Abstract
Ataxia telangiectasia (AT) and ataxia oculomotor apraxia type 2 (AOA2) are autosomal recessive ataxias caused by mutations in genes involved in maintaining DNA integrity. Lifespan in AT is greatly shortened (20s–30s) due to increased susceptibility to malignancies (leukemia/lymphoma). Lifespan in AOA2 is uncertain. We describe a woman with variant AT with two novel mutations in ATM (IVS14 + 2 T > G and 5825C > T, p.A1942V) who died at age 48 with pancreatic adenocarcinoma. Her mutations are associated with an unusually long life for AT and with a cancer rarely associated with that disease. We also describe two siblings with AOA2, heterozygous for two novel mutations in senataxin (3 bp deletion c.343–345 and 1398 T > G, p.I466M) who have survived into their 70s, allowing us to characterize the longitudinal course of AOA2. In contrast to AT, we show that persons with AOA2 can experience a prolonged lifespan with considerable motor disability.
1. INTRODUCTION
Ataxia telangiectasia (AT) and ataxia oculomotor apraxia type 2 (AOA2) are autosomal recessive causes of ataxia that share several clinical features, including sensorimotor neuropathy, gait ataxia, oculomotor apraxia, and elevated alpha fetoprotein (AFP) level. AT is an ataxia syndrome characterized by gait and truncal ataxia developing in childhood, often with individuals becoming wheelchair bound by 10 years of age [1]. Individuals with AT typically develop dysarthria and oculomotor apraxia. Unlike AOA2, AT is also associated with oculocutaneous telangiectasias, immunodeficiency, increased sensitivity to ionizing radiation and susceptibility to malignancies, particularly lymphomas and leukemias [1,2]. AT is caused by mutations in ATM, a gene that encodes a serine/threonine kinase of the phosphatidylinositol-3- kinase (PI3K) family [3]. ATM has been implicated in multiple pathways essential for maintaining cellular homeostasis and genome stability, including repair of double-stranded DNA breaks, regulation of cell cycle checkpoints and apoptosis, oxidative stress, mitochondrial homeostasis, telomere maintenance, and cellular protein turnover [4,5]. Since sequencing of ATM has become available, the range in clinical phenotype of AT has broadened, with recognition of variant AT in individuals with mutations in ATM but milder disease course or later onset. Recently, Canadian Mennonite families presenting with primary dystonia were found to have mutations in ATM, further broadening the clinical spectrum of variant AT [6]. Mutations in splice sites causing early truncation and nonsense mutations have been found in the classic AT phenotype [7,8], whereas missense mutations in ATM are being increasingly identified in variant AT. We present an individual with novel mutations causing variant AT who had unusual longevity and who died at age 48with pancreatic adenocarcinoma, an unusual malignancy for AT.
AOA2 is an autosomal recessive syndrome clinically characterized by ataxia onset during adolescence, cerebellar atrophy, sensorimotor peripheral neuropathy, and elevated serum AFP. Oculomotor apraxia, characterized by difficulty initiating saccades, is present in about 50% of patients with AOA2 [9]. AOA2 is caused by mutations in the senataxin (SETX) gene on chromosome 9q34, which encodes for a putative DNA helicase [10,11]. Although its function in the nervous system remains unclear, recent studies suggest that SETX functions in DNA break repair, RNA metabolism, and telomere stability [12,13]. Interestingly, dominant mutations in SETX are associated with juvenile amyotrophic lateral sclerosis type 4 [11]. Missense, nonsense and deletion mutations in both the conserved helicase domain and outside of the helicase domain, as well as non-coding missense mutations leading to frame shifts, have been identified in AOA2 patients [9,14]. We report 2 siblings with AOA2 who are compound heterozygotes for a previously described pathologic 3 bp deletion and a novel missense mutation in the C-terminus of SETX. They are both in their 70s, with medical records since the 1950s, allowing us to characterize the longitudinal course of AOA2. Unlike AT, individuals with AOA2 do not appear to have shortened lifespan, or increased susceptibility to malignancy.
2. CASE 1 –VARIANT ATAXIA TELANGIECTASIA
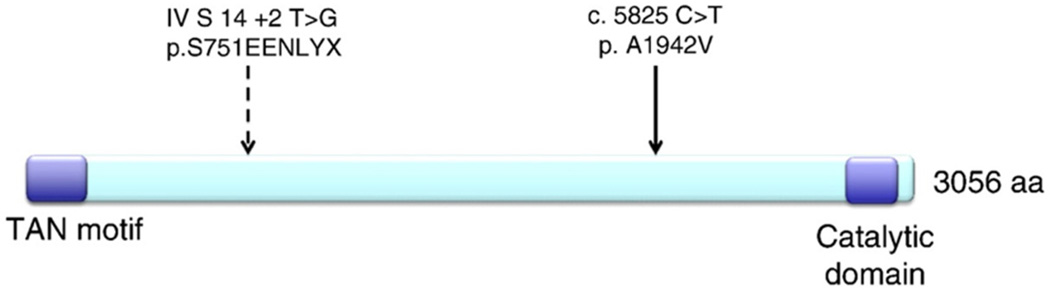
Patient I was a Caucasian female and was the product of a normal delivery. She started walking at 18 months. By age 2, she was noted to have mild athetosis and clumsiness, but had normal sensation and deep tendon reflexes. She developed dysarthric speech in early childhood. At age 9, she was noted to have lateral nystagmus, increased tone in her lower extremities, 2+ deep tendon reflexes and choreoathetosis of the upper limbs. She was considered to have low normal intellectual abilities. She graduated from high school with special education assistance. Walking became progressively more difficult because of severe ataxia and she was wheelchair bound by age 11, although she became an avid horseback rider at age 13 and was able to ride unassisted through her late 30s. A uterine fibroid was removed at age 33. At age 37, an EMG was performed due to severe sensory loss and weakness in the upper and lower extremities, revealing severe sensorimotor axonal and demyelinating peripheral neuropathy. Sensory and motor nerve responses were absent in the lower extremities. By age 37 she had not experienced cognitive deterioration that interfered with social interaction, but did not have formal neuropsychological testing. She was never employed, primarily due to severe dysarthria and ataxia. She had a pleasant and cheerful personality, and did not have any behavioral or emotional problems throughout her life. A brain MRI obtained at age 38 revealed midline cerebellar atrophy, without abnormalities in other structures of the brain. She was found to have an elevated AFP of 509.9 ng/dL at age 45. As she was never noted to develop telangiectasias, she underwent genetic testing for SETX, which was normal. Analysis of sequencing of the ATM gene using the UCSC genome browser (http://genome.ucsc.edu) revealed one ATM allele with a splice site mutation at conserved position IVS14 + 2 T > G and a missense mutation 5825C > T in exon 41 in the second ATM allele resulting in A1942V, a conserved codon (Fig. 1). Both are novel variants that are predicted to be deleterious. The patient was diagnosed with pancreatic adenocarcinoma five months prior to dying at age 48.
Figure 1.
2 novel heterozygous mutations in ATM causing variant AT. A mutation was found in the consensus splice donor site in intron 14 at position+2, T > G, predicting addition of EENLYX with premature stop. A second missense mutation C5825T was found, leading to A1942V in conserved exon 41. Neither mutation is located in the catalytic serine threonine kinase domain or TAN motif, which is a p53 binding site involved in response to double stranded DNA breaks and regulating telomere length.
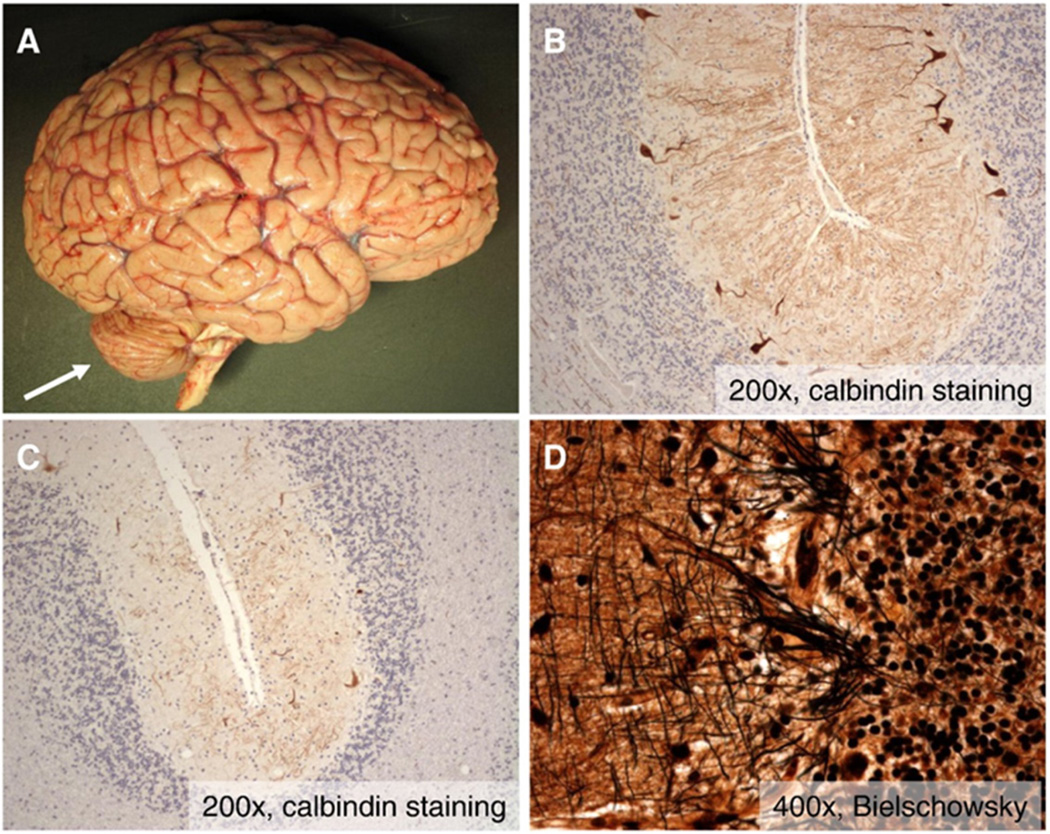
An autopsy revealed pancreatic adenocarcinoma involving the head of the pancreas and extending into the duodenum with metastasis to pancreatic lymph nodes. Neuropathologic examination of the brain revealed severe, symmetric cerebellar atrophy (Fig. 2A – arrow), which was confirmed with weight of the cerebellum and brainstem (100 g) being 44% less than predicted by whole brain weight (1325 g). There was no cortical or hippocampal atrophy, and deep cerebral nuclei, including neostriatum, amygdala, hippocampus, thalamus, and hypothalamus, in addition to white matter tracts, were grossly and histopathologically normal. This correlated with prior findings on MRI, where volume loss was restricted to the cerebellum. There was moderate (Fig. 2B) to severe (Fig. 2C) loss of Purkinje cells associated with the socalled “empty baskets” indicative of Purkinje neuron dropout (Fig. 2D) and Bergman gliosis, preferentially involving the superior-medial cerebellar cortex. There was severe atrophy of the spinal cord with severe axonal loss in the dorsal columns, greater in the gracile fasciculus than the cuneate fasciculus. There were moderate to severe loss of neurons in Clarke's column and less severe loss of anterior horn neurons. These findings are consistent with prior descriptions of ataxia telangiectasia [15]. Pathologic changes of Alzheimer's disease, including neuritic plaques and neurofibrillary tangles, were not identified, and Lewy bodies were not appreciated.
Figure 2.
Cerebellar pathology in variant AT. A) Gross photograph of fresh brain reveals marked cerebellar atrophy (arrow) with no evidence of cortical atrophy or other abnormalities. B–C) Photomicrographs at intermediate magnification (200×) of calbindin immunohistochemistry (which specifically labels Purkinje neurons) demonstrate moderate loss of Purkinje neurons in some areas (B) and severe loss in others (C). D) High magnification photomicrographs of Bielschowsky silver stained sections of cerebellar cortex reveals the presence of scattered “empty baskets” indicating Purkinje neuron dropout.
3. CASES 2 AND 3 – ATAXIA OCULOMOTOR APRAXIA TYPE 2
Patient II-2 is a 73 year old Caucasian female who developed unsteady gait at age 14. She was the product of a normal pregnancy and delivery, and had average intellect, completing a high school education. At age 37 she had a moderately severe wide-based ataxic gait with positive Romberg and was noted to have vertical and horizontal nystagmus and ocular apraxia. She had moderate, but comprehensible, dysarthria. Mild dysmetria was present in the upper extremities bilaterally. Deep tendon reflexes were absent in the upper extremities, with preserved 2+ patellar reflexes and mute plantar responses. She had mildly decreased sensation to vibration, sensation to light touch and joint position in the lower extremities, and moderately high arches were noted in her feet. She was initially diagnosed with a familial spinocerebellar ataxia. Progression of ataxia led to wheelchair use by age 48 and she became wheelchair bound by age 57. An MRI of the brain at age 59 revealed severe bilateral diffuse cerebellar atrophy.
At age 45, patient II-2 had an electro-nystagmogram that was interpreted as consistent with central origin vestibular abnormalities. Both left and right directional gaze nystagmus were present with eyes open and positional nystagmus was present with eyes closed. Caloric studies revealed an initial appropriate response to both cold and hot water in both ears, followed by direction reversal nystagmus.
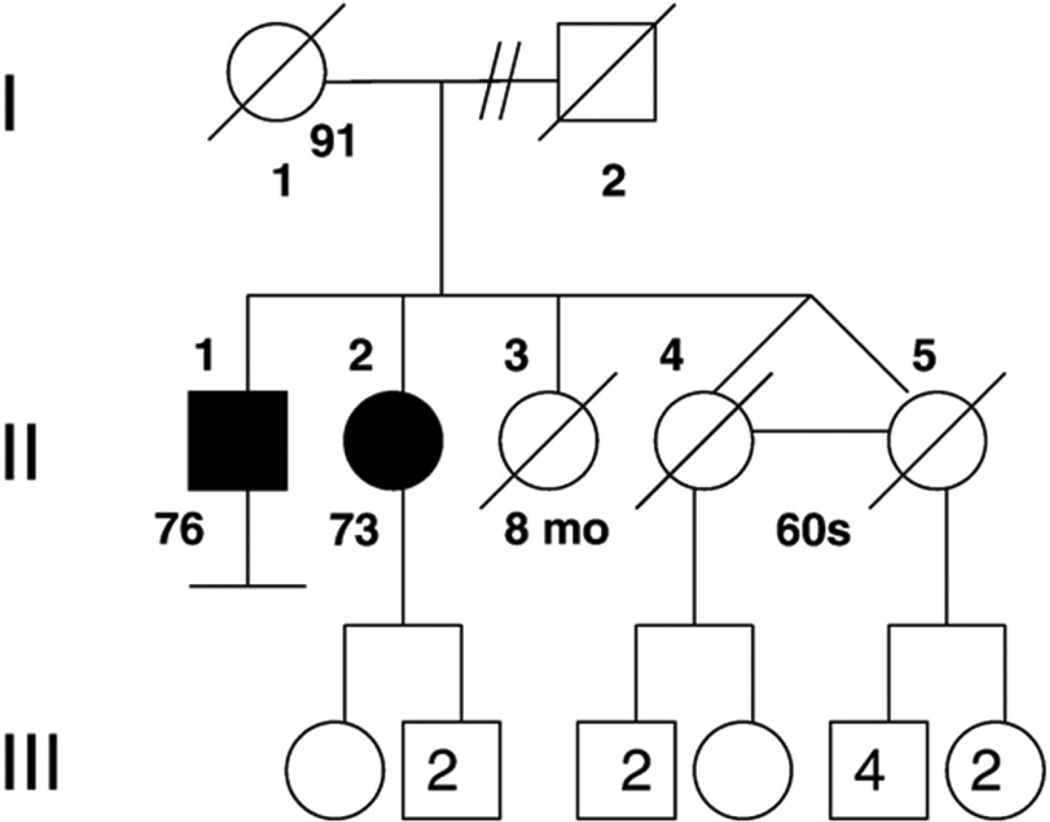
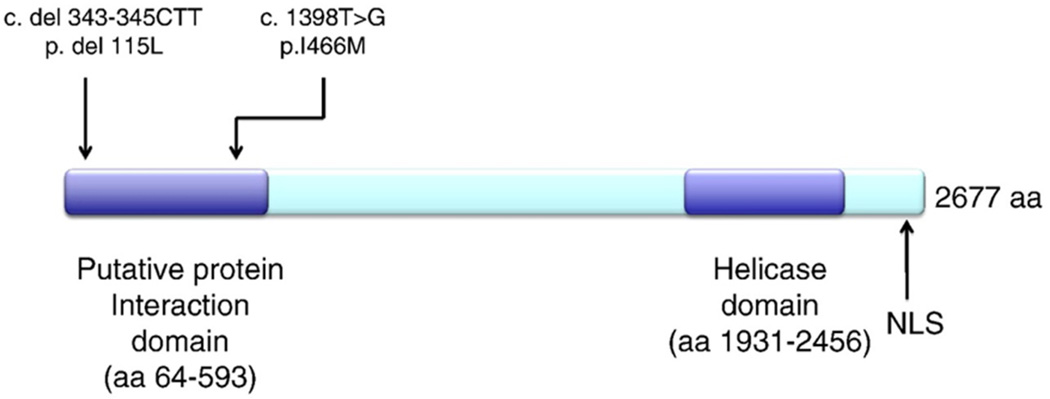
Family history revealed a brother with ataxia (see below), with no known consanguinity in the parents (Fig. 3). She was initially tested for Friedrich's ataxia, which revealed two normal GAA repeat lengths in FRDA. Genetic testing for spinocerebellar ataxias 1, 2, 3, 6 and 7, and POLG1 was normal. Her vitamin E level was within normal limits. AFP level was elevated at 70.5 ng/mL. Given an absence of telangiectasias on exam, SETX was sequenced and analyzed using the UCSC genome browser (http://genome.ucsc.edu), revealing mutations in both copies of SETX (Fig. 4). The first mutation is a 3 bp deletion at nucleotide positions 343 to 345 of CTT in exon 4, which is a known disease-associated mutation for AOA2. The second mutation is a missense mutation T1398G resulting in amino acid change isoleucine466methionine in exon 10, a previously unreported mutation, but predicted to be pathologic by SIFT and PolyPhen-2.1.0 (HumVar).
Figure 3.
Pedigree of AOA2 siblings. Shaded circle or square indicates an individual with ataxia.
Figure 4.
A novel missense mutation and known deleterious microdeletion in SETX causing AOA2. A 3 bp deletion was found at nucleotide positions 343 to 345 of CTT in exon 4, which is a known disease-associated mutation for AOA2. A second missense mutation T1398G was found, resulting in amino acid change I466M in exon 10, a previously unreported mutation. Both mutations are located within the putative protein interaction domain near the N-terminus of the SETX.
At age 71, patient II-2 endorsed stable symptoms for the past decade and was living independently. Her most recent neurologic exam at age 71 found full extra-ocular eye movements with horizontal nystagmus with lateral gaze. She had hypermetric saccades but no oculomotor apraxia or vertical nystagmus. Fasciculations were noted in the lower eyelids. She had mild dysarthria and scanning speech, which remained comprehensible. Her peripheral neuropathy slowly progressed and was worse distally than proximally. There was severe wasting of the intrinsic muscles of the hands bilaterally, and she had bilateral foot drop. Sensation to light touch was absent in the feet, and pinprick sensation was decreased distally in the lower legs in a stocking distribution. Vibration sense was absent distally in the upper and lower extremities, affecting the legs greater than the arms. Joint position was absent in the toes. Her ataxia slowly progressed, with severe symmetric dysmetria in the upper and lower extremities. Deep tendon reflexes were absent throughout, with normal tone. Plantar reflexes were mute to stimulation. Skin exam did not reveal any telangiectasias. She was fully oriented, had intact memory and was able to carry on a reasonable and appropriate conversation, but no formal neuropsychological testing was available.
Patient II-1 is a 76 year old Caucasian male and the older sibling of patient II-2 (Fig. 3). He has had similar but more severe symptoms compared to his sister. His onset of gait ataxia was at age 5. He dropped out of high school due to his disability from gait abnormalities. He was admitted to the neurology service at King County Hospital – Seattle (now Harborview Medical Center) in June 1958 at age 23 for neurologic workup of his gait abnormalities. He was noted at the time to have nystagmus at rest, which was exacerbated by lateral and up gaze. He was noted to have normal intellect. He had a wide-based stance and ataxic gait, and significant ataxia with gross and fine movements of the upper extremities. His deep tendon reflexes were absent throughout, and plantar responses were equivocal. He was discharged with the diagnosis of spinocerebellar degeneration.
Patient II-1 had a slowly progressive course. He became wheelchair bound at age 48 and retired at age 50. He was noted to have atrophy of the intrinsic hand muscles and weak grip at age 62. He continued to drive until his early 60s and was able to transfer without assistance. Currently at age 76, he lives in an assisted living facility where he requires one-person assistance for all transfers and activities of daily living except for eating, which he is able to do independently with adaptive utensils. On his most recent neurologic exam at age 75, he had severe bilateral foot drop and hand weakness with severe atrophy of the intrinsic muscles of the hands. He did not have truncal ataxia. He had severe dysmetria of the upper and lower extremities. He had no difficulty swallowing and no evidence of tongue atrophy. His eye movements were full with horizontal nystagmus. He had hypometric saccades and saccadic intrusions with lateral gaze pursuit. His speech was mildly dysarthric, which he believed was unchanged since his mid-30s. Sensation to pinprick and sensation to vibration and joint position were absent in the feet but present at the knees. Deep tendon reflexes remained absent throughout, with normal tone and equivocal response to plantar stimulation. He did not have any cognitive impairment that interfered with his daily social activities. He had good language fluency and followed commands without difficulty. He was oriented to person, place and time, could name recent presidents and perform simple arithmetic tasks. He had no difficulty with naming or repetition, could spell “world” forward and backward, and had intact short-term memory. Writing or drawing tasks could not be completed due to his ataxia and neuropathy.
He developed heart failure at age 74, which is now controlled on minimal antihypertensive medication. He is also on low dose aspirin and a statin for secondary prevention of cardiac disease.
Patient II-1 was found to have the same compound heterozygous mutations in the SETX gene as his sister. DNA from I-1, the mother of the siblings, was found to be heterozygous for the 3 bp deletion but not the missense mutation.
These siblings also had a sister who died at the age of 8 months of pneumonia, and identical twin sisters who both died of lung cancer in their late 60s, with no history of ataxia or neuropathy (Fig. 3). Their mother, who is of Italian descent, had a normal neurologic exam documented at age 62, with intact deep tendon reflexes, no foot deformities, no nystagmus or dysarthria, and no dysmetria. She died at age 91 of stomach cancer. The father, whose family was from the British Isles, was not available. There is no history of ataxia on the maternal or paternal side (Table 1).
Table 1.
Comparison of clinical features of AOA2 and AT patients with prolonged survival.
| I (AT) | II-1 (AOA2) | II-2 (AOA2) | |
|---|---|---|---|
| Ataxia onset | Noted by age 2 | Noted by age 5 | Noted by age 14 |
| Wheelchair bound | Age 11 | Age 48 | Age 57 |
| Sensorimotor neuropathy | Yes | Yes | Yes |
| Oculomotor apraxia | No | No | Yes |
| Nystagmus | Yes, lateral | Yes, horizontal and lateral | Yes, horizontal and lateral |
| Dysarthria | Yes | Yes | Yes |
| Cerebellar atrophy | Age 38, MRI brain with midline cerebellar atrophy | Not obtained | Age 59, MRI brain with marked cerebellar atrophy |
| Elevated AFP | 509.9 ng/mL at age 45, repeat 434.6 ng/mL | Not obtained | 70.5 ng/mL at age 70 |
| Cognition | Low normal as a child, not demented in adulthood | Normal intellect | Normal intellect |
| Lifespan | Diagnosed with pancreatic cancer, died 5 months after diagnosis at age 48 | Currently alive at age 76, with well- controlled heart failure | Currently alive and well at age 73 |
4. DISCUSSION
We have identified novel variants in ATM and senataxin associated with longer survival in AT and AOA2, respectively. In addition, our patient with variant AT developed a malignancy that is unusual for AT patients. Most AT patients develop hematologic malignancies and respiratory failure, often dying by the second or third decade of life. We are aware of only one other variant AT patient with longer longevity of 51 years, who died from a hematologic malignancy [7]. Pancreatic adenocarcinoma has not been reported in variant AT to date.
Patient I had multiple cerebellar signs in early childhood—gait ataxia, nystagmus, dysarthria and dysmetria, consistent with classic AT. However, this patient also had clinical findings suggestive of variant AT: choreoathetosis early in disease course, atypical longevity, atypical solid tumor malignancy, and absence of telangiectasias. It is possible that our patient may have expressed some ATM protein with functional kinase activity, as one mutation is a missense mutation that may still be translated, with no mutations present in the catalytic domain. This would further support the diagnosis of variant rather than classic AT, as a recent study found that classic AT was associated with absence or severe reduction in ATM protein and kinase activity, while variant AT patients with increased longevity were associated with residual presence of ATM protein and ATM kinase activity and developed predominantly solid tumors rather than hematologic malignancies [7]. Unfortunately, lymphocytic cell lines derived from our patient were not available to measure ATM kinase activity.
We describe two siblings with AOA2 with compound heterozygous mutations in senataxin, one of which is a previously unreported missense mutation. The missense mutation T1398G resulting in amino acid change isoleucine466methionine is most likely pathologic, as it segregates with the two siblings with symptoms consistent with AOA2, and is not present in the asymptomatic mother, who is heterozygous for the 3 bp deletion c.343–345, a known disease associated mutation. Both of these mutations are located in the N-terminus of SETX, rather than the helical domain. The function of the N-terminus of SETX remains unclear, but is conserved in yeast and vertebrates, and is predicted to include a protein interaction domain [11]. SETX is expressed widely in neurons in the mouse brain, with highest levels in the cerebellum and hippocampus [11]. Loss of function of SETX in human cell lines results in transcription termination and disrupted mRNA processing [13,16]. Work by De Amicis et.al. demonstrated that leukocyte cell lines from AOA2 patients with absent expression of SETX did not have increased sensitivity to induction of chromosomal aberrations. However, a fraction of SETX was found to localize to telomeric sequences, and leukocyte cell lines from AOA2 patients had shorter telomeres compared to age-matched wild type cell lines, suggesting that SETX may be involved in telomere stability via RNA transcription and chromatin compaction [12]. Recent work using the yeast homologue Sen1 and human SETX in cell culture demonstrated an important role for Sen1 in preventing stalling of replication forks at genomic sites where DNA–RNA hybrids (R-loops) form when replication collides head on with transcription [17,18]. Prolonged R-loops were found to recruit double-stranded break repair proteins, likely leading to increased risk of deletions, mutations and rearrangements [18]. Similar findings in the setting of meiotic recombination during spermatogenesis were found in SETX mutant male mice, where accumulation of R-loops in the absence of SETX was associated with unrepaired double-stranded breaks formed during meiotic recombination [19]. The unrepaired double-stranded breaks during meiosis led to arrest of spermatogenesis and apoptosis [19]. In addition, SETX was found to have a necessary role in meiotic sex chromosome inactivation. These findings suggest that the pathology of AOA2 may be due to increased genomic instability, however it remains unclear how SETX functions in the nervous system, as neurons are not thought to undergo active DNA replication or recombination in adults. These results also suggest that genomic areas where transcription is more difficult, such as telomeres and repetitive sequences, may be more susceptible to genomic instability, particularly in the absence of SETX, and could explain why SETX was found to localize to telomeres [17,18].
AT and AOA2 have several clinical features in common, including ataxia, oculomotor apraxia, and severe sensorimotor neuropathy. AFP level is elevated in both diseases and can be used as a screening test when either of these hereditary recessive ataxias are clinically suspected. ATM has been implicated in multiple cell functions overlapping with SETX, including genomic instability, although the two genes have not been found to interact directly. Accelerated shortening of telomeres in lymphocytes from AT patients has been also observed [20]. In addition to the recruitment and activation of ATM in response to double stranded DNA breaks, recent work suggests that unstable telomeres also recruit ATM in a separate pathway, activating the G2/M cell cycle checkpoint and preventing progression to mitosis [21]. Cell lines from both AOA2 and AT patients demonstrate increased sensitivity to oxidative stress, although unlike in AT, AOA2 cells are not sensitive to oxidizing radiation [22]. Recent work has suggested that ATM may play a role in sensing reactive oxygen species independently of the DNA damage response pathway [19]. Current research is revealing complex, multifaceted roles for ATM and SETX in maintaining cell homeostasis and DNA integrity. Further understanding of the functions of these genes in the nervous system can hopefully elucidate the disease mechanisms underlying ataxia.
Our reported patients with AOA2 are unique in that they have been followed longitudinally for over 60 years. Interestingly, despite having the same mutations in SETX, patient II-1 had a more severe phenotype compared to his sibling, patient II-2, with earlier onset of ataxia, and more severe debilitation. Oculomotor abnormalities seemed to improve in both patients in later age. In addition, both siblings have not developed any cognitive impairment that has interfered with social functioning, although formal neuropsychiatric testing could not be obtained. Neuropsychiatric testing of a single AOA2 patient suggested that deficits in executive function, working memory and implicit sequence learning could be part of a cerebellar cognitive process in AOA2 [23]. Recent studies suggest that the cerebellum may have more influence on cognition than previously thought, and it would be interesting to investigate this further in a larger cohort of AOA2 patients with cerebellar degeneration and extended longevity [24,25]. Both of our AOA2 patients demonstrate the slowly progressive ataxia and neuropathy as reported in other AOA2 patients, through the 8th decade of life, but contrary to AT, do not appear to have significant life-shortening complications that would suggest decreased life expectancy with AOA2. As more individuals are identified to have genetic mutations in SETX and ATM, our understanding of the phenotypic spectrum in these autosomal recessive ataxias broadens and will help to elucidate the role of SETX and ATM in the nervous system.
ACKNOWLEDGMENTS
This study is supported by the Department of Veterans Affairs Research and Fellowship funds, and NIH grant P50AG05136 (UW ADRC). The authors would like to thank Ms. Kim Howard for excellent technical support.
Footnotes
CONFLICT OF INTEREST
Dr Bird receives licensing fees from Athena Diagnostics, Inc.
REFERENCES
- 1.Chun HH, Gatti RA. Ataxia–telangiectasia, an evolving phenotype. DNA Repair (Amst) 2004;3(8–9):1187–1196. doi: 10.1016/j.dnarep.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Gatti R. Pagon RA, Bird TD, Dolan CR, Stephens K, Adam MP. Ataxia–telangiectasia. GeneReviews. 1993 [Google Scholar]
- 3.Savitsky K, Bar-Shira A, Gilad S, Rotman G, Ziv Y, Vanagaite L, et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995;268(5218):1749–1753. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- 4.McKinnon PJ. ATM and the molecular pathogenesis of ataxia telangiectasia. Annu Rev Pathol. 2012;7:303–321. doi: 10.1146/annurev-pathol-011811-132509. [DOI] [PubMed] [Google Scholar]
- 5.Hoche F, Seidel K, Theis M, Vlaho S, Schubert R, Zielen S, et al. Neurodegeneration in ataxia telangiectasia: what is new? What is evident? Neuropediatrics. 2012;43(3):119–129. doi: 10.1055/s-0032-1313915. [DOI] [PubMed] [Google Scholar]
- 6.Saunders-Pullman R, Raymond D, Stoessl AJ, Hobson D, Nakamura K, Pullman S, et al. Variant ataxia–telangiectasia presenting as primary-appearing dystonia in Canadian Mennonites. Neurology. 2012;78(9):649–657. doi: 10.1212/WNL.0b013e3182494d51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verhagen MM, Last JI, Hogervorst FB, Smeets DF, Roeleveld N, Verheijen F, et al. Presence of ATM protein and residual kinase activity correlateswith the phenotype in ataxia– telangiectasia: a genotype–phenotype study. HumMutat. 2012;33(3):561–571. doi: 10.1002/humu.22016. [DOI] [PubMed] [Google Scholar]
- 8.Gilad S, Chessa L, Khosravi R, Russell P, Galanty Y, Piane M, et al. Genotype–phenotype relationships in ataxia–telangiectasia and variants. Am J Hum Genet. 1998;62(3):551–561. doi: 10.1086/301755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anheim M, Monga B, Fleury M, Charles P, Barbot C, Salih M, et al. Ataxia with oculomotor apraxia type 2: clinical, biological and genotype/phenotype correlation study of a cohort of 90 patients. Brain. 2009;132(Pt 10):2688–2698. doi: 10.1093/brain/awp211. [DOI] [PubMed] [Google Scholar]
- 10.MoreiraMC, Klur S,WatanabeM, Nemeth AH, Le Ber I, Moniz JC, et al. Senataxin, the ortholog of a yeast RNA helicase, is mutant in ataxia–ocular apraxia 2. Nat Genet. 2004;36(3):225–227. doi: 10.1038/ng1303. [DOI] [PubMed] [Google Scholar]
- 11.Chen YZ, Hashemi SH, Anderson SK, Huang Y, Moreira MC, Lynch DR, et al. Senataxin, the yeast Sen1p orthologue: characterization of a unique protein in which recessive mutations cause ataxia and dominant mutations cause motor neuron disease. Neurobiol Dis. 2006;23(1):97–108. doi: 10.1016/j.nbd.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 12.De Amicis A, Piane M, Ferrari F, Fanciulli M, Delia D, Chessa L. Role of senataxin in DNA damage and telomeric stability. DNA Repair (Amst) 10(2):199–209. doi: 10.1016/j.dnarep.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Suraweera A, Lim Y, Woods R, Birrell GW, Nasim T, Becherel OJ, et al. Functional role for senataxin, defective in ataxia oculomotor apraxia type 2, in transcriptional regulation. Hum Mol Genet. 2009;18(18):3384–3396. doi: 10.1093/hmg/ddp278. [DOI] [PubMed] [Google Scholar]
- 14.Fogel BL, Lee JY, Perlman S. Aberrant splicing of the senataxin gene in a patient with ataxia with oculomotor apraxia type 2. Cerebellum. 2009;8(4):448–453. doi: 10.1007/s12311-009-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verhagen MM, Martin JJ, van Deuren M, Ceuterick-de Groote C, Weemaes CM, Kremer BH, et al. Neuropathology in classical and variant ataxia–telangiectasia. Neuropathology. 2012;32(3):234–244. doi: 10.1111/j.1440-1789.2011.01263.x. [DOI] [PubMed] [Google Scholar]
- 16.Skourti-Stathaki K, Proudfoot NJ, Gromak N. Human senataxin resolves RNA/DNA hybrids formed at transcriptional pause sites to promote Xrn2-dependent termination. Mol Cell. 42(6):794–805. doi: 10.1016/j.molcel.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuce O, West SC. Senataxin, defective in the neurodegenerative disorder ataxia with oculomotor apraxia 2, lies at the interface of transcription and the DNA damage response. Mol Cell Biol. 2013;33(2):406–417. doi: 10.1128/MCB.01195-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alzu A, Bermejo R, Begnis M, Lucca C, Piccini D, Carotenuto W, et al. Senataxin associates with replication forks to protect fork integrity across RNA-polymerase-IItranscribed genes. Cell. 2012;151(4):835–846. doi: 10.1016/j.cell.2012.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becherel OJ, Yeo AJ, Stellati A, Heng EY, Luff J, Suraweera AM, et al. Senataxin plays an essential role with DNA damage response proteins in meiotic recombination and gene silencing. PLoS Genet. 2013;9(4):e1003435. doi: 10.1371/journal.pgen.1003435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metcalfe JA, Parkhill J, Campbell L, Stacey M, Biggs P, Byrd PJ, et al. Accelerated telomere shortening in ataxia telangiectasia. Nat Genet. 1996;13(3):350–353. doi: 10.1038/ng0796-350. [DOI] [PubMed] [Google Scholar]
- 21.Thanasoula M, Escandell JM, Suwaki N, Tarsounas M. ATM/ATR checkpoint activation downregulates CDC25C to prevent mitotic entry with uncapped telomeres. EMBO J. 2012;31(16):3398–3410. doi: 10.1038/emboj.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suraweera A, Becherel OJ, Chen P, Rundle N, Woods R, Nakamura J, et al. Senataxin, defective in ataxia oculomotor apraxia type 2, is involved in the defense against oxidative DNA damage. J Cell Biol. 2007;177(6):969–979. doi: 10.1083/jcb.200701042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klivenyi P, Nemeth D, Sefcsik T, Janacsek K, Hoffmann I, Haden GP, et al. Cognitive functions in ataxia with oculomotor apraxia type 2. Front Neurol. 2012;3:125. doi: 10.3389/fneur.2012.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46(7):831–844. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage. 2012;59(2):1560–1570. doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]