Abstract
Rationale
Healing after myocardial infarction (MI) involves the biphasic accumulation of inflammatory Ly-6Chigh and reparative Ly-6Clow monocytes/macrophages (Mo/MΦ). According to one model, Mo/MΦ heterogeneity in the heart originates in the blood and involves the sequential recruitment of distinct monocyte subsets that differentiate to distinct macrophages. Alternatively, heterogeneity may arise in tissue from one circulating subset via local macrophage differentiation and polarization. The orphan nuclear hormone receptor, Nr4a1, is essential to Ly-6Clow monocyte production but dispensable to Ly-6Clow macrophage differentiation; dependence on Nr4a1 can thus discriminate between systemic and local origins of macrophage heterogeneity.
Objective
This study tested the role of Nr4a1 in MI in the context of the two Mo/MΦ accumulation scenarios.
Methods and Results
We show that Ly-6Chigh monocytes infiltrate the infarcted myocardium and, unlike Ly-6Clow monocytes, differentiate to cardiac macrophages. In the early, inflammatory phase of acute myocardial ischemic injury, Ly-6Chigh monocytes accrue in response to a brief Ccl2 burst. In the second, reparative phase, accumulated Ly-6Chigh monocytes give rise to reparative Ly-6Clow F4/80high macrophages that proliferate locally. In the absence of Nr4a1, Ly-6Chigh monocytes express heightened levels of Ccr2 on their surface, avidly infiltrate the myocardium, and differentiate to abnormally inflammatory macrophages, which results in defective healing and compromised heart function.
Conclusions
Ly-6Chigh monocytes orchestrate both inflammatory and reparative phases during MI and depend on Nr4a1 to limit their influx and inflammatory cytokine expression.
Keywords: Monocyte, macrophage, myocardial infarction, nuclear hormone receptor, healing
INTRODUCTION
Myocardial infarction (MI) is a leading cause of death worldwide1, 2. Although the mortality rate from MI has declined steadily over the last 50 years due to the application of medical innovations, scientific discoveries, and improvements in public health, the total number of deaths is on the rise. The healing of acute ischemic myocardial injury influences the outcomes post-infarction decisively. Adverse remodeling of the infarcted left ventricle associates with the development of heart failure due to systolic dysfunction and ischemic mitral regurgitation, whereas avoidance of excessive expansion of the infarcted left ventricle associates with better long-term prognosis. Recent experimental work suggests that this critical healing process requires a precise balance between removal of debris and regulation of scar formation. Macrophages may contribute essentially to the healing and regenerative process, but their polyfunctionality requires caution. On the one hand, macrophage depletion in infarcted hearts impairs collagen deposition, necrotic cell clearance, and angiogenesis, predesposing to cardiac rupture and death3. On the other hand, macrophage-induced inflammation can be harmful and cause post-MI heart failure4, 5. The molecular pathways that balance inflammatory and reparative macrophage functions therefore comprise novel potential targets of therapeutic intervention after MI.
Shortly after onset of ischemia, endothelial cells augment adhesion molecule expression that, along with released chemokines, trigger leukocyte mobilization and extravasation6. Within days, numerous leukocytes of medullary and extramedullary origins accumulate in the infarcting myocardium in massive numbers7–11. Among them, phagocytic monocyte-derived macrophages digest dead tissue, efferocytose, influence collagen deposition, and promote angiogenesis. Optimal healing of the myocardium involves the biphasic accumulation of myeloid cells expressing the surface glycoprotein Ly-6C: inflammatory Ly-6Chigh Mo/MΦ accumulate early, while reparative Ly-6Clow Mo/MΦ accumulate later. It is unclear, however, whether the biphasic response occurs via the sequential recruitment of different monocyte subsets which then differentiate to various macrophage subsets, as has been proposed in the MI setting9, or whether the response evolves via local differentiation and polarization of macrophages, as has been shown in other situations12–15. Distinguishing between these scenarios has been difficult to address given the obstacles in tracking monocytes influx and macrophage differentiation in vivo.
The orphan nuclear hormone receptor Nr4a1 (also known as Nur77) has gained attention as a molecular switch that controls many cellular functions involved in inflammation, apoptosis, and proliferation16–19. Recent studies have shown that Nr4a1 is essential to the development of Ly-6Clow monocytes20, but is dispensable to the differentiation of Ly-6Clow macrophages15. We reasoned that if the sequential recruitment hypothesis adequately explains the biphasic response, then the infarcted myocardium should lack Ly-6Clow macrophages in Nr4a1−/− mice. If, however, the biphasic response occurs via local macrophage differentiation and polarization, then Ly-6Clow macrophages should accumulate regardless of Nr4a1 expression. Therefore, studying the inflammatory and healing response in the context of Nr4a1 provided the double advantage of determining the role of Nr4a1 in MI – which is unknown – while also addressing whether the biphasic response after myocardial infarction occurs via the sequential differentiation of monocyte subsets or via the local differentiation of macrophages.
METHODS
For further details, see the online Supplemental Material.
Animals and animal experiments
Female C57BL/6J (WT), B6.SJL-Ptprca Pepcb/BoyJ (CD45.1+) and C57BL/6-Tg(UBC-GFP)30Scha/J mice (GFP+) were purchased from the Jackson Laboratory (Bar Harbor, ME). Nr4a1-deficient mice (Nr4a−/−) were kindly provided by Cathrine C. Hedrick, La Jolla Institute for Allergy and Immunology, USA. All protocols were approved by the Animal Review Committee at Massachusetts General Hospital. C57BL/6J were lethally irradiated and reconstituted with WT and Nr4a−/− bone marrow to generate respective chimeric mice. Myocardial infarction (MI) was induced by permanent ligation of the left anterior descending artery. For adoptive transfer studies monocyte subsets were sorted from blood and spleens of CD45.2+ GFP+ mice and injected into CD45.1+ mice on day 3 post MI.
Histology
Murine hearts were embedded in Tissue-Tek O.C.T compound (Sakura Finetek) and paraffin for sectioning and staining.
Flow cytometry and flow assisted cell sorting
Antibodies used for flow cytometry are listed in the online Supplemental Material. Data were acquired on a BD LSRII and analyzed with FlowJo. Cells were sorted with BD FACS AriaII.
Reverse transcription PCR
RNA was isolated from sorted cells with the RNeasy Micro Kit (Qiagen). Quantitative real-time TaqMan PCR was run on a 7500 PCR thermal cycler (Applied Biosystems).
Echocardiography
Images were acquired with a 13-MHz linear-array transducer (Vivid 7, GE Medical Systems) and analyzed with EchoPacs (GE Medical System). Fractional shortening (FS) was calculated using end-diastolic (ED) and end-systolic (ES) left ventricular inner diameters (LVID) as FS = (LVIDED − LVIDES)/LVIDED. Left ventricular ejection fraction (LVEF) was calculated using end-diastolic and end-systolic volumes as LVEF = (LVEDV − LVESV)/LVEDV × 100%.
Statistics
Results are shown as mean ± SEM. The unpaired Student’s t test was applied to evaluate differences between two study groups. One-way ANOVA with post-hoc Tuckey’s multiple comparisons test was performed when comparing more than two groups. P-values of 0.05 and less denote significant changes.
RESULTS
Nr4a1 expressing myeloid cells accumulate in the heart after MI and promote healing
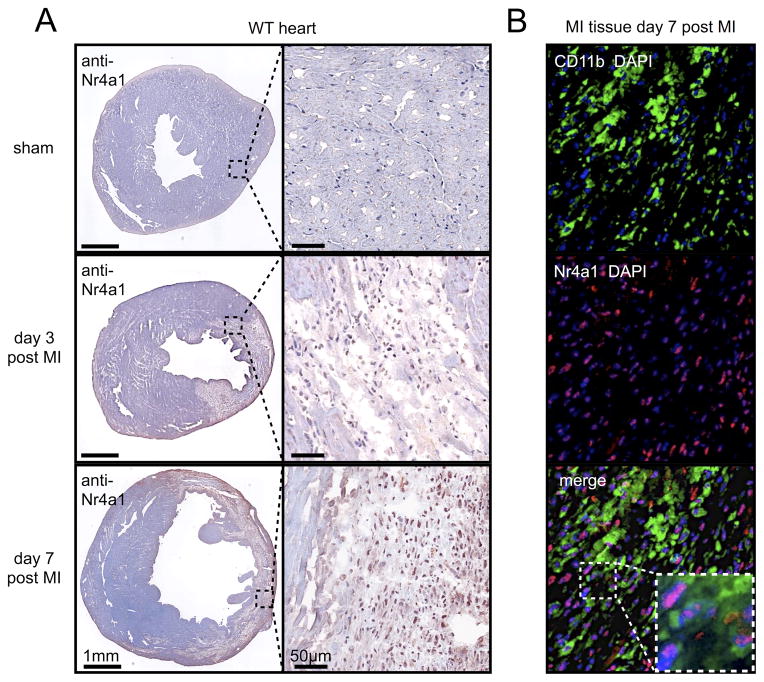
Testing the two concepts of macrophage accumulation in MI required determining whether Nr4a1-expressing myeloid cells infiltrate the infarcted myocardium. First, immunohistochemistry showed enrichment of Nr4a1 in the infarcted area on days 3 and 7 after permanent ligation of the left anterior descending coronary artery (LAD) compared to sham controls (Figure 1A). Second, immunofluorescence revealed that myeloid CD11b+ cells dominated the infiltrate in the infarct and expressed Nr4a1 in the nucleus (Figure 1B). Other cells, including endothelial cells and T cells, which were relatively rare, also stained for Nr4a1 (Online Figure I).
Figure 1. Nr4a1 expressing myeloid cells accumulate in myocardial infarction tissue.
A. Cross sections of heart tissue 3 and 7 days after permanent ligation of the left anterior descending coronary artery (LAD) or sham surgery. Overview and high magnification images of immunohistochemical staining for Nr4a1. B. Immunofluorescence co-staining for Nr4a1 and CD11b of MI tissue 7 days after coronary artery ligation. Representative images from one out of three samples are shown.
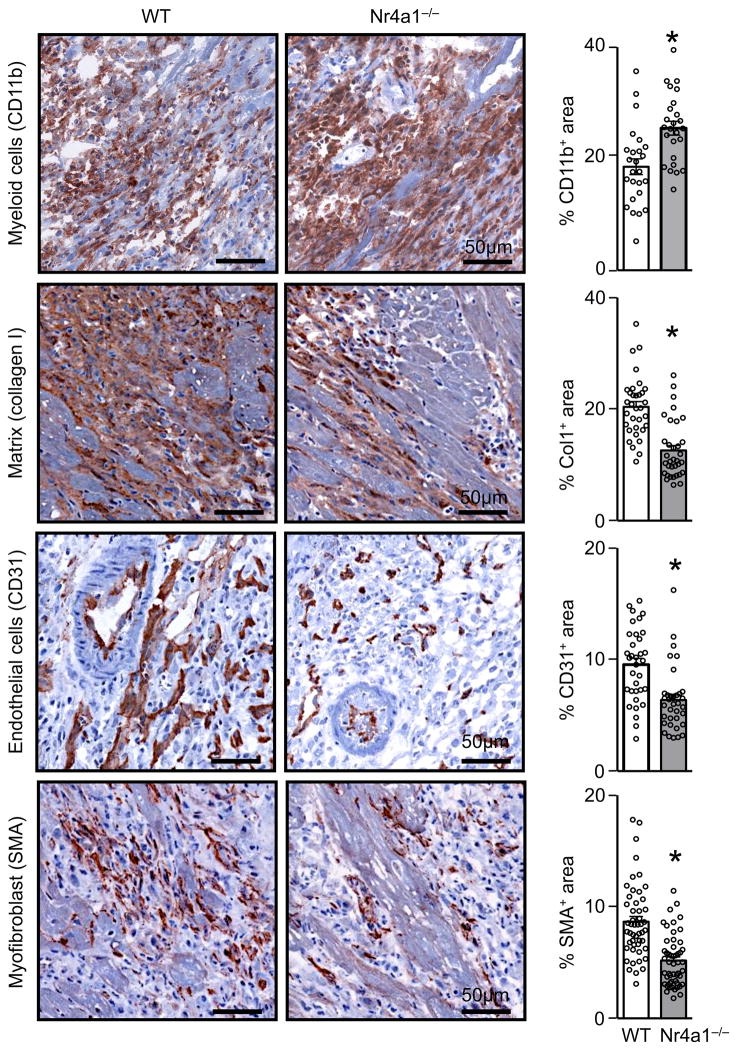
Next we asked whether the receptor is functionally important in the myocardial infiltrate. We generated chimeric mice that lacked Nr4a1 in bone marrow derived, hematopoietic cells, including cardiac macrophages (for simplicity we call these mice Nr4a1−/−) (Online Figure IIA-C). The approach allowed us to test the role of Nr4a1 in hematopoietic cells, while controling for Nr4a1 in stromal cells such as cardiomyocytes, fibroblasts, or endothelial cells. After reconstitution, we permanently ligated the LAD of these mice, along with controls (WT), and evaluated the healing response 7 days later. Compared to WT controls, the myocardium of Nr4a1−/− mice accumulated surprisingly more myeloid CD11b+ cells, but had smaller regions of extracellular matrix deposition, reflected by collagen I, fewer reparative smooth muscle actin (SMA)+ myofibroblasts, and less neovascularization, as determined by staining for CD31+ endothelial cells (Figure 2). Collectively, such a phenotype points to a defect in the healing process, suggesting that Nr4a1 deficiency retards the resolution of inflammation during MI.
Figure 2. Adverse cardiac remodeling in Nr4a1−/− chimeric mice 7 days after myocardial infarction.
Immunohistochemical staining of MI tissue for CD11b, Collagen 1 (Col1), CD31, and non-vascular smooth muscle alpha actin (SMA) in wild-type (WT) and Nr4a1−/− chimeric mice 7 days post MI Quantification of 10 randomly selected fields of view per sample. Results are presented as mean ± SEM, * p ≤ 0.05, n =5 per group.
The biphasic Ly-6Chigh monocyte and Ly-6Clow macrophage response after MI
The Mo/MΦ response after MI is biphasic9. In the first phase, inflammatory Ly-6Chigh Mo/MΦ accumulate and participate in inflammation. In the second phase, reparative Ly-6Clow Mo/MΦ contribute to collagen deposition and scar formation. The two phases may arise either via the sequential recruitment of circulating monocyte subsets or via local macrophage differentiation and polarization9, 12–15. The recent observations that Ly-6Clow monocytes do not differentiate to macrophages, as was previously thought, but patrol vessels and mark endothelial cells for elimination21–23 argues against the sequential recruitment model and necessitates its re-evaluation. We focused on Nr4a1 because Nr4a1−/− mice lack Ly-6Clow monocytes in the blood20 but can generate Ly-6Clow macrophages in tissue15.
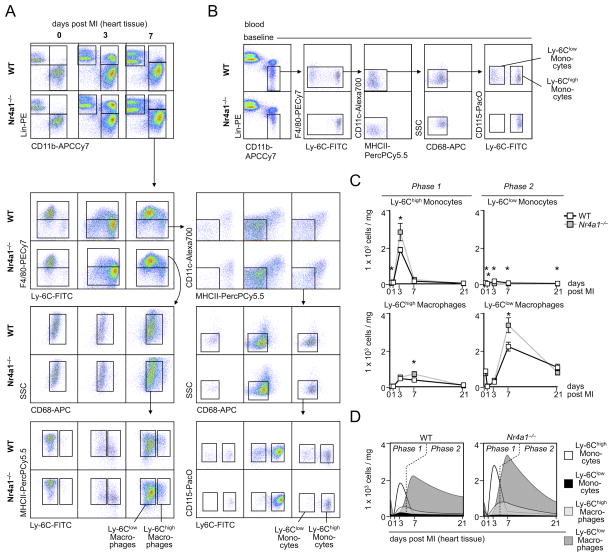
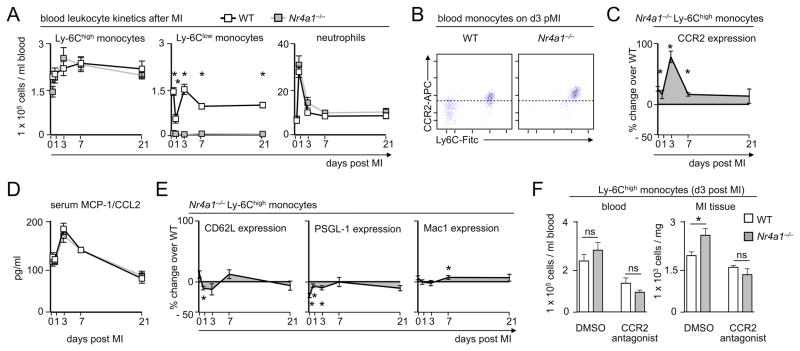
To track cell influx and differentiation, we developed a 9-color flow cytometry method (an improvement over the 4-color method in the original description of the biphasic response9). We profiled the leukocyte population in the myocardium in the steady state, and 3 and 7 days after coronary artery ligation, in both WT and Nr4a1−/− mice. In WT animals, we observed a peak of Ly-6Chigh monocytes on day 3 that waned by day 7, and a surge of Ly-6Clow cells that peaked at day 7 (Figure 3A). This finding agrees with our previous observation9. Nr4a1−/− mice, however, showed an early peak of Ly-6Chigh monocytes and, surprisingly, a late peak of Ly-6Clow macrophages, although these mice did not have Ly-6Clow monocytes in the heart (Figure 3A) or the blood (Figure 3B). Moreover, Nr4a1−/− mice had more, not less, monocytes and macrophages at the two time-points (Figure 3C), which agrees with our histological data (Figure 2). Neutrophil infiltration peaked in MI tissue during the first 3 days, completely regressed thereafter, and was more prominent in Nr4a1−/− mice, while lymphocytes accumulated similarly in both groups (Online Figure III). Given the revised and stringent gating strategy, which discriminates between monocytes and macrophages by the expression of F4/80, CD68, and MHCII, the data also indicate that the early Ly-6Chigh monocyte peak consists predominantly of Ly-6Chigh monocytes that have recently infiltrated the myocardium but have not yet differentiated to F4/80high CD68high MHCII+ macrophages. In contrast, the Ly-6Clow cell peak consists predominantly of mature F4/80high CD68high MHCII+ macrophages. Ly6Clow monocytes infiltrated the tissue at low numbers. Thus, while these data agree with the original description of a biphasic Mo/MΦ response in MI, they argue against sequential recruitment and in favor of a Ly-6Chigh monocyte-dominant phase 1 followed a Ly-6Clow macrophage-dominant phase 2 (Figure 3D).
Figure 3. Enhanced accumulation of Nr4a1−/− monocytes and macrophages in myocardial infarct tissue.
A. Representative images for flow cytometric analysis of MI tissue cell suspensions before, 3 and 7 days after permanent LAD ligation in WT and Nr4a1−/− mice. B. Flow cytometry and analogous gating strategy identifying blood monocyte subsets in WT and Nr4a1−/− chimeras at steady state. C. Flow cytometry based quantification of monocyte and macrophage numbers in MI tissue of WT versus Nr4a1−/− mice before and 1, 3, 7, and 21 days post MI. Results are presented as mean ± SEM, * p ≤ 0.05, n ≥ 4 per group and time point. D. Graphs illustrating flux of monocyte and macrophage subsets during phase 1 (≤ 4 days) and phase 2 (days 4–21) of infarct healing.
Ly-6Chigh monocytes give rise to both the inflammatory and reparative phase
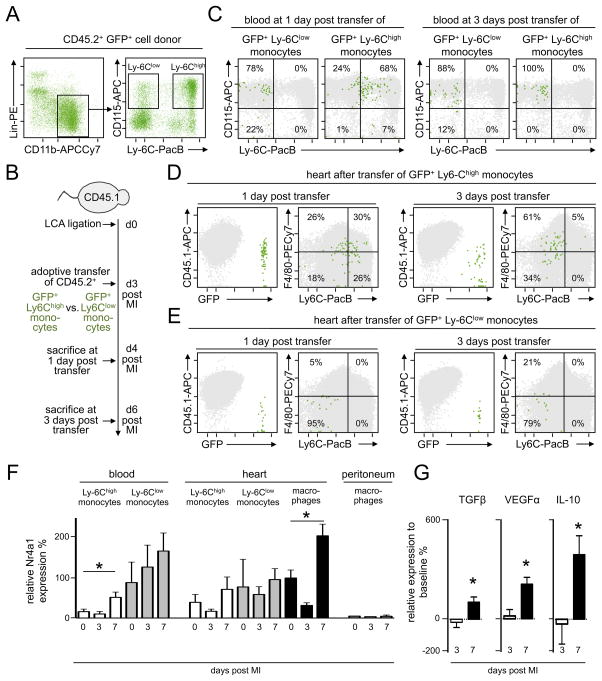
The data in Nr4a1−/− mice precluded the possibility that Ly-6Clow macrophages arise from Ly-6Clow monocytes in MI, and prompted us to identify the precursors of Ly-6Clow macrophages directly with a fate-mapping strategy in WT mice. We injected either Ly-6Chigh or Ly-6Clow monocytes from CD45.2+ GFP+ mice (Figure 4A) to CD45.1+ WT mice that had undergone coronary artery ligation 3 days earlier (Figure 4B). We chose to inject monocytes on day 3 post MI because this time-point corresponded to the monocyte peak in the infarct for both subsets (Figure 3). We then sacrificed the animals 1 day (i.e. 4 days after MI onset) and 3 days (i.e., 6 days after MI onset) later, and tracked the fate of the GFP+ cells. In the blood, we observed GFP+ cells on day 1 and day 3 in both the Ly-6Chigh and Ly-6Clow groups (Figure 4C). Ly-6Clow monocytes remained relatively unchanged between day 1 and day 3, insofar as they continued to be CD115+ Ly-6Clow. However, Ly-6Chigh monocytes were mostly CD115+ Ly-6Chigh on day 1, but entirely CD115+ Ly-6Clow by day 3. These data agree with the long-held assertion that Ly-6Chigh monocytes can convert to Ly-6Clow monocytes24, 25.
Figure 4. Nr4a1low Ly-6Chigh monocytes give rise to Nr4a1high Ly-6Clow macrophages in myocardial infarct tissue.
A. Identification of Ly-6Chigh and Ly-6Clow monocyte subsets in pooled CD45.2+ GFP+ blood and spleen samples for flow assisted cell sorting. B. Illustration of experimental approach with separate adoptive transfer of sorted GFP+ Ly-6Chigh monocytes (1×106) and GFP+ Ly-6Clow monocytes (0.5×106) 3 days after permanent ligation of the LAD in CD45.1+ mice. Sacrifice 1 and 3 days after adoptive transfer. C. Flow cytometric analysis of blood from CD45.1+ recipients of GFP+ monocyte subsets on days 1 and 3 after transfer. Quantification of the percentage of GFP+ cells within the respective gates. Pooled data of two independent experiments. D. Flow cytometric analysis of MI tissue from CD45.1+ recipients of GFP+ Ly-6Chigh monocytes on days 1 and 3 after transfer. Quantification of the percentage of GFP+ cells within the respective gates. Pooled data of two independent experiments. E. Flow cytometric analysis of MI tissue from CD45.1+ recipients of GFP+ Ly-6Clow monocytes on days 1 and 3 after transfer. Quantification of the percentage of GFP+ cells within the respective gates. Pooled data of two independent experiments. F. Quantification (qRT-PCR) of Nr4a1 expression in cells sorted from the blood, MI tissue and unstimulated peritoneal lavage on days 3 and 7 after permanent LAD ligation compared to sham operated C57Bl/6 mice (0 days post MI). Results are presented as mean ± SEM, * p ≤ 0.05, n ≥ 4 per time point. G. Relative expression of VEGFα, TGFβ and IL-10 in sorted Ly-6Chigh monocytes on day 3 (phase 1) and macrophages on day 7 (phase 2) post MI compared to baseline cardiac macrophages. Results are presented as mean ± SEM, * p ≤ 0.05, n ≥ 4 per time point.
We then evaluated the identity of GFP+ cells in the infarct. As in the blood, we detected GFP+ cells in both groups at both times. There were crucial differences, however, between the groups injected with Ly-6Chigh versus Ly-6Clow monocytes. Already 1 day after MI, ~50% of the accumulated Ly-6Chigh monocytes augmented F4/80. By day 3 (i.e., day 6 after MI, which corresponds to phase 2), almost all of the Ly-6Chigh monocytes had lowered Ly-6C and most of those became F4/80high macrophages (i.e., Ly-6Clow F4/80high macrophages) (Figure 4D). In contrast, Ly-6Clow monocytes accumulated but did not increase F4/80 (Figure 4E). These data, as well as data generated using Nr4a1−/− mice, strongly argue that Ly-6Chigh monocytes give rise to both the inflammatory Ly-6Chigh -dominant and reparative Ly-6Clow -dominant Mo/MΦ accumulation phases.
N4ra1 deficiency reduces monocyte recruitment into the myocardium
Having determined that Ly-6Chigh monocytes stem both the inflammatory and reparative phases in the infarcted myocardium, and having observed that in the absence of Nr4a1 the myocardium accumulates higher numbers of monocytes, we returned our attention to the role of Nr4a1, a receptor that has been implicated in many processes involved in cell proliferation and differentiation. We found major fluctuations of Nr4a1 expression in monocytes and macrophages sorted from the blood and the heart during infarct healing (Figure 4F). Considering the results of the previous fate mapping experiment, our data show that Nr4alow Ly-6Chigh monocytes that infiltrate during the inflammatory phase give rise to Nr4a1high macrophages that accumulate in the infarcted tissue during the reparative phase. Macrophages at a distant site such as the peritoneal cavity showed low Nr4a1 expression without modulation (Figure 4G), emphasizing the local nature of the response. Importantly, augmented expression of Nr4a1 by cardiac macrophages in the second phase associated with the rise of IL-10, TGFβ and VEGF (Figure 4G), which are key mediators of the reparative phase9. These data suggest a functional role for Nr4a1 in the cells that participate in MI healing. Future studies will need to determine how Nr4a1 is regulated. Although our data argue that regulation depends on the local environment, the precise mechanism responsible for Nr4a1 fluctuations need further study and may involve Toll-like receptor and NF-κB-dependent signaling26.
We hypothesized that the increased monocyte number in the infarcted myocardium of Nr4a1−/− mice reflects either heightened monocyte production or heightened monocyte recruitment. A number of studies have shown that monocytes infiltrating the myocardium derive from the bone marrow and a splenic reservoir through medullary and extramedullary hematopoiesis8–10, 27. Yet, we found no significant differences in the number of circulating Ly6Chigh monocytes after MI, despite somewhat lower counts in the bone marrow of Nr4a1−/− mice (Figure 5A, Online Figure IV), suggesting that monocyte production does not account for the differences in the infarct. To evaluate recruitment, we first observed that monocytes from Nr4a1−/− mice had a higher surface expression of the chemokine receptor Ccr2 (Figure 5B), which fluctuated and peaked on day 3 (Figure 5C). Ccr2 is critical to the mobilization of Ly-6Chigh monocytes out of the bone marrow and their accumulation in the infarct28–30. The observation that Nr4a1−/− Ly-6Chigh monocytes expressed abnormally high levels of Ccr2 on their surface thus argued for monocyte mobilization and recruitment as a major mechanism. Moreover, coinciding with the surge of Ccr2 expression in Nr4a1−/− mice, we observed a peak of serum Ccl2 (MCP-1), the ligand for Ccr2, in both chimeric groups on day 3 (Figure 5D). We detected relatively minor differences between WT and Nr4a1−/− Ly-6Chigh monocyte surface expression of other adhesion molecules implicated in monocyte recruitment, such as CD62L, PSGL1, or Mac1 (Figure 5E). These data imply that mobilization and recruitment, rather than production, accounted for differences in monocyte infiltration. Indeed, in vivo antagonism of Ccr2 reduced the number of Ly-6Chigh monocytes in the blood and infarct on day 3 and eliminated the differences between the WT and Nr4a1−/− mice (Figure 5F). Thus, Nr4a1 limits monocyte influx by limiting the expression of Ccr2 on Ly-6Chigh monocytes during the inflammatory phase of infarct healing.
Figure 5. Nr4a1-deficiency promotes CCR2-mediated monocyte recruitment to the myocardial infarct tissue.
A. Quantification of monocyte subsets and neutrophils in the blood of WT and Nr4a1−/− mice before and on days 1, 3, 7 and 21 post MI. Results are presented as mean ± SEM, * p ≤ 0.05, n ≥ 5 per group and time point. B. Representative dot plot of flow cytometric staining for CCR2 expression on circulating monocytes in WT and Nr4a1−/− mice 3 days post MI. C. Quantification of CCR2 expression on Ly-6Chigh monocytes by mean fluorescence intensity at indicated time points after MI. Results are shown as mean ± SEM percent change of CCR2 expression in Nr4a1−/− compared to WT control mice, * p ≤ 0.05, n ≥ 5 per group and time point. D. Quantification of serum MCP-1/CCL2 levels in WT and Nr4a1−/− mice before and on days 1, 3, 7 and 21 post MI. Results are presented as mean ± SEM, n ≥ 5 per group and time point. E. Quantification of expression of L-selectin (CD62L), P-selectin glycoprotein ligand-1 (PSGL-1) and integrin Mac1 on Ly-6Chigh monocytes by mean fluorescence intensity at indicated time points after MI. Results are shown as mean ± SEM percent change of marker expression in Nr4a1−/− compared to WT control mice, * p ≤ 0.05, n ≥ 5 per group and time point. F. Quantification of Ly-6Chigh monocyte numbers in peripheral blood and MI tissue on day 3 after permanent LAD ligation in WT and Nr4a1−/− mice treated with CCR2 antagonist (RS504393, 2mg/kg ip. b.i.d.) or vehicle (30% DMSO ip) alone. Results are presented as mean ± SEM, * p ≤ 0.05, n =5 per group.
Nr4a1-macrophages proliferate and are more inflammatory
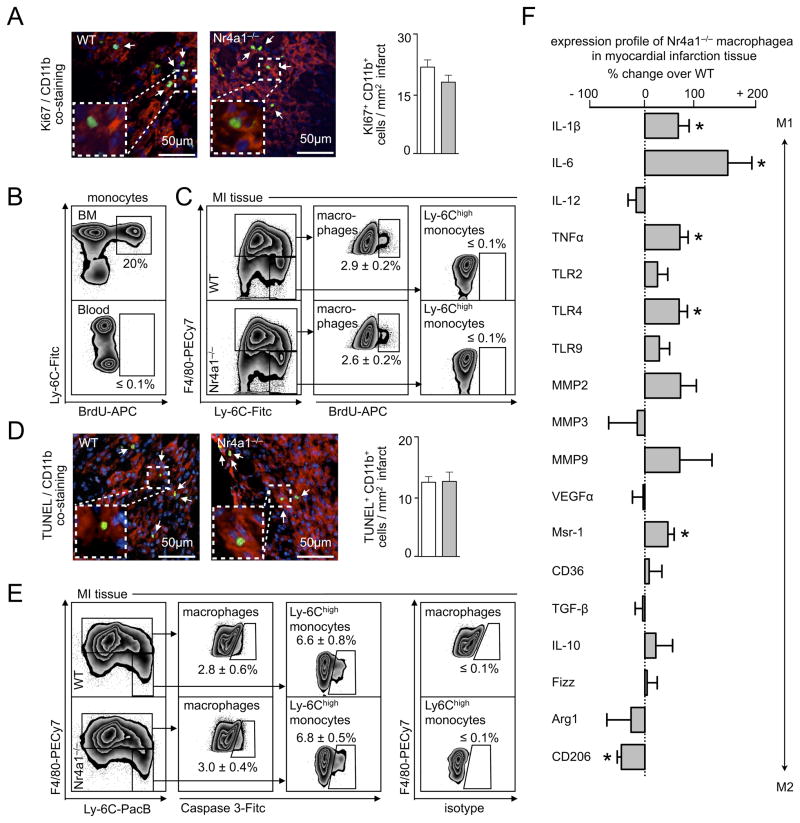
Our data show that Nr4a1 limits monocyte influx by limiting Ccr2 expression. Is Nr4a1 important in differentiated, Nr4a1high, cardiac macrophages (Figure 4F)? Given the reported functions of Nr4a1 in other cells, we first focused on macrophage proliferation and survival. To study proliferation, we utilized Ki-67, a marker whose nuclear expression identifies cells in mitosis, and BrdU, a nucleotide analogue that, if delivered as a short pulse, identifies locally proliferating cells25, 31, 32. We tested WT and Nr4a1−/− mice 7 days after MI, which corresponded to the macrophage peak. Remarkably, we found abundant Ki-67+ cells co-localized with CD11b in the myocardium (Figure 6A), indicating local proliferation of myeloid cells. To identify the cells more precisely, we used a flow cytometry approach with a BrdU-pulse. 2 h after injection of BrdU, when monocytes in the blood (Figure 6B) and myocardium were still BrdU−, already a substantial fraction (i.e., ~3%, which is in the range reported for lesional macrophage proliferation after such a pulse31) of mature F4/80high macrophages in the myocardium were BrdU+ (Figure 6C), indicating local proliferation. Recent studies have identified proliferation as a major mechanism by which macrophages replenish themselves in infection and atherosclerosis31, 33. We now show that monocyte-derived cardiac macrophages also proliferate after MI. Cardiac macrophage proliferation, however, did not depend on Nr4a1 because the rate of proliferation between the two groups did not differ, as determined with either Ki67 (Figure 6A) or BrdU (Figure 6C).
Figure 6. Nr4a1-deficient macrophages are pro-inflammatory, proliferate and die in infarct tissue.
A. Quantification of Ki67+ CD11b+ cells in MI tissue 7 days after permanent LAD ligation in WT and Nr4a1−/− mice on the right and representative merged images for immunofluorescent co-staining on the left. Results are presented as mean ± SEM, n =5 per group. B. Flow cytometric analysis of BrdU incorporation into WT blood and bone marrow (BM) monocytes 2 hours after iv injection of 200μg BrdU. C. Flow cytometric analysis of BrdU incorporation into WT and Nr4a1−/− Ly-6Chigh monocytes and macrophages in MI tissue 7 days after permanent LAD ligation. Results are presented as mean ± SEM, n =5 per group. D. Quantification of TUNEL+ CD11b+ cells in MI tissue 7 days after permanent LAD ligation in WT and Nr4a1−/− mice on the right and representative merged images for immunofluorescent co-staining on the left. Results are presented as mean ± SEM, n =5 per group. E. Flow cytometric analysis of Caspase 3 expression in WT and Nr4a1−/− Ly-6Chigh monocytes and macrophages in MI tissue 7 days after permanent LAD ligation. Results are presented as mean ± SEM, n =5 per group. Isotype staining is shown on the right. F. Gene expression profiling of WT and Nr4a1−/− macrophages sorted from MI tissue 7 days after permanent LAD ligation. Results are presented as mean ± SEM percent change of marker expression in Nr4a1−/− compared to WT control mice, * p ≤ 0.05, n ≥ 5 per group.
To profile survival, we performed TUNEL assays on tissue sections and measured expression of caspase-3 on cardiac monocytes and macrophages in WT and Nr4a1−/− mice. The TUNEL assays identified dying CD11b+ cells, with no differences between the groups (Figure 6D). Flow cytometric analysis of apoptosis using caspase-3 expression supported our TUNEL assay data, and showed that ~7% of monocytes and ~3% of macrophages in both WT and Nr4a1−/− mice were dying (Figure 6E). Together, these data suggest that cardiac macrophages proliferate and die in the infarcted myocardium independently of Nr4a1.
Aside from proliferation and survival, Nr4a1 has also been implicated in controlling expression of various inflammatory genes. Compared to WT controls, cardiac macrophages sorted from Nr4a1−/− mice exhibited a more inflammatory signature, as defined by the transcription of Il1β, Il6, Il2, Tnfα, Tlr2, Tlr4, Tlr9, Mmp2, Mmp3, Mmp9, Vegfα, Msr1, Tgfβ, Il10, Fizz, Arg1, and CD206 (Figure 6F). The data agree with the observation that Nr4a1 limits inflammation during the reparative phase (Figures 2 & 3).
Nr4a1 protects against left ventricular remodeling and dysfunction
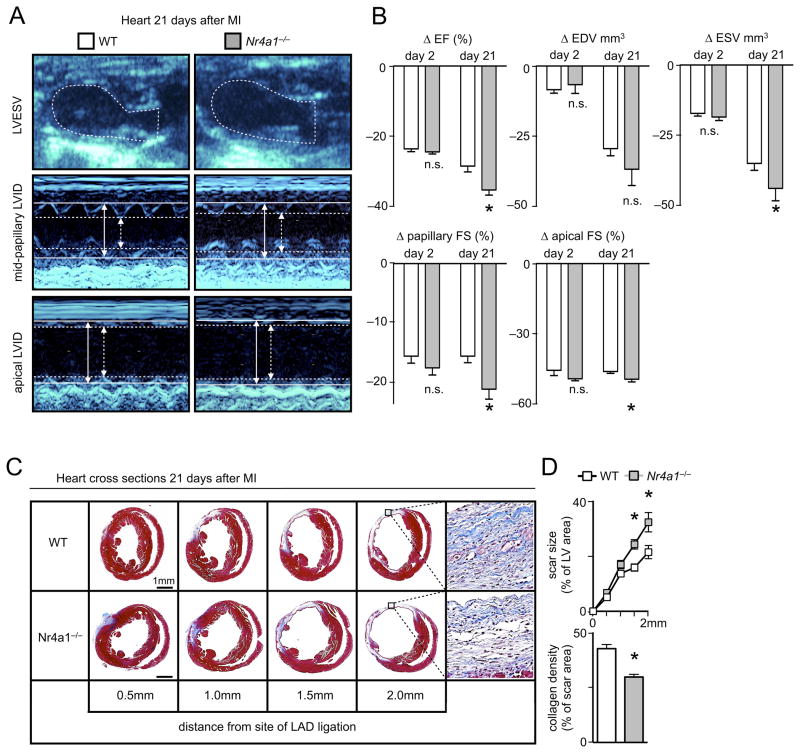
Would Nr4a1-dependent attenuation of cardiac inflammation and promotion of healing translate into better heart function? To test this hypothesis, we performed serial echocardiograms on WT and Nr4a1−/− mice before, and at 2 and 21 days after MI. We tracked individual changes in left ventricular volume, ejection fraction, and fractional shortening during infarct healing and cardiac remodeling. As expected, within 2 days after permanent LAD ligation, acute myocardial ischemia reduced the ejection fraction similarly in both groups, thereby excluding a potential bias by differences in surgery between the groups. By day 21 post coronary artery ligation, however, Nr4a1−/− mice developed more severe left ventricular dysfunction than WT mice, judged by indices such as ejection fraction, fractional shortening, and left ventricular volumes (Figure 7A, B, Online Table I), findings that agree with an increased myocardial scar size with reduced collagen density in Nr4a1−/− mice (Figure 7C, D). Therefore, Nr4a1 attenuates impaired left ventricular function and adverse cardiac remodeling via its actions on monocytes and macrophages in both inflammatory and reparative phases of the infarcted myocardium (Figure 8).
Figure 7. Nr4a1 limits left ventricular dysfunction after myocardial infarction.
A. Representative echocardiography images with long axis B-mode (upper panel) and short axis M-mode views (lower panels) of infarcted hearts from WT and Nr4a1−/− mice on day 21 after permanent LAD ligation. Arrows and lines mark left ventricular inner diameters (LVID) in systole (dashed) and diastole (firm). B. Quantification of individual changes (Δ) in heart parameters compared to baseline on days 2 and 21 post MI. Results are presented as mean ± SEM percent change of marker expression in Nr4a1−/− compared to WT control mice, * p ≤ 0.05, n ≥ 8 per group. C. Representative images of heart cross sections 21 days post MI at increasing distance from the site of coronary artery ligation in Nr4a1−/− and WT control mice, n =5 per group. D. Quantification of scar size in heart cross sections at different levels depending on the distance from the site of coronary artery ligation. Data are presented as a fraction of the left ventricle including the septum, mean ± SEM, * p ≤ 0.05, n =5 per group. Collagen density is quantified in 5 randomly selected fields of view per sample within the scar at 2 mm distance from the site of coronary artery ligation. Results are presented as mean ± SEM, * p ≤ 0.05, n =5 per group.
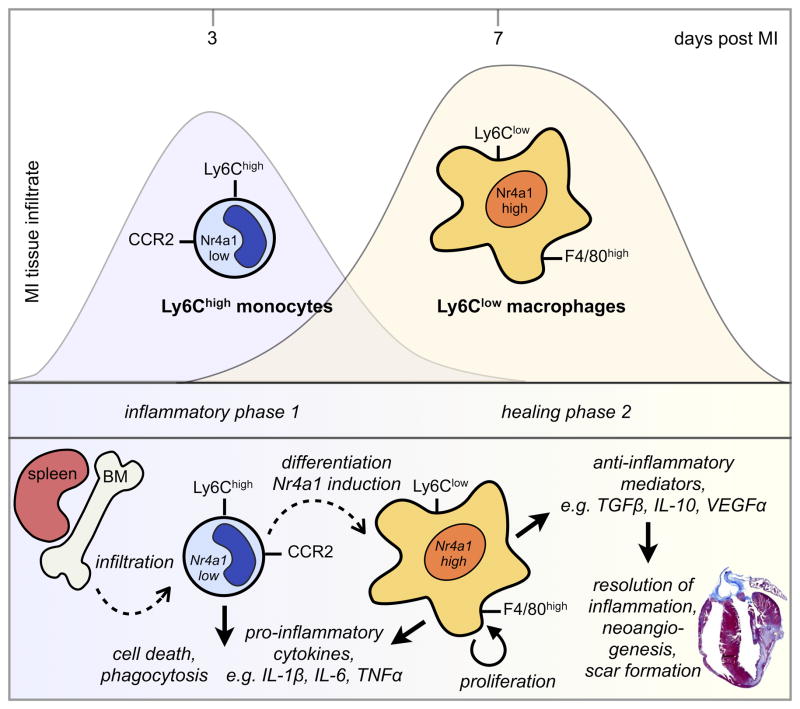
Figure 8. Biphasic model of monocyte/macrophage accumulation in MI tissue.
During the early phase of myocardial infarct healing inflammatory Nr4a1low Ly-6Chigh monocytes from the bone marrow and spleen infiltrate the MI tissue via CCR2. Consequently they differentiate into proliferating Nr4a1high Ly-6Clow macrophages that participate in the resolution of inflammation and cardiac remodeling in the second phase of infarct healing.
DISCUSSION
Nr4a1 is emerging as an important regulator of gene expression in macrophages, but consensus on its function in cardiovascular disease is still lacking. An early report showed that Nr4a1 activates genes involved in inflammation, apoptosis, and cell cycle control18. In murine atherosclerosis, the absence of Nr4a1 aggravated atherosclerosis34, 35, while its over-expression in lesional macrophages reduced inflammatory cytokine production36. The hypothesis that Nr4a1 is predominantly anti-inflammatory is also consistent with arterial injury and restenosis studies concluding that the receptor inhibits vascular outward remodeling and reduces macrophage accumulation37. Recent challenges on the importance of Nr4a1 in atherosclerosis 38, however, have reignited interest in the receptor’s range of influence.
In this study we have shown that the inflammatory response during MI is heightened, healing is compromised, and indices of heart failure worsen in mice lacking expression of Nr4a1 on hematopoietic cells. The events that influence macrophage accumulation and function in response to injury involve monocyte production in the bone marrow, monocyte mobilization from the bone marrow and a splenic reservoir, influx of monocytes into tissue, and differentiation to macrophages. Once differentiated, macrophages influence their environment through production of various mediators including cytokines, proteases, oxidases, and scavenger receptors10. Eventually macrophages die in situ27. This study confirmed that Nr4a1 contributes to Ly-6Clow – but also to Ly-6Chigh – monocyte numbers in the bone marrow20. In response to MI we found that Nr4a1-deficient Ly-6Chigh monocytes express high surface levels of Ccr2, which augments cell mobilization and influx, possibly explaining why, despite somewhat lower numbers in the steady state, monocyte infiltration rises compared to wild type. Once differentiated, cardiac macrophages rely on Nr4a1 to limit inflammation, but Nr4a1 does not appear to regulate apoptosis or proliferation. Thus, Nr4a1 limits both the influx of inflammatory monocytes to the myocardium and the expression of inflammatory mediators by monocyte-derived macrophages. Future studies will need to determine whether those processes are linked and how Nr4a1 influences other cells in MI, such as cardiomyocytes, endothelial cells, and T cells 39–42. It will also be important to discern whether additional mechanisms, perhaps mediated by neutrophils, contribute to the heightened influx of monocytes43.
In addition to providing novel insights into the role of Nr4a1 during MI, this study also revisited the biphasic model of macrophage accumulation, originally described by us and interpreted as evidence for sequential monocyte subset recruitment. We used Nr4a1, which is essential to Ly-6Clow monocytes but dispensable to Ly-6Clow macrophages, as a tool to discriminate between sequential recruitment and local macrophage differentiation. Combining advanced flow cytometry and fate mapping approaches, we show that the biphasic response originates from Ly-6Chigh monocytes, which accumulate in the infarct early and, over time, as they differentiate to macrophages, lose Ly-6C expression. Although this revised model agrees with the initial description of biphasic macrophage accumulation, it departs from it in one critical interpretation: the first Ly-6Chigh monocyte-dominant phase is followed not by a Ly-6Clow monocyte, but by a Ly-6Chigh monocyte-derived Ly-6Clow macrophage-dominant phase. This expanded concept highlights the importance of the local environment in orchestrating inflammation and healing.
Macrophage proliferation, which has garnered considerable attention recently33, 44, 45, is one example of a process that depends on the local environment. Our own observations in experimental atherosclerosis have revealed that proliferation of monocyte-derived macrophages dominates accumulation of lesional macrophages in established disease31. Here, we build on these and other observations46 by providing evidence that monocyte-derived cardiac macrophages proliferate robustly – and independently of Nr4a1 – in the infarcted myocardium. The data suggest that after initial Ly-6Chigh monocyte recruitment and differentiation, macrophages can also replenish themselves locally. Future studies will therefore need to determine the relative contribution and relevance of macrophage proliferation over the course of healing after MI. Our enumeration of Ly-6Chigh and Ly-6Clow monocytes in the myocardium over the first 7 days after MI, combined with data on Ccr2 and Ccl2 expression, supports the concept that the first phase requires intense Ly-6Chigh monocyte infiltration that sharply drops in the second phase. In light of these findings, the role of Ly-6Clow monocytes in MI remains uncertain. Ly-6Clow monocytes might nevertheless contribute indispensably, for example by patrolling the vasculature and marking damaged endothelial cells for disposal21–23.
MI and ischemic cardiomyopathy are major causes of morbidity and mortality worldwide. Decades of work explored the role of cardiomyocytes, cardiac fibroblasts, stem cells, and the extracelluar matrix47–50 in heart function and its failure post MI. Macrophages, which have received much less attention in this context, are emerging as key protagonists that can either promote inflammation or contribute to its resolution. This study has shed new mechanistic light onto monocyte and macrophage functions during MI by showing that the nuclear hormone receptor, Nr4a1, modulates both the early Ly-6Chigh monocyte-dominant inflammatory and the later Ly-6Clow macrophage-dominant reparative phases in the infarcted myocardium.
Supplementary Material
Novelty and Significance.
What Is Known?
Transcription factor Nr4a1 is essential to Ly-6Clow but not Ly-6Chigh monocyte development.
Accumulation of monocytes and macrophages in the infarcted myocardium is biphasic, involving an early Ly-6Chigh inflammatory phase and a later Ly-6Clow reparative phase.
The inflammatory response is exaggerated in LPS-stimulated Nr4a1-deficient bone marrow-derived macrophages.
What New Information Does This Article Contribute?
Ly-6Chigh monocytes give rise to both the Ly-6Chigh inflammatory and Ly-6Clow reparative phases.
Nr4a1-expression limits CCR2-mediated recruitment of Ly-6Chigh monocytes to the infarcting tissue.
Ly-6Clow macrophages derive from Ly-6Chigh monocytes, proliferate, and utilize Nr4a1 to dampen inflammation.
Myocardial infarction involves accumulation of monocytes and macrophages in tissue. Monocytes in mice comprise at least two functionally distinct subsets exhibiting different expression of Ly-6C. The orphan nuclear receptor Nr4a1 is essential to the development of Ly-6Clow but not Ly-6Chigh monocytes. We therefore utilized Nr4a1-deficient mice to study how Ly-6Clow monocytes and Nr4a1 expression in cardiac macrophages contribute to myocardial infarct healing. Ly-6Clow monocytes accumulated at low numbers in the infarct tissue and did not differentiate into cardiac macrophages. In contrast, inflammatory Ly-6Chigh monocytes dominated the infiltrate within the first three days after induction of myocardial infarction, differentiated into Ly-6Clow macrophages, dominated the second healing phase, and proliferated. This process coincided with elevated Nr4a1 expression in cardiac macrophages and increased expression of regenerative factors. Nr4a1 expression, albeit lower in Ly-6Chigh monocytes, corresponded with suppressed expression of chemokine receptor CCR2, limiting recruitment of inflammatory cells to the infarct. Consequently, Nr4a1 deficiency resulted in adverse cardiac remodeling during myocardial infarct healing and impaired cardiac function. Nr4a1 thus regulates the coordinated biphasic monocyte/macrophage response during myocardial infarction.
Acknowledgments
The authors thank Michael Waring and Adam Chicoine for sorting cells (Harvard Medical School). The authors thank Melissa Greene for technical assistance.
SOURCES OF FUNDING
This work was supported in part by NIH grants 1R01HL095612 and R56AI104695 (to F.K.S.). I.H. was supported by the DFG postdoctoral fellowship and the GTH Rudolf-Marx scholarship. L.MS.G. was supported by the Boehringer Ingelheim Fonds and the German Cardiac Society.
Nonstandard Abbreviations and Acronyms
- Arg1
arginase 1
- BrdU
bromodeoxyuridine
- Ccl
C–C chemokine ligand
- Ccr
C–C chemokine receptor
- CD
cluster of differentiation
- ED
end diastolic
- ES
end systolic
- FS
fractional shortening
- IL
interleukin
- LAD
left anterior descending coronary artery
- LPS
lipopolysaccharide
- LVID
left ventricular inner diameter
- Ly-6C
lymphocyte antigen 6C
- Mac1
macrophage antigen 1
- MI
myocardial infarction
- Mo
monocyte
- MΦ
macrophage
- Nr4a1
nuclear receptor subfamily 4, group a, member 1
- PSGL1
P-selectin glycoprotein ligand-1
- TGF
tissue growth factor
- Tlr
toll like receptor
- TNF
tumor necrosis factor
- TUNEL
TdT-mediated dUTP nick end labeling
- VEGF
vascular endothelial growth factor
- WT
wild type
Footnotes
DISCLOSURES
None.
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De Leon FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloom DE, Cafiero ET, Jane-Llopis E, Abrahams-Gessel S, Bloom LR, Fathima S, Feigl AB, Gaziano T, Mowafi M, Pandya A, Prettner K, Rosenberg L, Seligman B, Stein AZ, Weinstein C. The Global Economic Burden of Noncommunicable Diseases. Geneva: World Economic Forum; 2011. [Google Scholar]
- 3.van Amerongen MJ, Harmsen MC, van Rooijen N, Petersen AH, van Luyn MJ. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am J Pathol. 2007;170:818–829. doi: 10.2353/ajpath.2007.060547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation. 2010;121:2437–2445. doi: 10.1161/CIRCULATIONAHA.109.916346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012;110:159–173. doi: 10.1161/CIRCRESAHA.111.243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zouggari Y, Ait-Oufella H, Bonnin P, Simon T, Sage AP, Guerin C, Vilar J, Caligiuri G, Tsiantoulas D, Laurans L, Dumeau E, Kotti S, Bruneval P, Charo IF, Binder CJ, Danchin N, Tedgui A, Tedder TF, Silvestre JS, Mallat Z. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med. 2013;19:1273–1280. doi: 10.1038/nm.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005;96:881–889. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 8.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–166. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan E, Yeap XY, Dehn S, Terry R, Novak M, Zhang S, Iwata S, Han X, Homma S, Drosatos K, Lomasney J, Engman DM, Miller SD, Vaughan DE, Morrow JP, Kishore R, Thorp EB. Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circ Res. 2013;113:1004–1012. doi: 10.1161/CIRCRESAHA.113.301198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avraham-Davidi I, Yona S, Grunewald M, Landsman L, Cochain C, Silvestre JS, Mizrahi H, Faroja M, Strauss-Ayali D, Mack M, Jung S, Keshet E. On-site education of VEGF-recruited monocytes improves their performance as angiogenic and arteriogenic accessory cells. J Exp Med. 2013;210:2611–2625. doi: 10.1084/jem.20120690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, Hartland SN, Snowdon VK, Cappon A, Gordon-Walker TT, Williams MJ, Dunbar DR, Manning JR, van Rooijen N, Fallowfield JA, Forbes SJ, Iredale JP. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:E3186–E3195. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varga T, Mounier R, Gogolak P, Poliska S, Chazaud B, Nagy L. Tissue LyC6- Macrophages Are Generated in the Absence of Circulating LyC6- Monocytes and Nur77 in a Model of Muscle Regeneration. J Immunol. 2013;191:5695–5701. doi: 10.4049/jimmunol.1301445. [DOI] [PubMed] [Google Scholar]
- 16.Li QX, Ke N, Sundaram R, Wong-Staal F. NR4A1, 2, 3--an orphan nuclear hormone receptor family involved in cell apoptosis and carcinogenesis. Histol Histopathol. 2006;21:533–540. doi: 10.14670/HH-21.533. [DOI] [PubMed] [Google Scholar]
- 17.McMorrow JP, Murphy EP. Inflammation: a role for NR4A orphan nuclear receptors? Biochem Soc Trans. 2011;39:688–693. doi: 10.1042/BST0390688. [DOI] [PubMed] [Google Scholar]
- 18.Pei L, Castrillo A, Tontonoz P. Regulation of macrophage inflammatory gene expression by the orphan nuclear receptor Nur77. Mol Endocrinol. 2006;20:786–794. doi: 10.1210/me.2005-0331. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Y, Bruemmer D. NR4A orphan nuclear receptors: transcriptional regulators of gene expression in metabolism and vascular biology. Arterioscler Thromb Vasc Biol. 2010;30:1535–1541. doi: 10.1161/ATVBAHA.109.191163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, Geissmann F, Hedrick CC. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nat Immunol. 2011;12:778–785. doi: 10.1038/ni.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 22.Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, Hedrick CC, Cook HT, Diebold S, Geissmann F. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell. 2013;153:362–375. doi: 10.1016/j.cell.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, Jais JP, D’Cruz D, Casanova JL, Trouillet C, Geissmann F. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 25.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pei L, Castrillo A, Chen M, Hoffmann A, Tontonoz P. Induction of NR4A orphan nuclear receptor expression in macrophages in response to inflammatory stimuli. J Biol Chem. 2005;280:29256–29262. doi: 10.1074/jbc.M502606200. [DOI] [PubMed] [Google Scholar]
- 27.Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, Dutta P, Wei Y, Robbins C, Iwamoto Y, Sena B, Chudnovskiy A, Panizzi P, Keliher E, Higgins JM, Libby P, Moskowitz MA, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. 2012;209:123–137. doi: 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leuschner F, Dutta P, Gorbatov R, Novobrantseva TI, Donahoe JS, Courties G, Lee KM, Kim JI, Markmann JF, Marinelli B, Panizzi P, Lee WW, Iwamoto Y, Milstein S, Epstein-Barash H, Cantley W, Wong J, Cortez-Retamozo V, Newton A, Love K, Libby P, Pittet MJ, Swirski FK, Koteliansky V, Langer R, Weissleder R, Anderson DG, Nahrendorf M. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29:1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 30.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D, Zavitz CC, Shikatani EA, Parsons M, Rooijen NV, Lin HY, Husain M, Libby P, Nahrendorf M, Weissleder R, Swirski FK. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19:1166–1172. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu SN, Chen M, Jongstra-Bilen J, Cybulsky MI. GM-CSF regulates intimal cell proliferation in nascent atherosclerotic lesions. J Exp Med. 2009;206:2141–2149. doi: 10.1084/jem.20090866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamers AA, Vos M, Rassam F, Marinkovic G, Kurakula K, van Gorp PJ, de Winther MP, Gijbels MJ, de Waard V, de Vries CJ. Bone marrow-specific deficiency of nuclear receptor Nur77 enhances atherosclerosis. Circ Res. 2012;110:428–438. doi: 10.1161/CIRCRESAHA.111.260760. [DOI] [PubMed] [Google Scholar]
- 35.Hanna RN, Shaked I, Hubbeling HG, Punt JA, Wu R, Herrley E, Zaugg C, Pei H, Geissmann F, Ley K, Hedrick CC. NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ Res. 2012;110:416–427. doi: 10.1161/CIRCRESAHA.111.253377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonta PI, van Tiel CM, Vos M, Pols TW, van Thienen JV, Ferreira V, Arkenbout EK, Seppen J, Spek CA, van der Poll T, Pannekoek H, de Vries CJ. Nuclear receptors Nur77, Nurr1, and NOR-1 expressed in atherosclerotic lesion macrophages reduce lipid loading and inflammatory responses. Arterioscler Thromb Vasc Biol. 2006;26:2288–2294. doi: 10.1161/01.ATV.0000238346.84458.5d. [DOI] [PubMed] [Google Scholar]
- 37.Bonta PI, Matlung HL, Vos M, Peters SL, Pannekoek H, Bakker EN, de Vries CJ. Nuclear receptor Nur77 inhibits vascular outward remodelling and reduces macrophage accumulation and matrix metalloproteinase levels. Cardiovasc Res. 2010;87:561–568. doi: 10.1093/cvr/cvq064. [DOI] [PubMed] [Google Scholar]
- 38.Chao LC, Soto E, Hong C, Ito A, Pei L, Chawla A, Conneely OM, Tangirala RK, Evans RM, Tontonoz P. Bone marrow NR4A expression is not a dominant factor in the development of atherosclerosis or macrophage polarization in mice. J Lipid Res. 2013;54:806–815. doi: 10.1194/jlr.M034157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng Z, Volkers M, Din S, Avitabile D, Khan M, Gude N, Mohsin S, Bo T, Truffa S, Alvarez R, Mason M, Fischer KM, Konstandin MH, Zhang XK, Heller Brown J, Sussman MA. Mitochondrial translocation of Nur77 mediates cardiomyocyte apoptosis. Eur Heart J. 2011;32:2179–2188. doi: 10.1093/eurheartj/ehq496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.You B, Jiang YY, Chen S, Yan G, Sun J. The orphan nuclear receptor Nur77 suppresses endothelial cell activation through induction of IkappaBalpha expression. Circ Res. 2009;104:742–749. doi: 10.1161/CIRCRESAHA.108.192286. [DOI] [PubMed] [Google Scholar]
- 41.Cunningham NR, Artim SC, Fornadel CM, Sellars MC, Edmonson SG, Scott G, Albino F, Mathur A, Punt JA. Immature CD4+CD8+ thymocytes and mature T cells regulate Nur77 distinctly in response to TCR stimulation. J Immunol. 2006;177:6660–6666. doi: 10.4049/jimmunol.177.10.6660. [DOI] [PubMed] [Google Scholar]
- 42.Fassett MS, Jiang W, D’Alise AM, Mathis D, Benoist C. Nuclear receptor Nr4a1 modulates both regulatory T-cell (Treg) differentiation and clonal deletion. Proc Natl Acad Sci U S A. 2012;109:3891–3896. doi: 10.1073/pnas.1200090109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swirski FK, Robbins CS. Neutrophils usher monocytes into sites of inflammation. Circ Res. 2013;112:744–745. doi: 10.1161/CIRCRESAHA.113.300867. [DOI] [PubMed] [Google Scholar]
- 44.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sieweke MH, Allen JE. Beyond stem cells: self-renewal of differentiated macrophages. Science. 2013;342:1242974. doi: 10.1126/science.1242974. [DOI] [PubMed] [Google Scholar]
- 46.Frangogiannis NG, Mendoza LH, Ren G, Akrivakis S, Jackson PL, Michael LH, Smith CW, Entman ML. MCSF expression is induced in healing myocardial infarcts and may regulate monocyte and endothelial cell phenotype. Am J Physiol Heart Circ Physiol. 2003;285:H483–H492. doi: 10.1152/ajpheart.01016.2002. [DOI] [PubMed] [Google Scholar]
- 47.Anversa P, Kajstura J, Rota M, Leri A. Regenerating new heart with stem cells. J Clin Invest. 2013;123:62–70. doi: 10.1172/JCI63068. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Chen W, Frangogiannis NG. Fibroblasts in post-infarction inflammation and cardiac repair. Biochim Biophys Acta. 2013;1833:945–953. doi: 10.1016/j.bbamcr.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xin M, Olson EN, Bassel-Duby R. Mending broken hearts: cardiac development as a basis for adult heart regeneration and repair. Nat Rev Mol Cell Biol. 2013;14:529–541. doi: 10.1038/nrm3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zamilpa R, Lindsey ML. Extracellular matrix turnover and signaling during cardiac remodeling following MI: causes and consequences. J Mol Cell Cardiol. 2010;48:558–563. doi: 10.1016/j.yjmcc.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.