Abstract
Objectives
Asthma is the most prevalent chronic disease among children enrolled in Medicaid. This study measured real-world adherence and outcomes after an initial prescription for inhaled corticosteroid therapy in a multi-state Medicaid population.
Methods
We conducted a retrospective study among Medicaid-enrolled children aged 5–12 years with asthma in 14 southern states using 2007 Medicaid Analytic eXtract file claims data to assess adherence and outcomes over the 3 months following an initial prescription drug claim for inhaled corticosteroids (ICS-Rx). Adherence was measured by the long-term controller-to-total asthma drug claims ratio.
Results
Only one-third of children (33.4%) with an initial ICS-Rx achieved a controller-to-total drug ratio >0.5 over the next 90 days. Children for whom long-term control drugs represented less than half of their total asthma drug claims had a 21% higher risk of emergency department (ED) visit (adjusted odds ratio (AOR) 1.21 [95% CI 1.14, 1.27]), and a 70% higher risk of hospital admission (AOR 1.70 [95% CI 1.45, 1.98]) than those with a controller-to-total asthma drug ratio >0.5.
Conclusion
Real-world adherence to long-term controller medications is quite low in this racially diverse, low-income segment of the population, despite Medicaid coverage of medications. Adherence to long-term controller therapy had a measurable impact on real-world outcomes. Medicaid programs are a potential surveillance system for both medication adherence and ED utilization.
Keywords: Adherence, asthma, emergency department use, low-income, Medicaid, outcomes
Introduction
Asthma is the most common chronic disease in childhood, affecting an estimated 10.2 million children nationally (95 per 1000) [1–3]. Across all age groups, asthma led to 1.9 million emergency department (ED) visits [4], 479 000 hospitalizations [5] and 3388 deaths in the year 2009 [6]. Asthma is both a high-disparity condition and a high-variance condition (gap between usual care and optimal care) [7]. Hospitalization rates are 3.4 times higher for African–American than for white patients, for example [8]. Medicaid clients and uninsured patients are both significantly more likely to be hospitalized and to present through the emergency room during acute flare-ups [9].
Clinical trials have demonstrated the effectiveness of inhaled corticosteroids (ICS) and leukotriene inhibitors (LIs) in reducing airway inflammation and controlling asthma symptoms, but medication adherence in clinical trials is unrealistically high [10]. Medication adherence under “real world” conditions is estimated to be as low as 20% [11]. Various studies have shown a correlation between poor medication adherence and adverse outcomes [12,13]. Therefore, we undertook this study to examine asthma controller medication adherence rates in a large multi-state Medicaid population and to study the association of low adherence with adverse outcomes such as ED visits and hospital admissions.
Methods
Study design
This was a retrospective study among Medicaid-enrolled children with asthma in 14 southern states, focusing on adherence and outcomes after receiving a new prescription for inhaled corticosteroid.
Data sources
Our data set comprised 100% of Medicaid claims for calendar year 2007 in 14 southern states (Alabama, Arkansas, Florida, Georgia, Kentucky, Louisiana, Maryland, Missouri, Mississippi, North Carolina, South Carolina, Tennessee, Texas and Virginia). Data were obtained from the Centers for Medicare and Medicaid Services (CMS) in a standard Medicaid Analytic eXtract (MAX) file format with records for enrollee demographics, Medicaid eligibility, service utilization, prescription drugs and payments. We used the MAX inpatient file, outpatient (other services) file, prescription drug file and personal summary file.
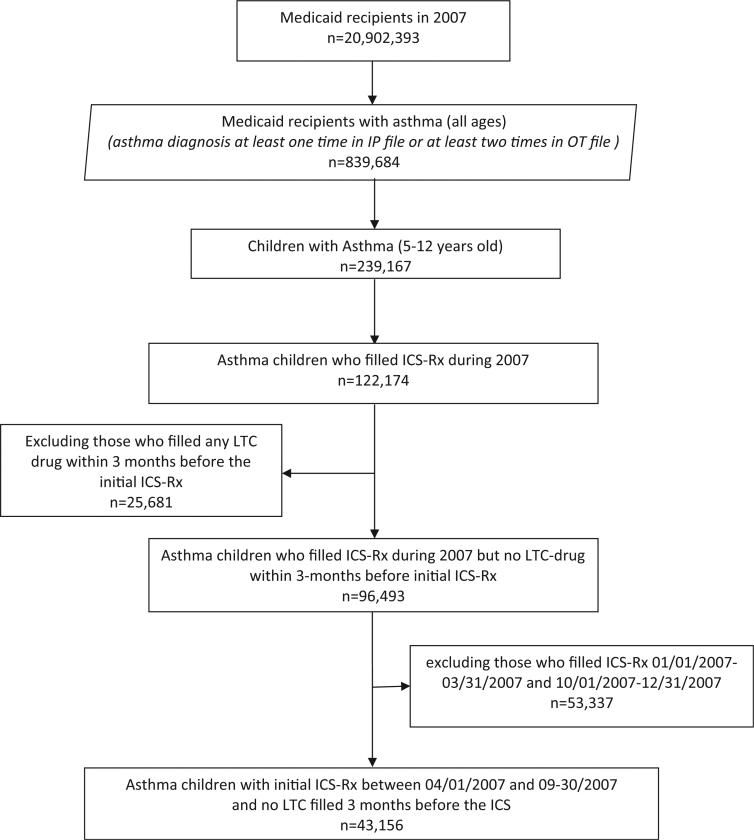
These 2007 Medicaid claims data included 20 902 393 enrollees. We selected a cohort of 839 684 persons who had a diagnosis of asthma for at least one inpatient admission or at least two records on different dates in the outpatient file (ICD-9 code: 493.xx, excluding 493.2x). The number of children age 5–12 years old with asthma was 239 167. Among the children with asthma, less than half (122 174) had any claim for an inhaled corticosteroid prescription (ICS-Rx). Among these, we selected children who had no record of any long-term control prescription drug claims (LTC-Rx, including, inhaled corticosteroids, LIs or oral corticosteroids) in Medicaid during the 90 days prior to their first ICS-Rx. This served as a practical marker or proxy for identifying children whose asthma was now being staged as “persistent” or who were newly deemed by their clinician to have asthma of sufficient severity and persistence to require ICS-Rx as a long-term controller medication. In order to have an adequate window of time for looking back 90 days and following forward 90 days from the first ICS-Rx, we included only children with an initial ICS-Rx during the period from 1 April 2007 to 30 September 2007. After all exclusion and inclusion criteria were applied, we had a cohort of 43 156 asthma children (Figure 1).
Figure 1.
Flow chart of subject inclusion.
Variables and measures
Outcome variable
Emergency room visits
Emergency department services within the 90 days period after ICS initiation were identified in inpatient and outpatient files depending on whether the Medicaid beneficiary was admitted or not admitted to the hospital. For those Medicaid beneficiaries seen in the ED, but not admitted to the hospital, services were identified in the Outpatient file, using revenue center code values of 0450–0459 and 0981. Those who were seen in the ED and then admitted to the hospital were identified from the Inpatient file, using the revenue center code values of 0450–0459 and 0981. Other charges associated with ED services were identified in the Inpatient file by place of service. We found all ED visits in both inpatient and outpatient settings and after de-duplication we added them together during the 90-day observational period. Then we categorized the ED visit variable as dichotomous (yes/no) for use in multivariate models.
Hospital admissions
Hospital admissions were captured in the inpatient files and categorized as dichotomous (yes/no).
Independent variables
Controller-to-total asthma medication ratio
This ratio expresses controller medications as a percentage of total asthma medication claims, which includes both controller and short-term reliever medications. This measure has been validated in administrative claims data, including high correlations (0.94) between use of two-quarter claims and full-year claims [14]. We categorized this variable into two groups (≥0.5, <0.5).
Adherence to ICS
The proportion of prescribed days covered (PPDC) was developed for this study to account for the adherence of ICS. The PPDC was defined as the total ICS days’ supply prescribed divided by the 90-day follow-up period. We stratified adherence of ICS by two categories (≥50% and <50%) assessed by using PPDC measure in the model. We also measured frequency of no refill, one refill and two or more refills of the initial ICS-Rx in the 90-day follow-up period.
Other independent variables
Gender
Race
We combined race and ethnicity into the categories of Non-Hispanic Whites, African–American, Hispanic and others based on records in the Medicaid personal summary file.
Physician office visits
Clinical services are found in outpatient files. We used procedure code values of 99201-205 and 99211-215 to find these claims in the file. We summed all doctor visits for an individual in the file during the 90-day period after the ICS initiation and then classified doctor visit count into three groups (no doctor visit, one time doctor visit and ≥2 times doctor visit);
Rural/urban status
Rural/urban status was determined by merging the MAX data with county level data from the area resources file (ARF). The ARF aggregates publically available data from multiple sources about socioeconomic and environmental characteristics. Federal Information Processing Standard codes were used to merge the ARF and MAX files. The 2003 Rural/Urban Continuum Codes are from Economic Research Service, Department of Agriculture. These codes form a classification scheme that distinguishes metropolitan (metro) counties by the population size of their metro area and non-metropolitan (non-metro) counties by degree of urbanization and adjacency to a metro area or non-metro areas. The ARFs classify the counties into three groups: Large metro area with at least 1 million residents or more; Small metro area with fewer than 1 million residents and Non-metro area;
Severity of asthma
Drug claims for two or more short-acting beta-agonist (SABA) rescue inhalers within the 90-day period prior to initial ICS prescription were considered an indicator of more severe asthma. Rescue drug SABAs included albuterol, levalbuterol and pirbuterol. To identify these drugs, we linked National Drug Code in Medicaid with LexiComp drug database to identify the specific drug ingredients and therapeutic class for each drug claim. Short-acting beta agonist drug use has been found to be one of the strongest predictors of asthma-related ED visits among patients who meet Healthcare Effectiveness Data and Information Set (HEDIS) criteria for persistent asthma (patients for whom long-term control drug therapy would be indicated) [15].
Statistical analysis
The difference in numerical variables among different controller-to-total asthma medication ratio groups were tested initially with ANOVA. Frequency variances of categorical variables were estimated by chi-squared test. The associated analysis of ED visit/hospital admission and controller-to-total asthma medication ratio group, drug adherence were tested by chi-squared test. Unadjusted odds ratio for ED visit/hospital admission were estimated through logistic regression using gender, race, rural/urban status, doctor visit count, severity of asthma, adherence of ICS treatment and controller-to-total asthma medication ratio group as the principal independent variable. Logistic regression models were repeated with adjustment for multiple covariates, which include age, gender, race, rural/urban status, doctor visit count, severity of asthma, adherence of ICS treatment, controller-to-total asthma medication ratio group and state. The level of statistical significance was set at 0.05 and all tests were two tailed. Analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC).
The difference in outcome variables among people who had no ICS refill, one ICS refill and two refills were tested initially with ANOVA. Frequency variances of categorical variables were estimated by chi-squared test. The associated analysis of ED visit and ICS refill drug adherence were tested by chi-squared test. Unadjusted odds ratios for ED visits were estimated through logistic regression using gender, race, rural/urban status, doctor visit count, severity of asthma and ICS refill level as the principal independent variable. Logistic regression models were repeated with adjustment for multiple covariates, which include gender, race, rural/urban status, doctor visit count, severity of asthma and ICS refill level. The level of statistical significance was set at 0.05 and all tests were two tailed. Analyses were conducted using SAS 9.2.
Results
Overall adherence rates as measured by paid pharmacy claims were low for various demographic sub-groups (Table 1). Thirty-five percent of female children had controller-to-total asthma medication ratios ≥0.5. Racial-ethnic variation in the proportion of children having a controller-to-total asthma medication ratio ≥0.5 ranged from 30.1% for Hispanic children to 35.0% for white children. Children who had more doctor visits and who had more severe asthma were more likely to be adherent to LTC-Rx (controller-to-total asthma medication ≥0.5).
Table 1.
Characteristics of asthma patients (age 5–12 years) who have file ICS drug in 14 Southern states in Medicaid, 2007.
| Controller-to-total asthma medication ratio ≥0.5 | Controller-to-total asthma medication ratio <0.5 | p Value | |
|---|---|---|---|
| N | 14391 (33.4%) | 28765 (66.6%) | |
| Age (mean, SD) | 8.1 (2.4) | 7.8 (2.3) | <0.01 |
| Gender | |||
| Female | 6029 (35.0%) | 11208 (65.0%) | <0.01 |
| Male | 8362 (32.3%) | 17555 (67.7%) | |
| Race | |||
| White | 4483 (35.7%) | 8082 (64.3%) | <0.01 |
| Black | 5927 (33.4%) | 11801 (66.6%) | |
| Hispanic | 2832 (30.1%) | 6570 (69.9%) | |
| Other | 1149 (33.2%) | 2312 (66.8%) | |
| Rural | |||
| Large metro | 5501 (32.7%) | 11325 (67.3%) | <0.01 |
| Small metro | 5233 (34.4%) | 9987 (65.6%) | |
| Non-metro | 3657 (32.9%) | 7453 (67.1%) | |
| Doctor visit count | |||
| No doctor visit | 4486 (37.5%) | 7468 (62.5%) | <0.01 |
| One time | 4101 (35.9%) | 7321 (64.1%) | |
| More than two times | 5804 (29.3%) | 13976 (70.7%) | |
| PPDCa | |||
| ≥50% | 4780 (33.3%) | 9555 (66.7%) | 0.99 |
| <50% | 9611 (33.4%) | 19210 (66.6%) | |
| Severity of asthmab | |||
| Yes | 2259 (19.4%) | 9386 (80.6%) | <0.01 |
| No | 12132 (38.5%) | 19379 (61.5%) |
Proportions of prescription days covered.
Filled SABA ≥2 times within prior 3 months before the initial ICS prescription.
Table 2 shows the crosstab of ED visit/hospital admission with controller-to-total asthma medication type and adherence to ICS drugs. Children who had lower controller-to-total asthma medication ratios were more likely to have ED visits after the initial ICS and also more likely to have a hospital admission.
Table 2.
Association of ED visit/hospital admission versus controller-to-total asthma medication and proportion of prescription days covered (PPDC).
| ED Visit | p Value | Hospital Admission | p Value | |
|---|---|---|---|---|
| Controller-to-total asthma medication ratio | ||||
| ≥0.5 | 2500 (17.4%) | <0.01 | 221 (1.5%) | <0.01 |
| <0.5 | 5971 (20.8%) | 769 (2.7%) | ||
| PPDCa | ||||
| ≥50% | 2946 (20.6%) | <0.01 | 454 (3.2%) | <0.01 |
| <50% | 5525 (19.2%) | 536 (1.9%) | ||
Proportion of prescription days covered.
Table 3 shows logistic regression results for the outcomes of ED visit and hospital admission. Hispanic children had a 30% lower risk of ED visit compared with non-Hispanic white children, but there was no difference in risk of ED visit between African–American children and white children. Small metro and rural area children were more likely to have an ED visit compared with children in large metro areas. Asthmatic children who had more than two doctor visits also had a 50% higher chance of having an ED visit compared with children with no doctor visits. Additionally, patients with higher adherence to LTC-Rx (higher controller-to-total asthma medication ratio) had a 25% lower risk of ED visit. The same pattern occurred in the odds of hospital admission. Children who had <50% controller-to-total asthma medication showed a 76% higher risk of having a hospital admission. African–American children had 18% higher odds of hospital admission compared with white children. Multivariate logistic regression demonstrated the same pattern. However, after controlling all the covariates, African–American children had a 12% higher risk of having an ED visit and 36% higher of hospital admission. Even adjusting for covariates, low adherence (controller-to-total asthma medication ratio <50%) was associated with a 21% higher risk of an ED visit and 70% higher risk of hospitalization. We also conducted a post hoc analysis of children who were initially excluded from our study cohort because they had received a long-term controller drug in the previous 90 days to assess any possible selection bias, but the same relationship was observed between low ICS-Rx adherence and outcomes (ED-visit or hospital admission).
Table 3.
Unadjusted and adjusted ORs of ED visit/hospital admission.
| ED |
IP |
|||
|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Gender | ||||
| Female | 1.00 | 1.00 | 1.00 | 1.00 |
| Male | 1.00 (0.95,1.05) | 1.00 (0.95,1.05) | 0.89 (0.79,1.01) | 0.88 (0.77,1.00) |
| Race/ethnicity | ||||
| White | 1.00 | 1.00 | 1.00 | 1.00 |
| Black | 0.95 (0.90,1.00) | 1.12 (1.05,1.19)a | 1.18 (1.01,1.38)a | 1.36 (1.14,1.60)a |
| Hispanic | 0.68 (0.64,0.73)a | 0.71 (0.65,0.78)a | 0.96 (0.79,1.16) | 1.01 (0.80,1.29) |
| Other | 1.12 (1.03,1.23)a | 1.21 (1.10,1.33)a | 2.19 (1.78,2.69)a | 2.42 (1.95,3.00)a |
| Rural/urban | ||||
| Large metro | 1.00 | 1.00 | 1.00 | 1.00 |
| Small metro | 1.16 (1.10,1.22)a | 0.94 (0.88,1.00) | 1.00 (0.86,1.16) | 1.13 (0.95,1.33) |
| Non-metro | 1.22 (1.15,1.30)a | 0.95 (0.89,1.02) | 1.03 (0.88,1.21) | 1.23 (0.94,1.35) |
| Doctor visit count | ||||
| No doctor visit | 1.00 | 1.00 | 1.00 | 1.00 |
| One time | 1.04 (0.97,1.11) | 0.87 (0.81,0.94)a | 0.73 (0.59,0.89)a | 0.90 (0.71,1.12) |
| More than two times | 1.50 (1.41,1.59)a | 1.33 (1.25,1.42)a | 1.67 (1.43,1.95)a | 1.99 (1.66,2.40)a |
| Severity of asthmab | ||||
| No | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 1.06 (1.01,1.12)a | 1.04 (0.98,1.10) | 1.16 (1.01,1.33)a | 1.04 (0.90,1.20) |
| PPDCc | ||||
| ≥0.5 | 1.00 | 1.00 | 1.00 | 1.00 |
| <0.5 | 0.92 (0.87,0.96)a | 0.93 (0.88,0.98)a | 0.58 (0.51,0.66)a | 0.62 (0.54,0.70)a |
| Controller-to-total asthma medication ratio | ||||
| ≥0.5 | 1.00 | 1.00 | 1.00 | 1.00 |
| <0.5 | 1.25 (1.18,1.31)a | 1.21 (1.14,1.27)a | 1.76 (1.51,2.05)a | 1.70 (1.45,1.98)a |
p < 0.05.
Filled SABA ≥2 times within prior 3 months before the initial ICS prescription.
Proportions of prescription days covered.
Discussion
A sea-change in the treatment of asthma occurred in the 1980s, with a new understanding of the inflammatory nature of asthma (as opposed to smooth-muscle bronchoconstriction). Chronic treatment with anti-inflammatory long-term controller medications, especially inhaled corticosteroids, quickly became the new standard of care [16]. Clinical trials clearly suggest that asthma outcomes, especially measured by hospitalization and death, should be significantly better with appropriate adherence to long-term controller therapy, especially inhaled corticosteroids. This has been one of the core recommendations of the National Asthma Education and Prevention Program guidelines since the 1990s [17]. Post hoc analysis of a multi-site clinical asthma trial found that inhaled corticosteroid use was associated with both lower impairment (persistent symptoms) and lower risk of a severe outcome such as hospitalization or ED visit [18]. An early prospective trial indicated that inhaled corticosteroids could be associated with up to a 40% reduction in hospitalizations in the first year after diagnosis of asthma [19].
However, real-world compliance with such recommendations is not easily achieved. Our major finding is that even among children who do receive an initial ICS-Rx, two-thirds of these asthmatic children do not sustain adherence through a 90-day follow-up period, even though ICS-Rx as daily long-term controller therapy is the foundation of national guidelines for asthma care.17 Our data confirm previous studies suggesting that asthma medication adherence, specifically long-term controller medication, is quite low in the Medicaid population. A study of children enrolled in Florida Medicaid or Child Health Insurance Program in 2001–2002 found very low adherence rates for long-term controller drugs as measured by medication possession ratios (only 0.16 for any controller medication and 0.08 for inhaled corticosteroid).
Low medication adherence reflects both provider and patient behaviors. A nine-state Medicaid data analysis demonstrated both low prescribing of appropriate medication (only 49% of adult asthma patients received even one Rx claim for any acceptable long-term controller therapy) and low adherence (only 27% filled the LTC-Rx at least twice). Even among those patients who did refill the LTC-Rx, only 16% were consistent in adhering to therapy for 6 months (i.e. medication possession ratio >0.8) [20]. In the four-state sample of the National Asthma Survey, over half (52.8%) of patients with persistent asthma did not report receiving an inhaled corticosteroid, even though they should have been receiving a long-term controller therapy. ICS treatment rates were even lower for African–Americans (odds ratio, 0.495) [21].
In addition, there are different rates of adherence depending on route of administration. While ICS-Rx is clearly the most effective form of long-term controller therapy in clinical trials, it is associated with even lower adherence rates than those for oral agents. Herndon11 demonstrated adherence rates of 20% for ICS-Rx treated children aged 2–18 years and 28% for leukotriene-inhibitor treated children in the Florida and Texas Medicaid population. Beyond adherence, effective delivery of ICS-Rx is technique-dependent. In a study among low-income, African–American children with persistent asthma, 11–15% of children were found to use inhalation techniques that would result in virtually no drug delivery [22].
How adherence is measured matters, because the association between ICS-Rx use and ED visits is bi-directional. More severe asthma and more frequent contact with prescribers (as occurs with either ED visits or physician office visits) both increase the likelihood of an inhaled corticosteroid prescription. In a study of 3435 Medicaid enrolled children age 2–12 years in South Carolina, the children with severe asthma (e.g. more likely to have ED visits) were nearly three times more likely to receive an inhaled corticosteroid and twice as likely to attend a follow-up appointment [23]. In fact, measuring ICS-Rx adherence simply by counting number of refills even in our own data would have shown a negative association with ICS-Rx adherence, even after controlling for SABA Rx claims, which would have contradicted previous research showing daily controller medication as a protective factor associated with lower ED visits.14
The bidirectional nature of this association can be teased out by using a ratio of long-term controller drug claims to total asthma-medication claims, in which the denominator and numerator increase with increasing severity, but the numerator is more specifically reflective of adherence to guideline-concordant therapy. For example, a comparative study of three different measures of long-term controller use in over 90 909 subjects meeting HEDIS criteria for persistent asthma in California and New York found that measuring LTC-Rx use simply by number of claims for a controller drug showed worse outcomes by number of LTC-Rx claims (odds ratio of 1.80 for one LTC-Rx claim and 1.44 for four claims). In contrast, when measured using the ratio of LTC-Rx claims to total asthma drug claims, LTC-Rx adherence was associated with a 23.0% lower likelihood of having an asthma exacerbation (adjusted odds ratio (AOR) = 0.77 [95% CI, 0.75–0.80]) [24]. National survey data indicate that this ratio has increased across the US population of persons with asthma from 0.5 in 1997 to a peak of 0.7 in 2004 [25].
The low adherence to controller medications observed in claims data surveillance of real-world behaviors represents a major impediment to successfully preventing asthma exacerbations and raises questions about the real-world translation of current national guidelines for asthma treatment. It also suggests that Medicaid programs could be an effective resource for improving asthma outcomes if quality of care surveillance and quality improvement interventions were more tightly integrated.
If higher adherence rates could be achieved, would they improve outcomes? Our data suggest that they would. High controller-medication adherence expressed as a proportion of total asthma drug use was associated with a decreased risk of ED utilization. In a study of adult managed care asthma patients, those with LTC-Rx claims representing more than half of their total asthma drug claims had less than half the risk (AOR 0.44 of asthma hospitalizations or ED visits than patients with lower LTC-Rx to total asthma drug ratios [26]. In the previously cited study of Florida and Texas Medicaid children with asthma, adherence with long-term controller medication was associated with a significantly lower rate of the ED visits, but not for hospitalizations. Previous studies of the Florida Medicaid population also showed that higher adherence to long-term controller therapy was associated with lower ED visit rates [27].
The overall low rate of hospitalization for asthma has been cited as a factor that would make outcome improvements unlikely to generate significant savings, given the cost of long-term controller medication when provided to a large population.11 However, a Markov model simulation study for adults with asthma (e.g. parameterized with a higher hospitalization rate than would be assumed with children) suggested that a 10% improvement in asthma long-term controller therapy adherence rates could result in an average savings of $1666 per year to employers, when counting both direct healthcare expenditures and indirect costs (absenteeism and lost productivity) [28].
Whether or not increasing long-term controller drug adherence as measured by the ratio of LTC-Rx claims to total asthma drug claims would actually improve asthma outcomes at a national population level is still open to question. A nationally representative sample of the US population using Medical Expenditure Panel Survey data found a 16.1% increase in the LTC-to-total drug claims ratio from 1997–1998 to 2004–2005, but the decline in exacerbation rates (from 0.27 per year to 0.23) was not statistically significant [29]. A recent study of pre-school children with frequent wheezing episodes also called into question the notion that all children with mild persistent asthma would achieve better outcomes than if they were to use inhaled corticosteroid therapy intermittently at times of wheezing or asthma exacerbation [30].
One area of concern is that racial disparities in adherence and ED utilization persist even after controlling for covariates, even though Medicaid enrollees have similar income levels and the same insurance coverage, provider panels, payment rates and drug formularies [27]. McQuaid et al. [31] demonstrated >7-fold differences in long-term controller adherence between Latino and non-Latino white children in Rhode Island and Puerto Rico. Racial disparities are well documented to be associated with poverty and other social determinants, as well as patient behaviors (adherence and self management), family beliefs and behaviors, environmental air quality and quality of healthcare. A recent review suggested that additional factors such as gene–environment interactions and differences in vitamin D metabolism may also play a role [32].
Assuming that adherence to guideline-concordant long-term controller therapy could impact asthma outcomes, how can adherence rates be improved? Strategies for improving quality of care, medication adherence and asthma outcomes have been subject to frequent study. Interventions can be conducted at the level of the clinical practice, the patient, the school, the community and across large managed care populations [33]. Patient financial incentives have been used to promote medication adherence in other high-impact clinical settings, such as anti-coagulant treatment [34]. A multidimensional intervention based on the pediatric chronic care model showed significant improvements in provider prescribing behaviors and in patient-level outcomes among Medicaid children receiving treatment in participating community health centers [35]. In the context of improving asthma outcomes in high-disparity populations, the Harlem Children's Zone Asthma Initiative has reported a 50% decline in visits to the ED for participants who completed a 12-month program of home visits and the proportion of participants hospitalized overnight decreased by 79% [36].
Limitations of our study are inherent in the use of administrative claims data for surveillance of asthma treatment adherence and adverse outcomes. Claims data are not able to provide clinical data such as peak flow rates or asthma staging. Therefore, the identification of patients who “should have” received long-term controller medication is subject to extrapolation from data available in the billed claims, such as repeated ED visits or frequent refills of short-acting beta agonist therapy. In general, the provision of one long-term controller drug Rx after an ED visit for asthma would be expected to generate on-going LTC-Rx for at least 3 months. Still, an advantage to using claims data to assess adherence is they measure real-world adherence in patients who do not believe they are being observed. Studies which explicitly assess adherence, whether by patient self-report, pill-counting or electronic means are likely to over-estimate real-world adherence.
Another limitation is that Medicaid claims may not capture every prescription fill or refill. Since only final action paid claims are in the data set, prescriptions which were filled but for which claims were rejected or not submitted or not paid are not captured. In a small study of 221 inner city children, Mudd et al. [37] found that there were significant discrepancies between Medicaid claims and pharmacy records for asthma medication, although Medicaid claims data missed fewer of the claims than did the pharmacy records (149 versus 371 out of 1998 prescriptions).
Conclusions
While national guidelines have consistently recommended inhaled corticosteroid and other long-term controller medications for children with persistent asthma, real-world adherence is quite low. Even so, adherence to long-term controller therapy has a measurable impact on real-world outcomes.
Acknowledgments
This research was supported in part by funding from the Agency for Healthcare Research and Quality (AHRQ grant #1R24HS019470) as part of the Multiple Chronic Conditions Research Collaborative. Participation of a student in this research was funded by the Jay Roman Medical Student Endowment funded by NIH/NIMHD 2S21MD000101.
Footnotes
Declaration of interest
Authors have no commercial interests or conflicts to disclose.
References
- 1.Asthma – United States, 1982–1992. MMWR Morb Mortal Wkly Rep. 1995;43:952–5. [PubMed] [Google Scholar]
- 2.Farber HJ, Berenson G, Wattigney W. Trends in asthma prevalence: the Bogalusa heart study. Ann Allergy Asthma Immunol. 1997;78:265–9. doi: 10.1016/S1081-1206(10)63179-1. [DOI] [PubMed] [Google Scholar]
- 3. [12 June 2012];National Health Interview Survey data as reported in Trends in Asthma Prevalence, Health Care Use, and Mortality in the United States. 2001–2010 Available at: http://www.cdc.gov/nchs/data/databriefs/db94.pdf.
- 4. [12 June 2012];National Hospital Ambulatory Medical Care Survey data as reported in Trends in Asthma Prevalence, Health Care Use, and Mortality in the United States. 2001–2010 Available at: http://www.cdc.gov/nchs/data/databriefs/db94.pdf.
- 5. [12 June 2012];National Hospital Discharge Survey data as reported in Trends in Asthma Prevalence, Health Care Use, and Mortality in the United States. 2001–2010 Available at: http://www.cdc.gov/nchs/data/databriefs/db94.pdf.
- 6.Centers for Disease Control and Prevention, National Center for Health Statistics Underlying Cause of Death 2009 on CDC WONDER Online Database, released 2012. [17 June 2012];Data for year 2009 are compiled from the Multiple Cause of Death File 2009, Series 20 No. 2O. 2012 Available at: http://wonder.cdc.gov/ucd-icd10.html.
- 7.Krishnan JA, Diette GB, Skinner EA, et al. Race and sex differences in consistency of care with national asthma guidelines in managed care organizations. Arch Intern Med. 2001;161:1660–8. doi: 10.1001/archinte.161.13.1660. [DOI] [PubMed] [Google Scholar]
- 8. [12 July 2012];National Hospital Discharge Survey data as reported in Asthma Prevalence, Health Care Use and Mortality: United States. 2003–05 Available at: http://www.cdc.gov/nchs/data/hestat/asthma03-05/asthma03-05.htm#fig6.
- 9.Targonski PV, Addington W, Kelleher P, Persky VW. Characteristics of hospitalization for asthma among persons less than 35 years of age in Chicago. J Asthma. 1995;32:365–72. doi: 10.3109/02770909509082761. [DOI] [PubMed] [Google Scholar]
- 10.Sorkness CA, Lemanske RF, Jr, Mauger DT, et al. Long-term comparison of 3 controller regimens for mild-moderate persistent childhood asthma: the Pediatric Asthma Controller Trial. J Allergy Clin Immunol. 2007;119:64–72. doi: 10.1016/j.jaci.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 11.Herndon JB, Mattke S, Evans Cuellar A, et al. Anti-inflammatory medication adherence, healthcare utilization and expenditures among medicaid and children's health insurance program enrollees with asthma. Pharmacoeconomics. 2012;30:397–412. doi: 10.2165/11586660-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Piecoro LT, Potoski T, Talbert JC, Doherty DE. Asthma prevalence, cost, and adherence with expert guidelines on the utilization of health care services and costs in a state Medicaid population. Health Serv Res. 2001;36:357–71. [PMC free article] [PubMed] [Google Scholar]
- 13.Adams RJ, Fuhlbrigge A, Finkelstein JA, et al. Impact of inhaled antiinflamatory therapy on hospitalization and emergency department visits for children with asthma. Pediatrics. 2001;107:706–11. doi: 10.1542/peds.107.4.706. [DOI] [PubMed] [Google Scholar]
- 14.Broder MS, Gutierrez B, Chang E, et al. Ratio of controller to total asthma medications: determinants of the measure. Am J Manag Care. 2010;16:170–8. [PubMed] [Google Scholar]
- 15.Schatz M, Zeiger RS, Yang SJ, et al. Relationship of asthma control to asthma exacerbations using surrogate markers within a managed care database. Am J Manag Care. 2010;16:327–33. [PubMed] [Google Scholar]
- 16.Busse WW. What role for inhaled steroids in chronic asthma? Chest. 1993;104:1565–71. doi: 10.1378/chest.104.5.1565. [DOI] [PubMed] [Google Scholar]
- 17.National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program. U.S. Department of Health and Human Services, National Institutes of Health; Aug, 2007. [13 January 2008]. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma – Full Report 2007. NIH Publication No. 07-4051. Available at: http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf. [Google Scholar]
- 18.Wu AC, Tantisira K, Li L, et al. Childhood Asthma Management Program Research Group. Predictors of symptoms are different from predictors of severe exacerbations from asthma in children. Chest. 2011;140:100–7. doi: 10.1378/chest.10-2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blais L, Suissa S, Boivin JF, Ernst P. First treatment with inhaled corticosteroids and the prevention of admissions to hospital for asthma. Thorax. 1998;53:1025–9. doi: 10.1136/thx.53.12.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Priest JL, Cantrell CR, Fincham J, et al. Quality of care associated with common chronic diseases in a 9-state Medicaid population utilizing claims data: an evaluation of medication and health care use and costs. Popul Health Manag. 2011;14:43–54. doi: 10.1089/pop.2010.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaidya V, Holiday-Goodman M, Pinto S. Demographic disparities in patient-reported use of inhaled corticosteroids among patients with persistent asthma. J Asthma Allergy. 2010;3:101–6. doi: 10.2147/JAA.S11683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Celano MP, Linzer JF, Demi A, et al. Treatment adherence among low-income, African American children with persistent asthma. J Asthma. 2010;47:317–22. doi: 10.3109/02770900903580850. [DOI] [PubMed] [Google Scholar]
- 23.Andrews AL, Teufel RJ, 2nd, Basco WT., Jr Low rates of controller medication initiation and outpatient follow-up after emergency department visits for asthma. J Pediatr. 2012;160:325–30. doi: 10.1016/j.jpeds.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 24.Yong PL, Werner RM. Process quality measures and asthma exacerbations in the medicaid population. J Allergy Clin Immunol. 2009;124:961–6. doi: 10.1016/j.jaci.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 25.Higashi A, Zhu S, Stafford RS, Alexander GC. National trends in ambulatory asthma treatment, 1997–2009. J Gen Intern Med. 2011;26:1465–70. doi: 10.1007/s11606-011-1796-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schatz M, Zeiger RS, Vollmer WM, et al. The controller-to-total asthma medication ratio is associated with patient-centered as well as utilization outcomes. Chest. 2006;130:43–50. doi: 10.1378/chest.130.1.43. [DOI] [PubMed] [Google Scholar]
- 27.Camargo CA, Jr, Ramachandran S, Ryskina KL, et al. Association between common asthma therapies and recurrent asthma exacerbations in children enrolled in a state Medicaid plan. Am J Health Syst Pharm. 2007;64:1054–61. doi: 10.2146/ajhp060256. [DOI] [PubMed] [Google Scholar]
- 28.Nerenz DR, Liu YW, Williams KL, et al. A simulation model approach to analysis of the business case for eliminating health care disparities. BMC Med Res Methodol. 2011;11:31. doi: 10.1186/1471-2288-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rank MA, Liesinger JT, Ziegenfuss JY, et al. The impact of asthma medication guidelines on asthma controller use and on asthma exacerbation rates comparing 1997–1998 and 2004–2005. Ann Allergy Asthma Immunol. 2012;108:9–13. doi: 10.1016/j.anai.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Zeiger RS, Mauger D, Bacharier LB, et al. Daily or intermittent budesonide in preschool children with recurrent wheezing. N Engl J Med. 2011;365:1990–2001. doi: 10.1056/NEJMoa1104647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McQuaid EL, Everhart RS, Seifer R, et al. Medication adherence among Latino and non-Latino white children with asthma. Pediatrics. 2012;129:e1404–10. doi: 10.1542/peds.2011-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill TD, Graham LM, Divgi V. Racial disparities in pediatric asthma: a review of the literature. Curr Allergy Asthma Rep. 2011;11:85–90. doi: 10.1007/s11882-010-0159-2. [DOI] [PubMed] [Google Scholar]
- 33.Tapp H, Hebert L, Dulin M. Comparative effectiveness of asthma interventions within a practice based research network. BMC Health Serv Res. 2011;11:188. doi: 10.1186/1472-6963-11-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volpp KG, Loewenstein G, Troxel AB, et al. A test of financial incentives to improve warfarin adherence. BMC Health Serv Res. 2008;8:272. doi: 10.1186/1472-6963-8-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fifield J, McQuillan J, Martin-Peele M, et al. Improving pediatric asthma control among minority children participating in medicaid: providing practice redesign support to deliver a chronic care model. J Asthma. 2010;47:718–27. doi: 10.3109/02770903.2010.486846. [DOI] [PubMed] [Google Scholar]
- 36. [7 November 2012];A.I.R. Harlem: Impact. Available at: http://www.harlemasthma.org/air/impact/
- 37.Mudd KE, Bollinger ME, Hsu VD, et al. Concordance of Medicaid and pharmacy record data in inner-city children with asthma. Contemp Clin Trials. 2008;29:13–20. doi: 10.1016/j.cct.2007.05.002. [DOI] [PubMed] [Google Scholar]