively. Ppt1−/− animals injected at 5 months of age and then reevaluated had a mean latency to fall similar to that of the previously unexposed group (29.34 seconds) on the first day of testing, but that value increased to 45.70 and 43.49 on the second and third days of evaluation. There is no doubt that some of this effect is due to motor learning, muscle memory, and familiarity with the rotarod task. However, the potential that repeated exposure to enrichment tasks may slow disease progression in Ppt1−/− mice and, by extension, patients with infantile neuronal ceroid lipofuscinosis, is something that warrants further investigation.
In summary, we have demonstrated that treatment with high doses of memantine improves motor learning in the Ppt1−/− mouse model of infantile neuronal ceroid lipofuscinosis. More studies that use repeated dosing of memantine are required before firm conclusions can be made. It is intriguing, however, that the effect we report has been demonstrated in older mice at an advanced stage in the disease. The potential therapeutic implications of memantine for infantile neuronal ceroid lipofuscinosis needs further study.
Acknowledgments
We thank Sarah Radel, Sarah Brink, Melanee Clark, and the staff of the Laboratory Animal Research Facility for providing technical assistance and aiding in animal care.
Funding
This work was supported in part by Haydens Hope and NIH R01 NS043310 and Sanford Health.
Footnotes
Author Contributions
RF conceived of the experiments, carried out the experiments, wrote the first draft of the article, and edited subsequent drafts. ADK aided in technical planning of the experiments and edited drafts of the manuscript. DAP coordinated the work and supervised writing.
Declaration of Conflicting Interests
The authors declare no potential conflicts of interest with respect to the research, authorship, or publication of this article.
Ethical Approval
All procedures were carried out in accordance with the guidelines of the Animal Welfare Act, National Institutes of Health policies, and the Sanford Health Animal Care and Use Committee (protocol number: 24-01-14B).
References
- 1.Cooper JD. Progress towards understanding the neurobiology of Batten disease or neuronal ceroid lipofuscinosis. Curr Opin Neurol. 2003;16(2):121–128. doi: 10.1097/01.wco.0000063762.15877.9b. [DOI] [PubMed] [Google Scholar]
- 2.Jalanko A, Braulke T. Neuronal ceroid lipofuscinoses. Biochim Biophys Acta. 2009;1793(4):697–709. doi: 10.1016/j.bbamcr.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Santavuori P, Haltia M, Rapola J, Raitta C. Infantile type of so-called neuronal ceroid-lipofuscinosis. 1. A clinical study of 15 patients. J Neurol Sci. 1973;18(3):257–267. doi: 10.1016/0022-510x(73)90075-0. [DOI] [PubMed] [Google Scholar]
- 4.Santavuori P. Neuronal ceroid-lipofuscinoses in childhood. Brain Dev. 1988;10(2):80–83. doi: 10.1016/s0387-7604(88)80075-5. [DOI] [PubMed] [Google Scholar]
- 5.Vesa J, Hellsten E, Verkruyse LA, et al. Mutations in the palmitoyl protein thioesterase gene causing infantile neuronal ceroid lipofuscinosis. Nature. 1995;376(6541):584–587. doi: 10.1038/376584a0. [DOI] [PubMed] [Google Scholar]
- 6.Camp LA, Hofmann SL. Purification and properties of a palmitoyl-protein thioesterase that cleaves palmitate from H-Ras. J Biol Chem. 1993;268(30):22566–22574. [PubMed] [Google Scholar]
- 7.Gupta P, Soyombo AA, Atashband A, et al. Disruption of PPT1 or PPT2 causes neuronal ceroid lipofuscinosis in knockout mice. Proc Natl Acad Sci U S A. 2001;98(24):13566–13571. doi: 10.1073/pnas.251485198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bible E, Gupta P, Hofmann SL, Cooper JD. Regional and cellular neuropathology in the palmitoyl protein thioesterase-1 null mutant mouse model of infantile neuronal ceroid lipofuscinosis. Neurobiol Dis. 2004;16(2):346–359. doi: 10.1016/j.nbd.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Kielar C, Maddox L, Bible E, et al. Successive neuron loss in the thalamus and cortex in a mouse model of infantile neuronal ceroid lipofuscinosis. Neurobiol Dis. 2007;25(1):150–162. doi: 10.1016/j.nbd.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macauley SL, Wozniak DF, Kielar C, et al. Cerebellar pathology and motor deficits in the palmitoyl protein thioesterase 1-deficient mouse. Exp Neurol. 2009;217(1):124–135. doi: 10.1016/j.expneurol.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffey MA, Wozniak D, Wong M, et al. CNS-directed AAV2-mediated gene therapy ameliorates functional deficits in a murine model of infantile neuronal ceroid lipofuscinosis. Mol Ther. 2006;13(3):538–547. doi: 10.1016/j.ymthe.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Michaelis EK. Molecular biology of glutamate receptors in the central nervous system and their role in excitotoxicity, oxidative stress and aging. Prog Neurobiol. 1998;54(4):369–415. doi: 10.1016/s0301-0082(97)00055-5. [DOI] [PubMed] [Google Scholar]
- 13.Gardoni F, Di Luca M. New targets for pharmacological intervention in the glutamatergic synapse. Eur J Pharmacol. 2006;545(1):2–10. doi: 10.1016/j.ejphar.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 14.Battaglioli G, Martin DL, Plummer J, Messer A. Synaptosomal glutamate uptake declines progressively in the spinal cord of a mutant mouse with motor neuron disease. J Neurochem. 1993;60(4):1567–1569. doi: 10.1111/j.1471-4159.1993.tb03323.x. [DOI] [PubMed] [Google Scholar]
- 15.Mennini T, Bastone A, Crespi D, et al. Spinal cord GLT-1 glutamate transporter and blood glutamic acid alterations in motor neuron degeneration (Mnd) mice. J Neurol Sci. 1998;157(1):31–36. doi: 10.1016/s0022-510x(98)00072-0. [DOI] [PubMed] [Google Scholar]
- 16.Boyce S, Webb JK, Carlson E, et al. Onset and progression of motor deficits in motor neuron degeneration (mnd) mice are unaltered by the glycine/NMDA receptor antagonist L-701,324 or the MAO-B inhibitor R(−)-deprenyl. Exp Neurol. 1999;155(1):49–58. doi: 10.1006/exnr.1998.6873. [DOI] [PubMed] [Google Scholar]
- 17.Pears MR, Cooper JD, Mitchison HM, et al. High resolution 1H NMR-based metabolomics indicates a neurotransmitter cycling deficit in cerebral tissue from a mouse model of Batten disease. J Biol Chem. 2005;280(52):42508–42514. doi: 10.1074/jbc.M507380200. [DOI] [PubMed] [Google Scholar]
- 18.Elger B, Schneider M, Winter E, et al. Optimized synthesis of AMPA receptor antagonist ZK 187638 and neurobehavioral activity in a mouse model of neuronal ceroid lipofuscinosis. ChemMedChem. 2006;1(10):1142–1148. doi: 10.1002/cmdc.200600144. [DOI] [PubMed] [Google Scholar]
- 19.Kovács AD, Weimer JM, Pearce DA. Selectively increased sensitivity of cerebellar granule cells to AMPA receptor-mediated excitotoxicity in a mouse model of Batten disease. Neurobiol Dis. 2006;22(3):575–585. doi: 10.1016/j.nbd.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 20.Ahtiainen L, Kolikova J, Mutka AL, et al. Palmitoyl protein thioesterase 1 (Ppt1)-deficient mouse neurons show alterations in cholesterol metabolism and calcium homeostasis prior to synaptic dysfunction. Neurobiol Dis. 2007;28(1):52–64. doi: 10.1016/j.nbd.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Kovács AD, Pearce DA. Attenuation of AMPA receptor activity improves motor skills in a mouse model of juvenile Batten disease. Exp Neurol. 2008;209(1):288–291. doi: 10.1016/j.expneurol.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolikova J, Afzalov R, Surin A, et al. Deficient mitochondrial Ca(2+) buffering in the Cln8(mnd) mouse model of neuronal ceroid lipofuscinosis. Cell Calcium. 2011;50(6):491–501. doi: 10.1016/j.ceca.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Kovács AD, Saje A, Wong A, et al. Temporary inhibition of AMPA receptors induces a prolonged improvement of motor performance in a mouse model of juvenile Batten disease. Neuropharmacology. 2011;60(2-3):405–409. doi: 10.1016/j.neuropharm.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovács AD, Saje A, Wong A, et al. Age-dependent therapeutic effect of memantine in a mouse model of juvenile Batten disease. Neuropharmacology. 2012;63(5):769–775. doi: 10.1016/j.neuropharm.2012.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finn R, Kovács AD, Pearce DA. Altered glutamate receptor function in the cerebellum of the Ppt1−/− mouse, a murine model of infantile neuronal ceroid lipofuscinosis. J Neurosci Res. 2012;90(2):367–375. doi: 10.1002/jnr.22763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284(5420):1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- 27.Parsons CG, Danysz W, Quack G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist--a review of preclinical data. Neuropharmacology. 1999;38(6):735–767. doi: 10.1016/s0028-3908(99)00019-2. [DOI] [PubMed] [Google Scholar]
- 28.Wu HM, Tzeng NS, Qian L, et al. Novel neuroprotective mechanisms of memantine: increase in neurotrophic factor release from astroglia and anti-inflammation by preventing microglial activation. Neuropsychopharmacology. 2009;34(10):2344–2357. doi: 10.1038/npp.2009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffey M, Bible E, Vogler C, et al. Adeno-associated virus 2-mediated gene therapy decreases autofluorescent storage material and increases brain mass in a murine model of infantile neuronal ceroid lipofuscinosis. Neurobiol Dis. 2004;16(2):360–369. doi: 10.1016/j.nbd.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Griffey M, Macauley SL, Ogilvie JM, Sands MS. AAV2-mediated ocular gene therapy for infantile neuronal ceroid lipofuscinosis. Mol Ther. 2005;12(3):413–421. doi: 10.1016/j.ymthe.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 31.Wei H, Zhang Z, Saha A, et al. Disruption of adaptive energy metabolism and elevated ribosomal p-S6K1 levels contribute to INCL pathogenesis: partial rescue by resveratrol. Hum Mol Genet. 2011;20(6):1111–1121. doi: 10.1093/hmg/ddq555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macauley SL, Roberts MS, Wong AM, et al. Synergistic effects of central nervous systemdirected gene therapy and bone marrow transplantation in the murine model of infantile neuronal ceroid lipofuscinosis. Ann Neurol. 2012;71(6):797–804. doi: 10.1002/ana.23545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts MS, Macauley SL, Wong AM, et al. Combination small molecule PPT1 mimetic and CNS-directed gene therapy as a treatment for infantile neuronal ceroid lipofuscinosis. J Inherit Metab Dis. 2012;35(5):847–857. doi: 10.1007/s10545-011-9446-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saha A, Sarkar C, Singh SP, et al. The blood-brain barrier is disrupted in a mouse model of infantile neuronal ceroid lipofuscinosis: amelioration by resveratrol. Hum Mol Genet. 2012;21(10):2233–2244. doi: 10.1093/hmg/dds038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat Rev Drug Discov. 2006;5(2):160–170. doi: 10.1038/nrd1958. [DOI] [PubMed] [Google Scholar]
- 36.Lipton SA. Pathologically activated therapeutics for neuroprotection. Nat Rev Neurosci. 2007;8(10):803–808. doi: 10.1038/nrn2229. [DOI] [PubMed] [Google Scholar]
- 37.Wöhrl R, Eisenach S, Manahan-Vaughan D, et al. Acute and long-term effects of MK-801 on direct cortical input evoked homosynaptic and heterosynaptic plasticity in the CA1 region of the female rat. Eur J Neurosci. 2007;26(10):2873–2883. doi: 10.1111/j.1460-9568.2007.05899.x. [DOI] [PubMed] [Google Scholar]
- 38.Manahan-Vaughan D, von Haebler D, Winter C, et al. A single application of MK801 causes symptoms of acute psychosis, deficits in spatial memory, and impairment of synaptic plasticity in rats. Hippocampus. 2008;18(2):125–134. doi: 10.1002/hipo.20367. [DOI] [PubMed] [Google Scholar]