Abstract
Background
Central venous catheters are frequently used for hemodialysis vascular access while patients await placement and maturation of an arteriovenous (AV) fistula or graft. Catheters may cause central vein stenosis, which can adversely affect vascular access outcomes. We compared the vascular access outcomes in patients with a history of ipsilateral and contralateral dialysis catheters.
Study design
Retrospective analysis of a prospective computerized vascular access database.
Setting & Participants
Patients at a large medical center who initiated hemodialysis with a catheter and subsequently received a fistula (n=233) or graft (n=89).
Predictor
History of central venous catheter placement ipsilateral vs. contralateral to the AV fistula or graft.
Outcome & Measurements
Primary access failure (access never suitable for dialysis) and cumulative access survival (time from successful cannulation until permanent access failure).
Results
Among patients receiving a fistula, the primary failure rate was similar for those with ipsilateral and contralateral catheters (50 vs 53%; HR, 0.94; 95% CI, 0.71–1.26; p=0.7), and the time to fistula maturation was similar (101±41 vs 107±39 days, p=0.5). However, the cumulative fistula survival was inferior in patients with ipsilateral catheters (HR, 2.48; 95% CI, 1.33–7.33; p=0.009). Among patients receiving a graft, the primary failure rate was similar for those with ipsilateral and contralateral catheter (35 vs 38%; HR, 0.92; 95% CI, 0.49–1.73; p=0.8), but the cumulative graft survival tended to be shorter with ipsilateral catheters (HR, 2.04; 95% CI, 0.92–5.38; p=0.07)
Limitations
Retrospective analysis, single medical center.
Conclusions
The primary failure rate of fistulas and grafts is not affected by the presence of an ipsilateral catheter. However, cumulative access survival is inferior in patients with prior ipsilateral catheters. Avoidance of ipsilateral catheters may improve long–term vascular access survival.
Central venous catheters are frequently used for hemodialysis access while patients await placement and maturation of an arteriovenous fistula (AVF) or graft (AVG). Despite significant nationwide efforts to reduce dialysis catheter use, a recent analysis of Centers for Medicare & Medicaid Services data documents their use in approximately 80% of incident and 24% of prevalent hemodialysis patients in the U.S (1). Hemodialysis catheters are associated with multiple complications, including central venous stenosis (2, 3), infection (4, 5), thrombosis (6), and decreased patient survival (7). Less attention has been paid to the potential negative effect of preexisting dialysis catheters on the outcomes of subsequent permanent vascular access. In one observational study, AVFs had a higher failure rate in patients with a prior history of dialysis catheters (8) – an unsettling fact for the nephrology community striving to maximize AVF use. The exact role the catheter plays in the pathogenesis of AVF failure has not been elucidated. One plausible hypothesis is that catheter-induced stenosis of the central vein that serves as an outflow tract for the AVF may lead to hemodynamic changes that preclude AVF maturation or induce its early thrombosis. If so, vascular access outcomes may be inferior when the dialysis catheter is ipsilateral, rather than contralateral, to the AVF or AVG.
To address this clinical issue, we retrospectively interrogated a prospective computerized vascular access database and compared primary failure and cumulative survival of upper extremity AVFs and AVGs placed in patients with a history of a dialysis catheter inserted via the ipsilateral vs contralateral internal jugular vein.
Methods
Study Population
The University of Alabama at Birmingham (UAB) serves approximately 500 hemodialysis patients who receive their routine care at 5 in-center dialysis units in metropolitan Birmingham supervised by UAB nephrologists. The vast majority of these patients’ hospitalizations occur at UAB Hospital, making it possible to track vascular access complications and outcomes. Two access coordinators employed by the UAB Division of Nephrology are responsible for scheduling all access procedures, communication between physicians and dialysis staff, and maintaining a prospective computerized access database of all vascular access procedures (9). We studied the vascular access outcomes in all patients who initiated HD with a dialysis catheter in the internal jugular vein and who received an AVF or AVG after starting dialysis.
Standard of care for access management
The usual practice at our medical center was for an interventional radiologist or nephrologist to insert a right or left internal jugular hemodialysis catheter shortly prior to initiation of HD, using fluoroscopy to ensure optimal positioning of the catheter tip in the right atrium. Subsequently, the transplant surgeons created a permanent access (AVF or AVG), guided by clinical evaluation and preoperative vascular mapping (10). The initial vascular access was placed in the non-dominant (usually, left) upper extremity, unless the mapping indicated unsuitable vessels in that extremity. The permanent access was revised percutaneously or surgically, when necessary to promote access suitability for dialysis. The dialysis catheter was typically removed after 3 consecutive successful cannulations of the permanent access. Patients underwent a fistulogram if there was a clinical suspicion of hemodynamically significant stenosis, with angioplasty performed if >50% stenosis was documented. Thrombectomy was performed surgically if the access clotted within a month of its creation and percutaneously if thrombosis occurred at later time periods. Elective surgical access revision was performed in patients with unsuccessful angioplasty or frequent access thrombosis. An access was deemed to have failed permanently if it could no longer be salvaged percutaneously or surgically to restore its suitability for hemodialysis.
Data Analysis
We retrospectively interrogated the prospective access database to identify 705 patients who initiated dialysis using a central venous catheter during the 6-year period from January 1, 2004 to December 31, 2009. We further narrowed our search to identify those patients fulfilling the following two criteria: (1) no vascular access procedures before HD initiation; and (2) creation of an upper extremity permanent access (AVF or AVG) after HD initiation in the presence of an ipsilateral or a contralateral dialysis catheter. Only the first AVF or AVG placed was included in the analysis, and it was labeled as ipsilateral or contralateral relative to the side the dialysis catheter. We excluded 63 patients with access surgery prior to initiation of HD and 319 patients who did not have a subsequent permanent vascular access created at our medical center. One patient was excluded from analysis because the catheter side was not specified in the database. The remaining 322 study patients included 233 patients receiving a first AVF and 89 receiving a first AVG. For the 23 patients who had serial dialysis catheters placed via both the right and left internal jugular veins prior to cannulation of the AVF or AVG, the catheter was considered to be ipsilateral to the access. The final study population included 69 patients with AVF and ipsilateral catheters, 164 with AVF and contralateral catheters, 27 with AVG and ipsilateral catheters, and 62 with AVG and contralateral catheters (Table 1).
Table 1.
Selection of patients for statistical analysis and primary failure rates
| Access type | total | unknown outcome |
successfully cannulated |
failed before cannulation |
Primary failure rate (%) |
|---|---|---|---|---|---|
| AVF with ipsilateral catheter | 69 | 7 | 31 | 31 | 50% |
| L AVF, L catheter | 28 | 3 | 14 | 11 | 44% |
| R AVF, R catheter | 41 | 4 | 17 | 20 | 54% |
| AVF with contralateral catheter | 164 | 13 | 71 | 80 | 53% |
| L AVF, R catheter | 156 | 12 | 68 | 76 | 53% |
| R AVF, L catheter | 8 | 1 | 3 | 4 | 57% |
| AVF without catheter | 224 | 32 | 87 | 105 | 55% |
| L AVF | 181 | 23 | 72 | 86 | 54% |
| R AVF | 43 | 9 | 15 | 19 | 56% |
| AVG with ipsilateral catheter | 27 | 1 | 17 | 9 | 35% |
| L AVG, L catheter | 10 | 0 | 6 | 4 | 40% |
| R AVG, R catheter | 17 | 1 | 11 | 5 | 31% |
| AVG with contralateral catheter | 62 | 6 | 35 | 21 | 38% |
| L AVG, R catheter | 61 | 6 | 34 | 21 | 38% |
| R AVG, L catheter | 1 | 0 | 1 | 0 | 0% |
Unless otherwise indicated values shown are number of patients.
AVF, arteriovenous fistula; AVG, arterioventous graft; L, left; R, right
Our preliminary analysis indicated that an ipsilateral catheter was more likely in patients receiving an AVF or AVG in the right, rather than left, upper extremity. Since the first fistula is typically placed in the non-dominant (usually, left) arm, patients whose fistula is placed in the right arm may have poor vessel quality. Thus, an inferior outcome of vascular accesses in patients with ipsilateral catheters could potentially reflect poor vessel quality, rather than the presence of a catheter. To address this potential confounder, we analyzed a separate control group of patients during the identical study period, who received the first AVF at least 3 months before initiation of dialysis, and did not require a central venous catheter prior to use of the AVF. This control group included 224 patients, and the AVF outcome was unknown in 32. Of the 192 patients with known AVF outcomes, 158 received a pre-ESRD fistula in the left and 34 in the right upper extremity.
We analyzed the primary failure and cumulative survival rates for all AVFs and AVGs using the computerized access database. A primary access (AVF or AVG) failure was defined as access failure before three consecutive successful cannulations for dialysis (2 needles and dialysis blood flow ≥250 ml/min). Primary AVF failure was typically due to early thrombosis or inadequate AVF maturation. Cumulative access survival was calculated from the first successful cannulation to permanent access failure, regardless of the number of interventions required to maintain access patency. Patient follow-up was censored at death, kidney transplant, transfer to an outside HD unit, or end of study follow-up (December 31, 2010). We obtained permission from the local Institutional Review Board to examine each patient’s medical records for research purposes.
Statistical Analysis
Baseline patient characteristics were compared using t tests for continuous variables and χ2 test for categorical variables. Multiple variable logistic regression analysis was used to evaluate which factors were associated with primary vascular access failure. Kaplan-Meier survival curves were generated to calculate cumulative access survival, and a preliminary analysis using the statistical test of Gill and Schumacher10a confirmed that the assumption of proportional hazards was met (p=0.4). Log rank tests were used to compare the differences between the patient subsets. Multivariate analysis was employed using the Cox proportional hazards model to examine the independent association between catheter location relative to permanent access and cumulative survival of permanent access. Hazard ratios and their confidence intervals (CI) were calculated. P<0.05 was considered statistically significant.
Results
Study Population
Baseline demographic and clinical characteristics of the patients with AVF and AVG and a history of prior dialysis catheters are summarized in Tables 2 and 3. Most study patients were black, reflecting the racial demographics of our dialysis patient population. Women comprised approximately 60% of the AVG group and 45% of the AVF group, reflecting the higher likelihood of graft placement in female patients. The vast majority of patients had hypertension and approximately half had diabetes. A substantial proportion of patients had vascular disease or congestive heart failure. The median time from catheter placement to access creation was 66 (25th–75th percentile, 35–109) days for AVFs and 72 (25th–75th percentile, 44–149) days for AVGs.
Table 2.
Demographic and clinical features of the patients with AVFs
| Variable | Ipsilateral catheter | Contralateral catheter | P value |
|---|---|---|---|
| No. of patients | 69 | 164 | |
| Age (y) | 54 ± 14 | 51 ± 15 | 0.2 |
| Male sex | 41 (59%) | 87 (53%) | 0.4 |
| Black race | 56 (81%) | 128 (78%) | 0.6 |
| Diabetes | 40 (58%) | 69 (42%) | 0.03 |
| Hypertension | 58 (84%) | 149 (91%) | 0.1 |
| CAD | 18 (26%) | 27 (16%) | 0.09 |
| PVD | 12 (17%) | 14 (9%) | 0.05 |
| CVD | 13 (19%) | 16 (11%) | 0.06 |
| CHF | 24 (35%) | 25 (15%) | 0.008 |
| AVF location | 0.4 | ||
| Forearm | 38 (55%) | 101 (62%) | |
| Upper arm | 31 (45%) | 63 (38%) | |
| AVF side | <0.001 | ||
| Left | 27 (39%) | 156 (95%) | |
| Right | 42 (61%) | 8 (5%) | |
| Catheter side | <0.001 | ||
| Left | 27 (39%) | 8 (5%) | |
| Right | 42 (61%) | 156 (95%) | |
Values for continuous variables given as mean +/− SD; values for categorical variables given as number (percentage). AVF had to have been placed after initiation of hemodialysis.
CAD, coronary artery disease; PVD, peripheral vascular disease; CVD, cerebrovascular disease, CHF, congestive heart failure; AVF, arteriovenous fistula.
Table 3.
Demographic and clinical features of the patients with AVGs
| Variable | Ipsilateral catheter | Contralateral catheter | P value |
|---|---|---|---|
| No. of patients | 27 | 62 | |
| Age (y) | 52 ± 11 | 55 ± 15 | 0.4 |
| Male sex | 11 (41%) | 24 (39%) | 0.9 |
| Black race | 23 (85%) | 49 (79%) | 0.5 |
| Diabetes | 17 (63%) | 32 (52%) | 0.3 |
| Hypertension | 23 (85%) | 57 (90%) | 0.5 |
| CAD | 6 (22%) | 12 (19%) | 0.7 |
| PVD | 4 (15%) | 9 (14%) | 0.9 |
| CVD | 6 (22%) | 10 (16%) | 0.5 |
| CHF | 4 (15%) | 16 (26%) | 0.3 |
| AVG location | 0.8 | ||
| Forearm | 8 (30%) | 20 (32%) | |
| Upper arm | 19 (70%) | 42 (68%) | |
| AVG side | <0.001 | ||
| Left | 10 (37%) | 61 (98%) | |
| Right | 17 (63%) | 1 (2%) | |
| Catheter side | <0.001 | ||
| Left | 10 (37%) | 1 (2%) | |
| Right | 17 (63%) | 61 (98%) | |
Values for continuous variables given as mean +/− SD; values for categorical variables given as number (percentage). AVF had to have been placed after initiation of hemodialysis.
CAD, coronary artery disease; PVD, peripheral vascular disease; CVD, cerebrovascular disease, CHF, congestive heart failure; AVG, arteriovenous graft.
AVF Outcomes in patients with prior dialysis catheters
Comparison of contralateral and ipsilateral catheter groups
As compared to AVF patients with contralateral catheters, those with ipsilateral catheters were more likely to have diabetes (58% vs 42%, p = 0.03) and congestive heart failure (35% vs 15%, p = 0.008) (Table 2). Moreover, the ipsilateral catheter group was more likely to have catheters placed in the left internal jugular vein (39% vs 5%, p < 0.001). Otherwise, the two groups were not significantly different with respect to their demographics and comorbid conditions.
Primary AVF Failures
There were 69 patients with an AVF and an ipsilateral catheter, and 7 had an unknown AVF outcome. Of the remaining 62 patients, 31 (or 50%) had a primary AVF failure (Table 1). There were 164 patients with an AVF and a contralateral catheter, and 13 had an unknown AVF outcome. Of the remaining 151 patients, 80 (or 53%) had a primary AVF failure. Thus, the primary AVF failure rate was similar between patients with ipsilateral and contralateral catheters (50% vs 53%; HR, 0.94; 95% CI, 0.71–1.26; p=0.7)(Table 4). The time to AVF maturation was similar for patients with ipsilateral and contralateral catheters (101 ± 41 vs 107 ± 39 days, p = 0.5).
Table 4.
Association of primary access failure and cumulative access survival with access and catheter location.
| Outcome | Access type |
Access timing |
Comparison | HR (95% CI) | p-value |
|---|---|---|---|---|---|
| Primary failure | AVF | Post-HD | Ipsilateral vs contralateral catheter | 0.94 (0.71–1.26) | 0.7 |
| Primary failure | AVG | Post-HD | Ipsilateral vs contralateral catheter | 0.94 (0.50–1.76) | 0.8 |
| Primary failure | AVF | Post-HD | Right vs left | 1.08 (0.80–1.46) | 0.6 |
| Primary failure | AVG | Post-HD | Right vs left | 0.76 (0.34–1.70) | 0.6 |
| Primary failure | AVF | Pre-HD | Right vs left | 1.03 (0.74–1.43) | 0.9 |
| Cumulative survival | AVF | Post-HD | Ipsilateral vs contralateral catheter | 2.48 (1.33–7.33) | 0.009 |
| Cumulative survival | AVG | Post-HD | Ipsilateral vs contralateral catheter | 2.04 (0.92–5.38) | 0.07 |
| Cumulative survival | AVF | Post-HD | Right vs left | 1.86 (0.79–4.38) | 0.2 |
| Cumulative survival | AVG | Post-HD | Right vs left | 1.22 (0.45–3.26) | 0.7 |
| Cumulative survival | AVF | Pre-HD | Right vs left | 1.56 (0.67–4.16) | 0.3 |
Pre-HD indicates prior to HD initiation; post-HD corresponds to after initiation of HD.
AVF, arteriovenous fistula; AVG, arteriovenous graft; HD, hemodialysis;
We ran a multiple variable logistic regression using stepwise selection process to evaluate factors associated with AVF non-maturation. The variables in this model included age, sex, race, diabetes, coronary artery disease, peripheral vascular disease, cerebrovascular disease, congestive heart failure, AVF location (forearm or upper arm), relative catheter location (ipsilateral or contralateral), and catheter side (left or right). In this model, three factors predicted AVF non-maturation: female sex (HR, 2.98; 95% CI, 1.64 –5.39; p<0.001); forearm location (HR, 2.62; 95% CI, 1.42–4.85; p=0.002); and age ≥ 65 years (HR, 2.41; 95% CI, 1.08–5.41; p=0.04).
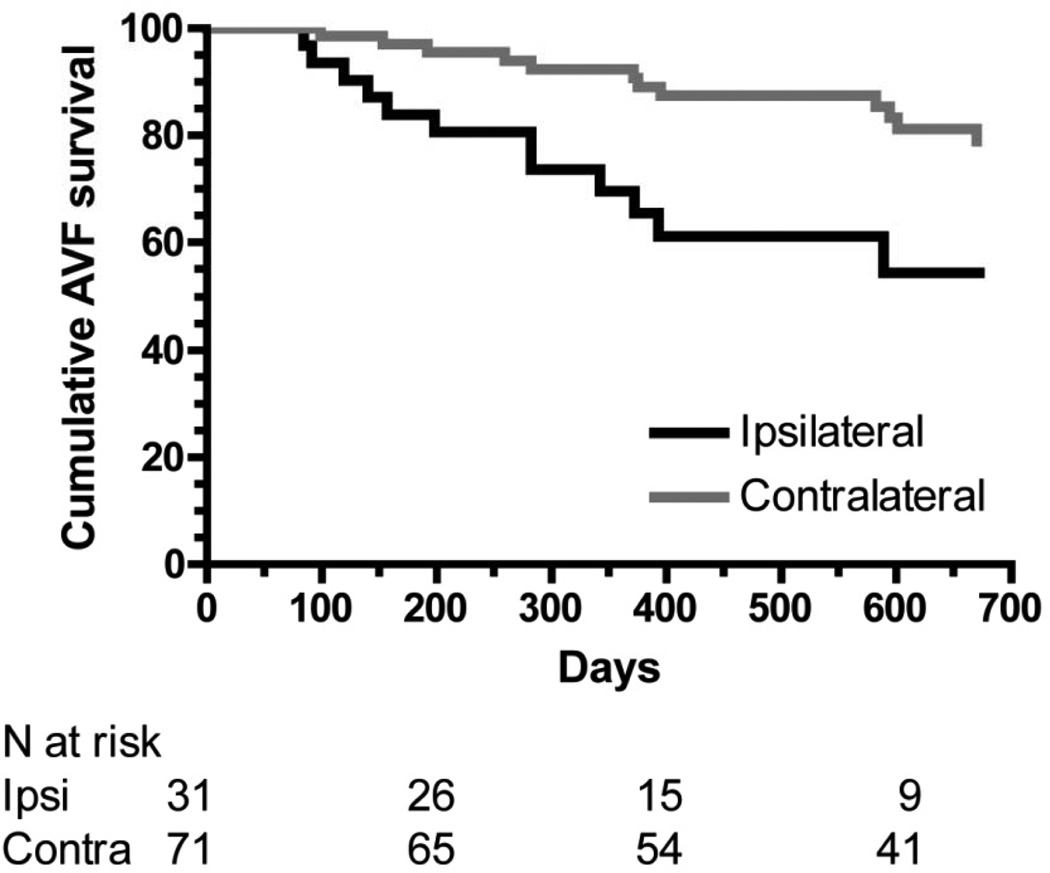
Cumulative AVF Survival
Among patients whose AVFs were used successfully for dialysis, the cumulative AVF survival was lower for the ipsilateral catheter group as compared with the contralateral catheter group (HR, 2.48; 95% CI, 1.33–7.33; p=0.009) (Figure 1). The cumulative AVF survival at 2 years was 54% vs 74%, respectively. Because there were significant baseline differences between the two groups, a multivariate analysis was performed. After building a multivariable model with age, sex, race, diabetes, coronary artery disease, peripheral vascular disease, cerebrovascular disease, congestive heart failure, catheter side, and fistula location, and using a step-wise selection process for AVF survival, the only significant variable in the model was catheter side (ipsilateral vs contralateral), with a HR of 2.55 (95% CI, 1.24–5.29; p = 0.01).
Figure 1.
Cumulative survival of fistulas with ipsilateral dialysis catheters (black line) and contralateral dialysis catheters (gray line). P = 0.009. Analysis is restricted to fistulas that successfully matured.
AVG Outcomes in patients with prior catheters
Comparison of contralateral and ipsilateral catheter groups
There were no significant differences in demographics and comorbidities between the patients with ipsilateral and contralateral catheters (Table 3). However, similar to patients with AVFs, there were more left-sided catheters in the ipsilateral catheter group than in the contralateral catheter group (37% vs 2%, p < 0.001) (Table 3).
Primary AVG Failures
There were 27 patients with AVG and ipsilateral catheters. The AVG outcome was unknown in 1 patient; of the remaining 26 patients, 9 (or 35%) had a primary AVG failure (Table 1). There were 63 patients with AVG and a contralateral catheter, of whom 6 had an unknown AVG outcome. Of the remaining 57 patients, 21 (or 37%) had a primary AVG failure. Thus, the rate of primary AVG failure was similar in patients with ipsilateral and contralateral catheters (35 vs 37%, HR, 0.92; 95% CI, 0.49–1.73; p=0.8).
We ran a multiple variable logistic regression analysis using stepwise selection process to evaluate factors associated with primary AVG failure. The variables in this model included age, sex, race, diabetes, coronary artery disease, peripheral vascular disease, cerebrovascular disease, congestive heart failure, AVF location (forearm or upper arm), relative catheter location (ipsilateral or contralateral), and catheter side (left or right). Only diabetes predicted primary AVG failure (HR, 3.57; 95% CI, 1.39–9.09; p=0.008).
Cumulative AVG Survival
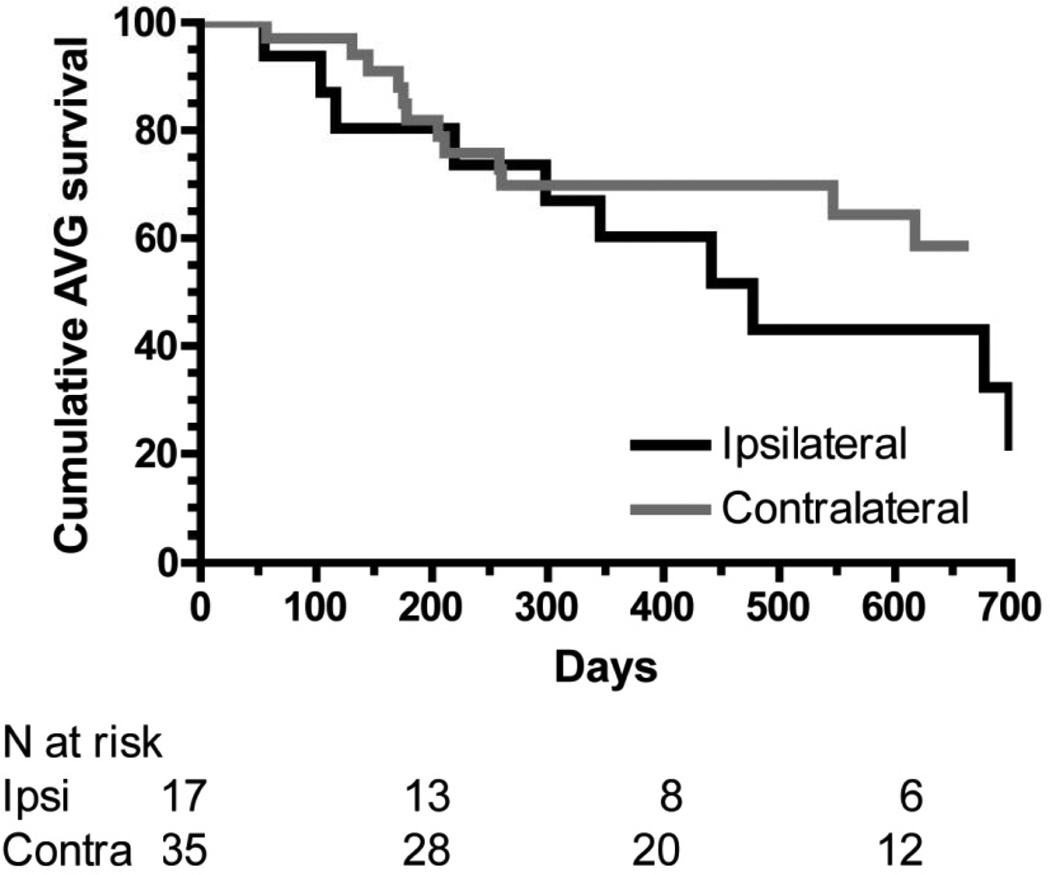
Among patients whose AVGs were used successfully for dialysis, there was a numerically lower cumulative AVG survival in patients with ipsilateral catheters as compared to those with contralateral catheters (HR, 2.04; 95% CI, 0.92–5.38), but this was not statistically significant (p=0.07; Figure 2; Table 4). The cumulative 2-year AVG survival was 22% and 58%, respectively.
Figure 2.
Cumulative survival of grafts with ipsilateral dialysis catheters (black line) and contralateral dialysis catheters (gray line). P = 0.07. Analysis is restricted to grafts that successfully matured.
After building a multivariable model with age, sex, race, diabetes, coronary artery disease, peripheral vascular disease, cerebrovascular disease, congestive heart failure, catheter side, and fistula location, and using a step-wise selection process for AVG survival, there were no significant predictors of AVG survival. The hazard ratio of cumulative AVG survival for patients with ipsilateral catheters vs those with contralateral catheters was 2.78 (95% CI, 0.86–8.93).
Outcomes of AVF in right vs left upper extremities
Among those patients receiving an AVF after initiating dialysis, neither the primary failure rate nor cumulative access survival differed significantly between AVFs placed in the right and left upper extremities (Table 4). Likewise, there was no difference in primary failure or cumulative access survival of AVFs placed prior to initiating dialysis (in the absence of a dialysis catheter) between the 2 extremities (Table 4).
Discussion
The presence of an ipsilateral dialysis catheter was not associated with the primary failure rates of AVFs or AVGs or with the AVF maturation time. In contrast, the presence of an ipsilateral catheter was associated with a substantially shortened cumulative AVF survival; this relationship was non-significant (p=0.07) for cumulative AVG survival. What are the potential explanations for the differential effects of an ipsilateral catheter on the short-term (primary access failure or non-maturation) and long-term (cumulative access survival) outcomes?
One possible short-term effect of a catheter on AVF maturation is its physical obstruction of the access outflow tract (brachiocephalic vein and superior vena cava). Obstruction of the outflow tract may produce dilation of accessory veins that competitively drain the AVF, thereby impeding AVF maturation (11). Our results indicate, however, that the presence of an ipsilateral catheter was not associated with a lower AVF maturation rate. Moreover, obliteration of accessory veins can convert immature AVFs to ones that are successfully cannulated for dialysis (12). Juxta-anastomotic stenosis may also contribute to AVF non-maturation (13), but is probably not affected by presence of an ipsilateral dialysis catheter. Alternatively, the presence of an ipsilateral catheter may simply be a surrogate marker for unmeasured clinical factors associated with fistula non-maturation. We observed an association of primary AVF failure (AVF non-maturation) with older age, female sex, and forearm fistulas, in agreement with previous reports (14–17). However, none of these factors was associated with the presence of an ipsilateral catheter (Table 2), nor was primary AVF failure associated with catheter location in multiple variable logistic regression.
A potential mechanism for the negative effect of ipsilateral catheters on long-term access survival is that catheters incite endothelial damage and venous stenosis that persists even after catheter removal (18–20). In our study, successful cannulation of AVF or AVG defined mature vascular access and was predicated by dialysis catheter removal. Thus, the inferior cumulative access survival was associated with a history of an ipsilateral dialysis catheter, rather than its ongoing presence. The persistent central vein stenosis impedes blood flow along the entire length of the outflow tract. In combination with co-existing venous stenoses commonly afflicting AVFs (21, 22), it may increase the risk of AVF thrombosis. Such a deleterious effect might be expected to be greater with AVGs, as they are more likely than AVFs to thrombose with low access blood flows. The lack of statistically significant association between ipsilateral catheters and cumulative AVG survival may reflect the small number of patients with AVGs or shorter duration of catheter dependence in patients with AVGs as compared to those with AVFs.
Previous publications reported a high incidence of central vein stenosis and decreased AVF survival in patients with prior dialysis catheter use, without addressing whether the catheter was ipsilateral or contralateral to the AVF (2, 8, 18, 23). Only one published study specifically addressed the long-term vascular access survival in the presence of an ipsilateral dialysis catheter (24). However, this study defined access failure as ligation necessitated by symptomatic central vein obstruction, rather than cumulative access survival.
The current NKF-KDOQI (National Kidney Foundation Kidney Disease Outcomes Quality Initiative) guidelines recommend placement of dialysis catheters preferentially in the right internal jugular vein and the initial AVF in the non-dominant upper extremity (usually, the left side)(25). It is therefore not surprising that the most common access combination in our study population was “left AVF + right catheter” (66.7%), followed by “right AVF + right catheter” (17.9%), “left AVF and left catheter” (11.5%), and finally “right AVF and left catheter” (3.4%). About 20% of our study patients had their first ever AVF created in the right upper extremity, presumably because of the left hand dominance or the vascular anatomy observed on preoperative ultrasound mapping. The unexpectedly high (15%) prevalence of left-sided HD catheters in patients initiating dialysis may reflect previous undocumented right-sided non-dialysis central vein catheters.
What are the clinical implications of the present study? Our observations expose serious pitfalls in the intuitively simple decision-making process of vascular access planning. They would favor preferential placement of a left-sided dialysis catheter in a patient starting HD with a maturing right upper extremity AVF. When a patient who has started dialysis with a right-sided catheter is subsequently determined to be most suitable for a right upper extremity (ipsilateral) AVF, the physician may be tempted to relocate the catheter to the left (contralateral) side to improve the chances of the AVF long-term survival. However, the existing catheter may have already damaged the central vein, and delayed catheter relocation may simply extend the damage to the other central vein. Unfortunately, there are no published data on the timing of endothelial injury associated with internal jugular catheters to guide optimal medical management in this scenario.
Our study has some limitations. First, due to its observational design, one must be cautious in inferring causality between the dialysis catheter and subsequent ipsilateral permanent access failure. We cannot exclude the possibility that the presence of an ipsilateral catheter was a surrogate marker for undefined patient characteristics that lead to early access failure. For example, the presence of an ipsilateral catheter was more common in patients whose initial access was in the right upper extremity, and could potentially be a surrogate marker for poor quality vessels. However, the lack of difference in outcomes of pre-hemodialysis initiation AVFs placed in the right or left upper extremities (in the absence of a catheter) makes this hypothesis less likely. Moreover, it would be difficult to justify a randomized clinical study in which patients were deliberately allocated to receive a catheter ipsilateral to the permanent vascular access. Second, this was a single-center study, and the outcomes observed may not generalize to other institutions. However, the demographic and clinical characteristics of our patients, as well their access survival, are comparable to those reported in similar studies (26–28). Finally, additional information, such as dialysis catheter type, imaging studies documenting central vein stenosis, and the types of interventions attempted to maintain a failing AVF or AVG would be helpful in further establishing the pathogenesis of catheter-associated permanent access failure.
In conclusion, our results suggest that an ipsilateral internal jugular vein dialysis catheter is not deleterious to AVF maturation, but may impair cumulative permanent access survival. Although the findings were not statistically significant, a similar effect may potentially occur with ipsilateral dialysis catheters and AVG outcomes. In agreement with these observations, a preliminary report observed a high incidence of central vein stenosis and catheter-dependence among patients receiving an ipsilateral transvenous cardiac device (29). Whenever possible, clinicians should place the dialysis catheter in the internal jugular vein contralateral to the existing or anticipated location of the vascular access, so as to optimize the access lifespan.
Acknowledgements
Portions of this manuscript were presented at the American Society of Nephrology meeting in Philadelphia, PA on November 8–13, 2011.
Support: This study was funded in part by NIH training grant 5T3DK007545-23 ("Interdisciplinary Training in Kidney Related Research") to Dr. Shingarev and by NIH grant 5R01DK085027 to Dr. Allon.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no relevant financial interests.
References
- 1.Collins AJ, Foley RN, Gilbertson DT, Chen SC. The state of chronic kidney disease, ESRD, and morbidity and mortality in the first year of dialysis. Clin J Am Soc Nephrol. 2009;4(Suppl 1):S5–11. doi: 10.2215/CJN.05980809. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal AK, Patel BM, Haddad NJ. Central vein stenosis: a nephrologist's perspective. Semin Dial. 2007;20:53–62. doi: 10.1111/j.1525-139X.2007.00242.x. [DOI] [PubMed] [Google Scholar]
- 3.Wilkin TD, Kraus MA, Lane KA, Trerotola SO. Internal jugular vein thrombosis associated with hemodialysis catheters. Radiology. 2003;228:697–700. doi: 10.1148/radiol.2283020681. [DOI] [PubMed] [Google Scholar]
- 4.Lee T, Barker J, Allon M. Tunneled catheters in hemodialysis patients: reasons and subsequent outcomes. Am J Kidney Dis. 2005;46:501–508. doi: 10.1053/j.ajkd.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 5.Stevenson KB, Hannah EL, Lowder CA, et al. Epidemiology of hemodialysis vascular access infections from longitudinal infection surveillance data: predicting the impact of NKF-DOQI clinical practice guidelines for vascular access. Am J Kidney Dis. 2002;39:549–555. doi: 10.1053/ajkd.2002.31405. [DOI] [PubMed] [Google Scholar]
- 6.Schwab SJ, Buller GL, McCann RL, Bollinger RR, Stickel DL. Prospective evaluation of a Dacron cuffed hemodialysis catheter for prolonged use. Am J Kidney Dis. 1988;11:166–169. doi: 10.1016/s0272-6386(88)80206-3. [DOI] [PubMed] [Google Scholar]
- 7.Xue JL, Dahl D, Ebben JP, Collins AJ. The association of initial hemodialysis access type with mortality outcomes in elderly Medicare ESRD patients. Am J Kidney Dis. 2003;42:1013–1019. doi: 10.1016/j.ajkd.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Rayner HC, Pisoni RL, Gillespie BW, et al. Creation, cannulation and survival of arteriovenous fistulae: data from the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2003;63:323–330. doi: 10.1046/j.1523-1755.2003.00724.x. [DOI] [PubMed] [Google Scholar]
- 9.Allon M, Bailey R, Ballard R, et al. A multidisciplinary approach to hemodialysis access: prospective evaluation. Kidney Int. 1998;53:473–479. doi: 10.1046/j.1523-1755.1998.00761.x. [DOI] [PubMed] [Google Scholar]
- 10.Allon M, Lockhart ME, Lilly RZ, et al. Effect of preoperative sonographic mapping on vascular access outcomes in hemodialysis patients. Kidney Int. 2001;60:2013–2020. doi: 10.1046/j.1523-1755.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- 10a.Gill R, Schumacher M. A simple test of the proportional hazards assumption. Biometrika. 1987;74:289–300. [Google Scholar]
- 11.Turmel-Rodrigues L, Mouton A, Birmelé B, et al. Salvage of immature forearm fistulas for haemodialysis by interventional radiology. Nephrol Dial Transplant. 2001;16:2365–2371. doi: 10.1093/ndt/16.12.2365. [DOI] [PubMed] [Google Scholar]
- 12.Beathard GA, Arnold P, Jackson J, Litchfield T. Lifeline POFoR: Aggressive treatment of early fistula failure. Kidney Int. 2003;64:1487–1494. doi: 10.1046/j.1523-1755.2003.00210.x. [DOI] [PubMed] [Google Scholar]
- 13.Beathard GA, Settle SM, Shields MW. Salvage of the nonfunctioning arteriovenous fistula. Am J Kidney Dis. 1999;33:910–916. doi: 10.1016/s0272-6386(99)70425-7. [DOI] [PubMed] [Google Scholar]
- 14.Lazarides MK, Georgiadis GS, Antoniou GA, Staramos DN. A meta-analysis of dialysis access outcome in elderly patients. J Vasc Surg. 2007;45:420–426. doi: 10.1016/j.jvs.2006.10.035. [DOI] [PubMed] [Google Scholar]
- 15.Miller CD, Robbin ML, Allon M. Gender differences in outcomes of arteriovenous fistulas in hemodialysis patients. Kidney Int. 2003;63:346–352. doi: 10.1046/j.1523-1755.2003.00740.x. [DOI] [PubMed] [Google Scholar]
- 16.Peterson WJ, Barker J, Allon M. Disparities in fistula maturation persist despite preoperative vascular mapping. Clin J Am Soc Nephrol. 2008;3:437–441. doi: 10.2215/CJN.03480807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huber TS, Ozaki CK, Flynn TC, et al. Prospective validation of an algorithm to maximize native arteriovenous fistulae for chronic hemodialysis access. J Vasc Surg. 2002;36:452–459. doi: 10.1067/mva.2002.127342. [DOI] [PubMed] [Google Scholar]
- 18.MacRae JM, Ahmed A, Johnson N, Levin A, Kiaii M. Central vein stenosis: a common problem in patients on hemodialysis. ASAIO J. 2005;51:77–81. doi: 10.1097/01.mat.0000151921.95165.1e. [DOI] [PubMed] [Google Scholar]
- 19.Palabrica T, Lobb R, Furie BC, et al. Leukocyte accumulation promoting fibrin deposition is mediated in vivo by P-selectin on adherent platelets. Nature. 1992;359:848–851. doi: 10.1038/359848a0. [DOI] [PubMed] [Google Scholar]
- 20.Forauer AR, Theoharis C. Histologic changes in the human vein wall adjacent toindwelling central venous catheters. J Vasc Interv Radiol. 2003;14:1163–1168. doi: 10.1097/01.rvi.0000086531.86489.4c. [DOI] [PubMed] [Google Scholar]
- 21.Falk A. Maintenance and salvage of arteriovenous fistulas. J Vasc Interv Radiol. 2006;17:807–813. doi: 10.1097/01.RVI.0000217928.43396.35. [DOI] [PubMed] [Google Scholar]
- 22.Roy-Chaudhury P, Spergel LM, Besarab A, Asif A, Ravani P. Biology of arteriovenous fistula failure. J Nephrol. 2007;20:150–163. [PubMed] [Google Scholar]
- 23.Schillinger F, Schillinger D, Montagnac R, Milcent T. Post catheterisation vein stenosis in haemodialysis: comparative angiographic study of 50 subclavian and 50 internal jugular accesses. Nephrol Dial Transplant. 1991;6:722–724. doi: 10.1093/ndt/6.10.722. [DOI] [PubMed] [Google Scholar]
- 24.Salgado OJ, Urdaneta B, Colmenares B, García R, Flores C. Right versus left internal jugular vein catheterization for hemodialysis: complications and impact on ipsilateral access creation. Artif Organs. 2004;28:728–733. doi: 10.1111/j.1525-1594.2004.07316.x. [DOI] [PubMed] [Google Scholar]
- 25.National Kidney Foundation. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Vascular Access. Am J Kidney Dis. 2006;48(suppl 1):S176–S322. doi: 10.1053/j.ajkd.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 26.Oliver MJ, McCann RL, Indridason OS, Butterly DW, Schwab SJ. Comparison of transposed brachiobasilic fistulas to upper arm grafts and brachiocephalic fistulas. Kidney Int. 2001;60:1532–1539. doi: 10.1046/j.1523-1755.2001.00956.x. [DOI] [PubMed] [Google Scholar]
- 27.Dixon BS, Novak L, Fangman J. Hemodialysis vascular access survival: upper-arm native arteriovenous fistula. Am J Kidney Dis. 2002;39:92–101. doi: 10.1053/ajkd.2002.29886. [DOI] [PubMed] [Google Scholar]
- 28.Salman L, Alex M, Unger SW, Contreras G, Lenz O, Asif A. Secondary autogenous arteriovenous fistulas in the "fistula first" era: results of a longterm prospective study. J Am Coll Surg. 2009;209:100–105. doi: 10.1016/j.jamcollsurg.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 29.Drew DA, Meyer KB, Weiner DE. Transvenous cardiac device wires and vascular access in hemodialysis patients. Am J Kidney Dis. 2011;58:494–496. doi: 10.1053/j.ajkd.2011.05.005. [DOI] [PubMed] [Google Scholar]