Abstract
Observational studies and trials from low-income countries indicate that measles vaccine has beneficial nonspecific effects, protecting against non–measles-related mortality. It is not known whether measles vaccine protects against hospital admissions. Between 2003 and 2007, 6417 children who had received the third dose of diphtheria, tetanus, and pertussis vaccine were randomly assigned to receive measles vaccine at 4.5 months or no measles vaccine; all children were offered measles vaccine at 9 months of age. Using hospital admission data from the national pediatric ward in Bissau, Guinea-Bissau, we compared admission rates between enrollment and the 9-month vaccination in Cox models, providing admission hazard rate ratios (HRRs) for measles vaccine versus no measles vaccine. All analyses were conducted stratified by sex and reception of neonatal vitamin A supplementation (NVAS). Before enrollment the 2 groups had similar admission rates. Following enrollment, the measles vaccine group had an admission HRR of 0.70 (95% confidence interval [CI], .52–.95), with a ratio of 0.53 (95% CI, .32–.86) for girls and 0.86 (95% CI, .58–1.26) for boys. For children who had not received NVAS, the admission HRR was 0.53 (95% CI, .34–.84), with an effect of 0.30 (95% CI, .13–.70) for girls and 0.73 (95% CI, .42–1.28) for boys (P = .08, interaction test). The reduction in admissions was separately significant for measles infection (admission HRR, 0 [95% CI, 0–.24]) and respiratory infections (admission HRR, 0.37 [95% CI, .16–.89]). Early measles vaccine may have major benefits for infant morbidity patterns and healthcare costs.
Clinical trials registration
Keywords: Edmonston-Zagreb, hospital admissions, measles infection, measles vaccination, morbidity reduction, nonspecific effects of vaccine
The current policy to vaccinate against measles at 9 months of age in low-income countries was formulated in the mid-1970s on the basis of studies of seroconversion at different ages; the overall effect of measles vaccine on morbidity and mortality was not examined. When measles vaccine was introduced at demographic surveillance sites in Africa in the late 1970s and early 1980s, several studies reported reductions in child mortality of ≥40% [1–7]. Studies in Bangladesh and Haiti provided similar results [8–10]. This effect was larger than expected since measles infection causes only 10% of all childhood deaths [11]. Subsequent randomized trials have confirmed that measles vaccine has beneficial nonspecific effects, and therefore measles vaccine probably reduces severity or provide protection against infections other than measles [12].
Little is known about the underlying mechanisms behind these nonspecific effects of measles vaccine; in particular, there is no information on how measles vaccine affects morbidity patterns. During 2004–2007, we conducted a trial of early measles vaccination, randomly assigning children to receive measles vaccine early, at 4.5 months of age, in addition to the recommended measles vaccine at 9 months of age or to receive no vaccine at 4.5 months of age and only the recommended measles vaccine at 9 months of age. As hypothesized, early receipt of measles vaccine reduced mortality between 4.5 and 36 months of age by 36% (95% confidence interval [CI], 2%–58%) for girls, but the effect was limited for boys (reduction, 5% [95% CI, −36% to 36%]) [13]. The trial also revealed that receipt of neonatal vitamin A supplementation (NVAS) removed the beneficial effect of early receipt of measles vaccine [13].
To understand possible biological mechanisms and the health implications of these findings, it will be important to determine which morbidity patterns are affected by the nonspecific effects. We therefore used data from the national pediatric ward in Bissau, Guinea-Bissau, to examine whether measles vaccine affected the rate of hospital admission and whether effects differed for different types of infection. We compared the admission rate from enrollment and until the 9-month measles vaccination for children randomly assigned to receive measles vaccine or no measles vaccine at 4.5 months of age, the latter children having the third dose of diphtheria, tetanus, and pertussis vaccine (DTP3) as their most recent vaccination.
METHODS
Setting and Study Population
The trial was conducted in Guinea-Bissau in the Bandim Health Project (BHP) study area, which covers 6 districts with approximately 102 000 inhabitants [14]. All houses are visited every month to register new pregnancies and births. All children are visited every 3 months until 3 years of age to collect information on breast-feeding status, infections, hospital admissions, and vaccination status. Coverage for DTP3 and measles vaccine was 89% and 88%, respectively, in the 12–23-month age group when the trial started [15].
Objectives and Study Design
The trial has been described in detail elsewhere [13, 16]. Briefly, the primary objective was to measure the effect of different measles vaccine schedules on overall mortality between 4.5 and 36 months of age. The children had to have received DTP3 at least 4 weeks before enrollment. The trial included 3 arms: standard-dose Edmonston-Zagreb (EZ) measles vaccine (Serum Institute of India [Pune, India]) at 4.5 and 9 months of age (group A), no vaccine at 4.5 months of age and standard-dose EZ measles vaccine at 9 months of age (group B), and no vaccine at 4.5 months of age and standard-dose Schwarz measles vaccine (Rouvax [Aventis, France]) at 9 months of age (group C). All children were invited back at 9 months of age to receive measles vaccine. EZ measles vaccine provides better seroconversion in the presence of maternal antibody and may boost better than Schwarz measles vaccine [17] and was therefore selected for the early measles vaccine arm.
The sample size was based on detecting a 25% difference in mortality between the early receipt group (group A) and the control groups (groups B and C). We hypothesized a priori that beneficial effects on child survival would be strongest for girls and in the dry season (December–May) [13]. The trial was funded to examine antibody responses, clinical protection, and survival. However, we maintained a registration of hospital admissions during the trial and therefore examined how measles vaccine affected admission patterns.
Enrollment, Informed Consent, and Randomization
Eligible infants were identified in the registration system. The mothers or guardians were invited to the local health center and received an oral and a written explanation of the study from a physician. The physician performed a medical examination. If agreeing to participate, the mothers selected an envelope with the allocation number.
Information on Hospital Admissions
Three health centers provide primary care in the study area. Severe cases are sent to the national hospital. The BHP has maintained a register for admissions to the pediatric ward since the 1990s. This is the only specialized pediatric ward in the country and is used as general pediatric ward for residents in Bissau. Staff from BHP register all admitted children by name, sex, date of birth, name of mother, residential area, and outcome. Using the ID-number on the vaccination card, the assistant assigns the BHP identification number. For children who do not bring a card and cannot be identified easily, a senior data manager identifies the children, using all available information. For the present analysis, it was verified that correct identification numbers had been assigned.
The responsible physician assigns primary and secondary diagnoses. We have used the primary diagnosis with the exception that 4 children who had “measles” as a secondary diagnosis were reclassified as having “measles” as the primary diagnosis. Malaria was to be diagnosed by microscopy of thick and thin blood slides, but a diagnosis of malaria was often made by clinicians even though parasites had not been detected [18].
At the 9-month vaccination, the mother was asked whether the child had been admitted, where, at what age and with which symptoms. These reports were examined individually in relation to the admission register. When the mother reported an “admission” not found in the register, reported symptoms were usually diarrhea and vomiting (85% [78/92]; measles vaccine, 29; no vaccine, 49). According to local practice, individuals with diarrhea are rehydrated and then sent home without being registered as having been admitted to the hospital. Mothers reported hospital admission of 14 children with fever or presumptive malaria (measles vaccine, 2; no vaccine, 12), data for which could not be found in the register. Maternal reports on admissions had no dates and could therefore not be included in the analysis. Inconsistencies between maternal reports and register could be due to the mother misinterpreting observation at the outpatient clinic as an “admission” or due to the mother reporting a wrong date of birth or bringing no or the wrong vaccination card to the hospital. Furthermore, children may have Portuguese names, ethnic names, or nicknames, and if a name differs from the one in the demographic files it may be difficult to identify the child.
Follow-up
All children were called for the 9-month vaccination. However, mothers often stay with relatives or take part in the harvest of cashew nuts in the rural areas; some children will therefore not be home when called. We kept calling to offer measles vaccine, until children were 18 months old. As in the survival analysis [13], we followed children from enrollment until they received the 9-month measles vaccine or were censored at 18 months of age. Because a long follow-up duration can dilute the effect of early measles vaccine, our results are limited to follow-up data up to 9 months of age (304 days).
Statistical Analyses
The statistical analyses were conducted in Stata 12. Follow-up started at enrollment and ended at death, migration, receipt of the 9-month vaccination, or at 18 months of age for children who were not yet vaccinated, whichever came first. Children did not contribute follow-time during admission; follow-up time resumed at discharge. We present follow-up time with admission hazard rate ratios (HRRs) and Wald 95% CIs estimated from a Cox proportional hazards model with age as underlying time. Use of time since inclusion as underlying time did not yield a difference in the results. The proportional hazards assumption was assessed graphically and was tested using Schoenfeld residuals (P = .95). Age was inherently controlled for in the model. Multiple admissions were allowed. Following an admission, the risk of a second admission was assumed to be the same as the risk of the first admission. As in previous analyses [13], we adjusted for district by stratification. In case there were 0 hospital admissions (admission HRR, 0), CIs were obtained by a profile likelihood method, inverting results of the likelihood ratio test into a CI. CIs for interactions involving 0 admissions were calculated similarly. In case there were no admissions in one group, groups were compared by a log-rank test. To account for death, competing risk methods were used to calculate cumulative incidence curves for group A versus groups B and C combined.
Exclusions
As in previous analyses [13, 16], 231 children were excluded. Eighty children reported measles virus infection before enrollment; 131 were enrolled within 25 days of DTP3 vaccination, owing to a programming error; 18 were enrolled twice; and 2 had the wrong age on record, being 1 year older than indicated in the demographic file.
Ethics
The protocol was approved by the Danish Central Ethical Committee, the Gambia/MRC scientific and ethics committees, and the Guinean Ministry of Health's Research Coordination Committee. Study participants had access to free consultations at the local health centers and to essential drugs free of charge. The clinical trials registration number was NCT00168558.
RESULTS
Participant Flow
We enrolled children between August 2003 and April 2007. The study profile has been described previously ([13] Figure 1); 2129 were randomly assigned to receive EZ measles vaccine at 4.5 and 9 months of age, and 4288 were randomly assigned to receive no vaccine at 4.5 months of age. There were no major differences in demographic, socioeconomic, and health related background factors for the early receipt group and the control group ([13] Table 1). The median age at the 9-month measles vaccine was 274 days (interquartile range, 271–278 days) in the early receipt group and 273 days (271–276 days) in the control group. Furthermore, there was no difference in hospital admissions before enrollment (relative risk [RR], 1.03 [95% CI, 0.80–1.33]). Between enrollment and the 9-month measles vaccine, 221 hospital admissions were detected.
Figure 1.
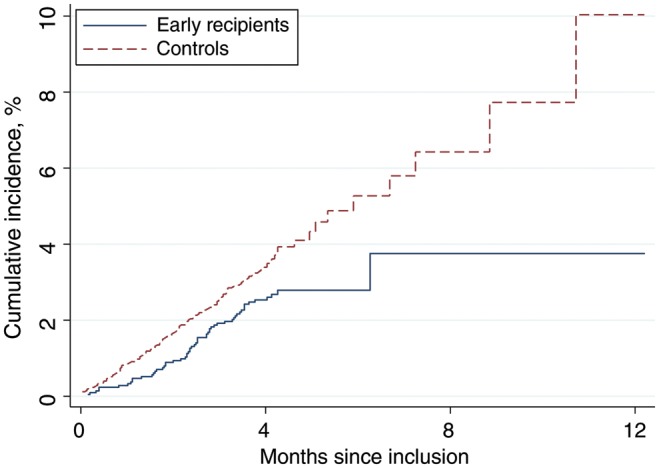
The cumulative incidence of hospital admissions, according to randomization group. Early recipients received measles vaccine at 4.5 and 9 months of age, and controls received vaccine at 9 months of age.
Table 1.
Admission Rates Between 4.5 and 9 Months of Age and Admission Hazard Rate Ratio (HRR) for Early Recipients of Measles Vaccine (Group A), Compared With Controls (Groups B and C), by Neonatal Vitamin A Supplementation (NVAS) and Sex
| NVAS Status, Sex | Group A |
Groups B and C |
Admission HRRa (95% CI) |
|||
|---|---|---|---|---|---|---|
| Admissions/100 Person- Years (Admissions/Person-Days), Children, No. | Enrolled Children, No. | Admissions/100 Person-Years (Admissions/Person-Days), Children, No. | Enrolled Children, No. | All Admissionsb | Excluding Measles Admissions | |
| Overall | ||||||
| Boys | 9.5 (37/142 037) | 1084 | 11.1 (86/282 311) | 2151 | 0.86 (0.58–1.26) | 0.96 (0.64–1.41) |
| Girls | 5.3 (20/137 120) | 1045 | 10.2 (78/278 905) | 2137 | 0.53 (0.32–0.86) | 0.59 (0.36–0.97) |
| All | 7.5 (57/279 157) | 2129 | 10.7 (164/561 216) | 4288 | 0.70 (0.52–0.95) | 0.78 (0.58–1.07) |
| No NVAS | ||||||
| Boys | 8.4 (17/73 485) | 558 | 11.4 (46/147 115) | 1130 | 0.73 (0.42–1.28) | 0.80 (0.46–1.41) |
| Girls | 3.0 (6/71 919) | 549 | 10.3 (43/152 213) | 1165 | 0.30 (0.13–0.70) | 0.35 (0.15–0.82) |
| All | 5.8 (23/145 404) | 1107 | 10.9 (89/299 328) | 2295 | 0.53 (0.34–0.84) | 0.60 (0.38–0.95) |
| NVAS | ||||||
| Boys | 10.6 (20/68 552) | 526 | 10.8 (40/135 196) | 1021 | 1.00 (0.58–1.71) | 1.14 (0.66–1.98) |
| Girls | 7.8 (14/65 201) | 496 | 10.1 (35/126 692) | 972 | 0.79 (0.42–1.46) | 0.83 (0.45–1.56) |
| All | 9.3 (34/133 753) | 1022 | 10.5 (75/261 888) | 1993 | 0.90 (0.60–1.35) | 0.99 (0.66–1.50) |
Data are from the national pediatric ward, Guinea-Bissau. Early recipients received measles vaccine at 4.5 and 9 months of age, and controls received vaccine at 9 months of age.
Abbreviation: CI, confidence interval.
a Calculated as A/[A + B].
b With follow-up to only 9 months of age, the admission HRR was 0.72 (95% CI, 0.53–0.97) for all children, 0.53 (95% CI, 0.32–0.88) for girls, and 0.87 (95% CI, 0.59–1.29) for boys; among children who did not receive NVAS, the HRR was 0.56 (95% CI, 0.35–0.88) for all children, 0.32 (95% CI, 0.13–0.74) for girls, and 0.76 (95% CI, 0.43–1.33) for boys; for children who received NVAS, the HRR was 0.89 (95% CI, 0.59–1.35) for all children, 0.77 (95% CI, 0.40–1.46) for girls, and 1.00 (95% CI, 0.58–1.72) for boys.
Main Results
Children randomly assigned to the early vaccine group had significantly lower risk of admission (n = 57) than children in the control group (n = 164; Table 1 and Figure 1). The effect on admissions was strongest and separately significant for girls, with a reduction of 47% (95% CI, 14%–68%) but not for boys (14% [95% CI, −26% to 42%]; Table 1). Results were similar when the children were followed only to 9 months of age (Table 1). The effect remained significant for girls when measles-associated admissions were excluded (Table 1). There was no difference in the median duration of admissions for early recipients (7 days [interquartile range, 4–9 days]) and controls (7 days [IQR, 5–9 days]). The hospital case-fatality rate was 7% (4/57) for early recipients and 9% (14/164) for controls.
Only 8% (17/221) of admissions were related to measles virus infection (Table 2). The 2 main categories were presumptive malaria (42%) and pneumonia/respiratory infections (35%). Both presumptive malaria (62%) and respiratory infections (58%) were slightly more common in the rainy season (Supplementary Table 1). Early receipt of measles vaccine had similar protective effects in the 2 main disease groups (Table 2). There was no sex-associated difference for admissions due to presumptive malaria, whereas the beneficial effect on admissions for respiratory infections differed significantly for girls (admission HRR, 0.31 [95% CI, 0.12–0.79]) and boys (admission HRR, 1.24 [95% CI, 0.65–2.36]; P = .02, test of interaction; Supplementary Table 2).
Table 2.
Hospital Admission Diagnoses Between 4.5 and 9 Months of Age, by Sex and Vaccination Group
| Diagnosis | Admitted Children, No., by Sex and Vaccination Group (No NVAS; NVAS) |
Admission HRR (95% CI) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Boys |
Girls |
All |
All Admissions | No NVAS | NVAS | ||||
| Early Recipients (n = 1084) | Controls (n = 2151) | Early Recipients (n = 1045) | Controls (n = 2137) | Early Recipients (n = 2129) | Controls (n = 4288) | ||||
| Pneumonia/ respiratory infection | 15 (6; 9) | 24 (14; 10) | 5 (0; 5) | 33 (19; 14) | 20 (6; 14) | 57 (33; 24) | 0.71 (0.42–1.17) | 0.37 (0.16–0.89) | 1.15 (0.59–2.21) |
| Diarrhea and dysentery | 7 (3; 4) | 11 (8; 3) | 3 (2; 1) | 4 (2; 2) | 10 (5; 5) | 15 (10; 5) | 1.35 (0.60–3.00) | 1.05 (0.36–3.06) | 1.94 (0.56–6.71) |
| Malaria, anemia | 12 (6; 6) | 39 (19; 20) | 10 (4; 6) | 31 (14; 17) | 22 (10; 12) | 70 (33; 37) | 0.64 (0.39–1.03) | 0.62 (0.31–1.26) | 0.65 (0.34–1.24) |
| Measles | 0 | 9 (4; 5) | 0 | 8 (6; 2) | 0 | 17 (10; 7) | 0 (0–0.24) | 0 (0–0.44) | 0 (0–0.61) |
| Othera | 3 (2; 1) | 3 (1; 2) | 2 (0; 2) | 2 (2; 0) | 5 (2; 3) | 5 (3; 2) | 2.05 (0.59–7.10) | 1.38 (0.23–8.27) | 3.05 (0.51–18.25) |
| All | 37 (17; 20) | 86 (46; 40) | 20 (6; 14) | 78 (43; 35) | 57 (23; 34) | 164 (89; 75) | 0.70 (0.52–0.95) | 0.53 (0.34–0.84) | 0.90 (0.60–1.35) |
Data are from the national pediatric ward, Guinea-Bissau. Early recipients received measles vaccine at 4.5 and 9 months of age, and controls received vaccine at 9 months of age.
Abbreviations: CI, confidence interval; HRR, hazard rate ratio; NVAS, neonatal vitamin A supplementation.
a Chicken pox (1 case), malnutrition (1), sepsis (2), febrile syndrome (2), throat abscess (2), impetigo (1), and intoxication (1).
As hypothesized, the beneficial effect was most pronounced for children receiving measles vaccine in the dry season (December–May), with a reduction of 47% (95% CI, 14%–67%), whereas there was little effect for children vaccinated in the rainy season (June–November; 14% [95% CI, −28% to 42%]; Table 3).
Table 3.
Admission Rates Between 4.5 and 9 Months of Age and Admission Hazard Rate Ratio (HRR) for Recipients of Early Measles Vaccine (Group A), Compared With Controls (Groups B and C), by Season of Enrollment
| NVAS Status, Season | Group A |
Groups B and C |
Admission HRR (95% CI) |
|||
|---|---|---|---|---|---|---|
| Admissions/100 Person-Years (Admissions/Person-Days), Children, No. | Enrolled Children, No. | Admissions/100 Person-Years (Admissions/Person-Days), Children, No. | Enrolled Children, No. | All Admissions | Excluding Measles Admissions | |
| Overall | ||||||
| Dry | 5.4 (21/142 294) | 1063 | 10.1 (80/287 701) | 2149 | 0.53 (0.33–0.86) | 0.62 (0.38–1.01) |
| Rainy | 9.6 (36/136 863) | 1066 | 11.2 (84/273 515) | 2139 | 0.86 (0.58–1.28) | 0.93 (0.63–1.38) |
| All | 7.5 (57/279 157) | 2129 | 10.7 (164/561 216) | 4288 | 0.70 (0.52–0.95) | 0.78 (0.58–1.07) |
| No NVAS | ||||||
| Dry | 5.2 (11/76 721) | 570 | 11.1 (47/154 524) | 1165 | 0.47 (0.24–0.91) | 0.54 (0.28–1.05) |
| Rainy | 6.4 (12/68 683) | 537 | 10.6 (42/144 804) | 1130 | 0.61 (0.32–1.15) | 0.67 (0.35–1.28) |
| All | 5.8 (23/145 404) | 1107 | 10.9 (89/299 328) | 2295 | 0.53 (0.34–0.84) | 0.60 (0.38–0.95) |
| NVAS | ||||||
| Dry | 5.6 (10/65 573) | 493 | 9.0 (33/133 177) | 984 | 0.62 (0.31–1.26) | 0.73 (0.36–1.51) |
| Rainy | 12.8 (24/68 180) | 529 | 11.9 (42/128 711) | 1009 | 1.09 (0.66–1.81) | 1.15 (0.69–1.91) |
| All | 9.3 (34/133 753) | 1022 | 10.5 (75/261 888) | 1993 | 0.90 (0.60–1.35) | 0.99 (0.66–1.50) |
Data are from the national pediatric ward, Guinea-Bissau. Early recipients received measles vaccine at 4.5 and 9 months of age, and controls received vaccine at 9 months of age.
Abbreviations: CI, confidence interval; NVAS, neonatal vitamin A supplementation.
Analysis by NVAS Status
NVAS neutralized the beneficial effect of early measles vaccine [13]; we therefore made separate analyses for children, by NVAS status (Tables 1–3 and Supplementary Figure 1). The beneficial effects of measles vaccine on admission was strong for children who had not received NVAS (admission HRR, 0.53 [95% CI, 0.34–0.84]), whereas there was little effect among those who had received NVAS (admission HRR, 0.90 [95% CI, 0.60–1.35]; Table 1). Among children who had not received NVAS, early receipt of measles vaccine had a significant effect on admissions for respiratory infections (admission HRR, 0.37 [95% CI, 0.16–0.89]) but not for presumptive malaria (admission HRR, 0.62 [95% CI, 0.31–1.26]; Table 2), and the beneficial effect on hospital admissions for respiratory infections differed significantly between girls (admission HRR, 0 [95% CI, 0–0.23]) and boys (admission HRR, 0.84 [95% CI, 0.32–2.20]; Supplementary Table 2), with a female-to-male admission HRR of 0 (95% CI, 0–0.33).
DISCUSSION
Early receipt of measles vaccine was associated with a lower risk of hospital admission; this effect was separately significant for girls. The reduction in overall and non–measles-related causes of admission were found mainly among children who had not received NVAS; the beneficial effect of early receipt of measles vaccine was particularly marked for respiratory infections, especially in girls.
This is the first randomized controlled trial of the nonspecific effects of measles vaccine in which hospital admissions have been monitored to detect the likely causes of the differential effect of measles vaccine on child survival. It was not a placebo-controlled trial because we did not want mothers to believe erroneously that their child had already received measles vaccine [13]. The physicians at the pediatric ward could have looked at the vaccination card to see whether the child had received an additional measles vaccine. However, they were unlikely to do so, and if it had affected the likelihood of being admitted it would presumably have affected all disease categories equally.
There is no official register of children being admitted or, for that matter, of children leaving the pediatric ward. The only register is the one that the BHP maintains and that BHP staff have to check daily about what happened to all currently admitted children, to detect who died, fled, or was discharged. As discussed above, a few admissions may not have been registered. Addition of the 14 cases of fever and presumptive malaria reported by others but not found in the register would strengthen the protective effect of measles vaccine against admissions.
Resources are limited at the ward, and most diagnoses are clinical rather than laboratory confirmed. The seasonal difference in malaria admission was not very marked (Supplementary Table 1), but malaria is transmitted all year round in Bissau, although it is more frequent in the late rainy season and early dry season [19]. Hence, diagnostic categories should be interpreted cautiously. The fever interpreted as malaria may well be sepsis, and malaria may well be misdiagnosed as respiratory infections in the rainy season, as found in the Gambia [20]. Still the beneficial effect of measles vaccine was strongest in the dry season, and it seems plausible that the majority of respiratory infections were indeed “respiratory” and that measles vaccine has a particularly strong effect on respiratory infections.
Around 6% in the control group against only 1% in the early receipt group received measles vaccine elsewhere before they turned up for the 9-month vaccination session (Figure 1) [13]. This information has not been taken into consideration in the analysis of admission rates because the information was only available retrospectively for children who survived. Hence, control children have been assigned too much follow-up time in the no-vaccine group (DTP3 vaccinated). The admission HRRs are therefore conservative, and the true effect of early receipt of measles vaccine may have been slightly stronger.
The protective effect of measles vaccine against admission was similar to what we have previously reported for mortality [13]. Early receipt of measles vaccine reduced both admissions and mortality among children who had not received NVAS. These effects were stronger and significant for girls but not for boys.
In the same pediatric department, we have previously conducted observational studies, comparing hospital case-fatality rates of different infections for measles vaccine recipients and nonrecipients, with the latter group usually having DTP as their most recent vaccination [21]. The adjusted case-fatality rate in the group aged 6–17 months was 2-fold lower for measles vaccine recipients (mortality ratio [MR], 0.51 [95% CI, 0.27–0.98]), and this effect was particularly marked for pneumonia (MR, 0.28 [95% CI, 0.07–0.91]). A similar nonsignificant trend was observed for fever or presumptive malaria (RR, 0.30 [95% CI, 0.13–1.18]), whereas nothing was found for diarrhea (RR, 1.38 [95% CI, 0.29–9.36]) and other conditions (RR, 0.75 [95% CI, 0.27–2.89]). This differential effect on relative severity of different diseases is similar to that in the present study.
There is yet no explanation of the beneficial nonspecific effects of measles vaccine, but some mechanisms are clearly possible. Animal studies of heterologous immunity have shown that priming with one pathogen may either enhance or reduce susceptibility to subsequent infection with unrelated pathogens [22, 23]. One explanation of why respiratory infections are more affected may be that beneficial heterologous cross-reactions are more common between measles virus and other related paramyxoviruses, such as respiratory syncytial virus, parainfluenza virus, and influenza virus. The effect was significant and more marked on admissions in the dry season, when admissions for respiratory infections are common [24] and when the diagnosis is less likely to be confounded by the concomitant presence of malaria [20]. Training of the innate immune system with BCG has been shown to induce nonspecific protection through epigenetic reprogramming of monocytes [25], and a similar mechanism may also apply to measles vaccine. Furthermore, children who had maternal antibodies at time of measles vaccination had better survival than children who received measles vaccine in the presence of no detectable antibodies [26].
In animal studies of live and inactivated vaccines of the same antigen, the live vaccine has generally been associated with a better capacity to handle a subsequent challenge [27–31].
Hence, the reduction in admissions for girls who received measles vaccine, compared with girls who received DTP3 as their most recent vaccine, could be related to differences in immune priming with a live or inactivated vaccine. It is not known why these effects would differ for girls and boys. However, the female and male immune systems are undoubtedly different. It is not common to report effects of interventions separately for girls and boys; however, we have found in many observational studies and randomized trials that the effects of measles vaccine, DTP, BCG, and vitamin A supplementation differ for girls and boys [32–34]. NVAS appears to reprogram the immune system in ways that interact with other interventions throughout infancy and that often differ for girls and boys [34–37]. Likewise, it is uncommon to report effects of vaccines separately by season. However, there are major differences in the immune system and exposure patterns in the dry and the rainy season [38, 39]. On the basis of previous experience, we had hypothesized that the beneficial effect of measles vaccine would be strongest in the dry season. In the present trial, children enrolled at 4.5 months of age in the dry season had a higher level of maternal antibodies than children enrolled in the rainy season [40]. Hence, the beneficial immune stimulation related to the presence of maternal antibodies may be better in the dry season [26].
Essentially all vaccine research and policy decisions are based on the assumption that vaccines protect against a specific pathogen and nothing else, apart from any adverse events linked to the initial challenge. The randomized trial of early measles vaccine has shown that measles vaccine has additional beneficial effects on child survival [13], and the present study suggests that early receipt of measles vaccine reduces hospital admissions for non–measles-related infections. Taking these effects into consideration could have major consequences for child survival and healthcare costs. Pursuing these observations, we found in a Danish nationwide register-based study of >500 000 children that measles, mumps, and rubella vaccine is associated with a reduction of 14% (95% CI, 12%–16%) in the risk of infectious diseases–associated hospital admissions [41]; this protective effect was significantly better for lower respiratory tract infections than for other kind of infections [42].
The current measles vaccine policy is based on the observation that seroconversion is better when maternal antibodies have waned, and it is therefore recommended to delay measles vaccine to 12 months of age once measles virus infection is under control. This recommendation is based on no evidence about the effect on overall health and survival. It might cost lives and increase healthcare costs if children between 9 and 12 months of age were no longer to have the beneficial nonspecific effects of measles vaccine. With current evidence it would be better to lower the age of the first measles vaccine and to follow receipt at this age with an additional measles vaccination at 9 or 18 months of age [13, 43]. NVAS clearly limited the beneficial effect of early receipt of measles vaccine. We have found in 3 trials in Guinea-Bissau (unpublished data) [34–36], that NVAS was associated with significantly higher female infant mortality. Although NVAS may have some benefit for boys [36], it is to be hoped that NVAS does not become general policy for all children.
The Strategic Advisory Group of Experts of the World Health Organization's immunization program recently initiated a review of the nonspecific effects of BCG, measles vaccine, and DTP [44]. This will hopefully stimulate research into the real-life effects of measles vaccine and other vaccines to understand the full extent of these effects. Childhood mortality has been declining in recent years, making it increasingly difficult to conduct studies with mortality as an outcome. It is therefore important that the present study suggests that effects are similar for mortality [13] and hospital admissions. For both outcomes, early receipt of measles vaccine had beneficial nonspecific effects that were stronger for girls than boys, tended to be more marked in the dry season, and were neutralized by NVAS.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgment. The research on the decline in maternal antibody levels, which inspired the present trial, was funded by the Thrasher Foundation.
M. L. G., C. L. M., H. C. W., and P. A. designed the study; C. L. M., M. L. G., and P. A. initiated the study routines; C. L. M. and C. B. treated the patients with measles during the outbreak; A. R. supervised the routine registration system; F. S. B. and V. A. D. helped with the hospital registration system; C. S. B. was principal investigator of the vitamin A trials and provided the data on these children; P. A., A. A., and H. R. analyzed the data; A. A. and H. R. were responsible for the statistical analyses; P. A. and C. L. M. wrote the first draft; and all authors contributed to the final version of the manuscript. P. A. is guarantor of the study.
Disclaimer. The funding agencies had no role in the study design, data collection, data analysis, data interpretation, or manuscript writing.
Financial support. This work was supported by the Council for Development Research, Ministry of Foreign Affairs, Denmark (grant 104.Dan.8.f.); the European Union Seventh Framework Programme (grant Health-F3-2011-261375 to C. M.); the European Research Council (starting grant ERC-2009-StG-243149 to C. S. B.); the Novo Nordisk Foundation (professorship grant to P. A.); and the Danish National Research Foundation (to the Research Center for Vitamins and Vaccines).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Potential conflicts of interest.: All authors: No potential conflicts of interest.
References
- 1.The Kasongo project team. Influence of measles vaccination on survival pattern of 7–35-month-old children in Kasongo, Zaire. Lancet. 1981;1:764–7. [PubMed] [Google Scholar]
- 2.Aaby P, Bukh J, Lisse IM, Smits AJ. Measles vaccination and child mortality. Lancet. 1981;ii:93. doi: 10.1016/s0140-6736(81)90443-8. [DOI] [PubMed] [Google Scholar]
- 3.Aaby P, Bukh J, Lisse IM, Smits AJ. Measles vaccination and reduction in child mortality: a community study from Guinea-Bissau. J Infect. 1984;8:13–21. doi: 10.1016/s0163-4453(84)93192-x. [DOI] [PubMed] [Google Scholar]
- 4.Desgrées du Loû A, Pison G, Aaby P. The role of immunizations in the recent decline in childhood mortality and the changes in the female/male mortality ratio in rural Senegal. Am J Epidemiol. 1995;142:643–52. doi: 10.1093/oxfordjournals.aje.a117688. [DOI] [PubMed] [Google Scholar]
- 5.Aaby P, Samb B, Simondon F, Knudsen K, Coll Seck AM, Bennett J, Whittle H. Divergent mortality for male and female recipients of low-titre and high-titre measles vaccines in rural Senegal. Am J Epidemiol. 1993;138:746–55. doi: 10.1093/oxfordjournals.aje.a116912. [DOI] [PubMed] [Google Scholar]
- 6.Aaby P, Bukh J, Lisse IM, Smits AJ, Gomes J, Fernandes MA, Indi F, Soares M. Determinants of measles mortality in a rural area of Guinea-Bissau: Crowding, age, and malnutrition. J Trop Pediatr. 1984;30:164–69. doi: 10.1093/tropej/30.3.164. [DOI] [PubMed] [Google Scholar]
- 7.Aaby P, Pedersen IR, Knudsen K, et al. Child mortality related to seroconversion or lack of seroconversion after measles vaccination. Pediatr Inf Dis J. 1989;8:197–200. [PubMed] [Google Scholar]
- 8.Holt EA, Boulos R, Halsey NA, Boulos IM, Boulos C. Childhood survival in Haiti: Protective effect of measles vaccination. Pediatrics. 1990;85:188–94. [PubMed] [Google Scholar]
- 9.Clemens JD, Stanton BF, Chakraborty J, Chowdhury S, Rao M, Ali M. Measles vaccination and childhood mortality in rural Bangladesh. Am J Epidemiol. 1988;128:1330–9. doi: 10.1093/oxfordjournals.aje.a115086. [DOI] [PubMed] [Google Scholar]
- 10.Koenig MA, Khan MA, Wojtyniak B, et al. The impact of measles vaccination upon childhood mortality in Matlab, Bangladesh. Bull WHO. 1990;68:441–7. [PMC free article] [PubMed] [Google Scholar]
- 11.de Quadros CA. Can measles be eradicated globally? Bull WHO. 2004;82:134–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Aaby P, Samb B, Simondon F, Coll Seck AM, Knudsen K, Whittle H. Non-specific beneficial effect of measles immunisation: analysis of mortality studies from developing countries. BMJ. 1995;311:481–5. doi: 10.1136/bmj.311.7003.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aaby P, Martins CL, Garly ML, et al. Non-specific effects of standard measles vaccine at 4.5 and 9 months of age on childhood mortality: Randomised controlled trial. BMJ. 2010;341:c6495. doi: 10.1136/bmj.c6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aaby P. Bandim: an unplanned longitudinal study. In: Das Gupta M, Aaby P, Pison G, Garenne M, editors. Prospective community studies in developing countries. Oxford: Clarendon Press; 1997. pp. 276–96. [Google Scholar]
- 15.Aaby P, Martins C, Bale C, et al. Sex differences in the effect of vaccines on the risk of hospitalisation due to measles in Guinea-Bissau. Pediatr Inf Dis J. 2010;29:324–8. doi: 10.1097/INF.0b013e3181c15367. [DOI] [PubMed] [Google Scholar]
- 16.Martins CL, Garly ML, Balé C, et al. Protective efficacy of standard Edmonston-Zagreb measles vaccination in infants aged 4.5 months: interim analysis of a randomised clinical trial. BMJ. 2008;337:a661. doi: 10.1136/bmj.a661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garly M-L, Balé C, Martins CL, et al. Measles antibody responses after early two dose trials in Guinea-Bissau with Edmonston-Zagreb and Schwartz standard titre measles vaccine: better antibody increase from booster dose of the Edmonston-Zagreb vaccine. Vaccine. 2001;19:1951–9. doi: 10.1016/s0264-410x(00)00431-x. [DOI] [PubMed] [Google Scholar]
- 18.Rodrigues A, Schellenberg JA, Kofoed PE, Aaby P, Greenwood B. Changing pattern of malaria in Bissau, Guinea-Bissau. Trop Med Int Hlth. 2008;13:410–17. doi: 10.1111/j.1365-3156.2008.02016.x. [DOI] [PubMed] [Google Scholar]
- 19.Ursing J, Kofoed PE, Rodrigues A, Rombo L. No seasonal accumulation of resistant P. falciparum when high-dose chloroquine is used. PLoS One. 2009;4:e6866. doi: 10.1371/journal.pone.0006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Dempsey TJ, McArdle TF, Laurence BE, Lamont AC, Tood JE, Greenwood BM. Overlap in the clinical features of pneumonia and malaria in African children. Trans R Soc Trop Med Hyg. 1993;87:662–5. doi: 10.1016/0035-9203(93)90279-y. [DOI] [PubMed] [Google Scholar]
- 21.Veirum JE, Sodemann M, Biai S, et al. Routine vaccinations associated with divergent effects on female and male mortality at the paediatric ward in Bissau, Guinea-Bissau. Vaccine. 2005;23:1197–203. doi: 10.1016/j.vaccine.2004.02.053. [DOI] [PubMed] [Google Scholar]
- 22.Shann F. Heterologous immunity and the non-specific effects of vaccines. A major medical advance? Pediatr Inf Dis J. 2004;23:555–8. doi: 10.1097/01.inf.0000130155.42392.04. [DOI] [PubMed] [Google Scholar]
- 23.Welsh RM, Selin LH. No one is naïve: The significance of heterologus T-cell immunity. Nat Rev Immunol. 2002;2:417–26. doi: 10.1038/nri820. [DOI] [PubMed] [Google Scholar]
- 24.Greenwood B. The epidemiology of pneumococcal infection in children in the developing world. Phil Trans R Soc Lond B. 1999;354:777–85. doi: 10.1098/rstb.1999.0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleinnijenhuis J, Quintin J, Preijers F, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A. 2012;109:17537–42. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aaby P, Martins CL, Garly ML, et al. Measles vaccination in the presence of maternal measles antibody associated with better child survival in two trials of early two-dose measles vaccination. (submitted) [Google Scholar]
- 27.Lindblad EB, Elhay MJ, Silva R, Appelberg R, Andersen P. Adjuvant modulation of immune responses to tuberculosis subunit vaccines. Infect Immun. 1997;65:623–9. doi: 10.1128/iai.65.2.623-629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnard A, Mahon BP, Watkins J, Redhead K, Mills KH. Th1/Th2 cell dichotomy in acquired immunity to Bordetella pertussis: variables in the in vivo priming and in vitro cytokine detection techniques affect the classification of T-cell subsets as Th1, Th2 or Th0. Immunology. 1996;87:372–80. doi: 10.1046/j.1365-2567.1996.497560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graham BS, Henderson GS, Tang YW, Lu X, Neuzil KM, Colley DG. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J Immunol. 1993;151:2032–40. [PubMed] [Google Scholar]
- 30.Fischer JE, Johnson JE, Johnson TR, Graham BS. Pertussis toxin sensitization alters the pathogenesis of subsequent respiratory syncytial virus infection. J Infect Dis. 2000;182:1029–38. doi: 10.1086/315806. [DOI] [PubMed] [Google Scholar]
- 31.Moran TM, Park H, Fernandez-Sesma A, Schulman JL. Th2 responses to inactivated Influenza virus can be converted to Th1 responses and facilitate recovery from heterosubtypic virus infection. J Infect Dis. 1999;180:579–85. doi: 10.1086/314952. [DOI] [PubMed] [Google Scholar]
- 32.Aaby P, Benn CS, Nielsen J, Lisse IM, Rodrigues A, Ravn H. Testing the hypothesis that diphtheria-tetanus-pertussis vaccine has negative non-specific and sex-differential effects on child survival in high-mortality countries. BMJ Open. 2012;2:e000707. doi: 10.1136/bmjopen-2011-000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benn CS, Aaby P, Nielsen J, Binka FN, Ross DA. Does vitamin A supplementation interact with routine vaccinations? An analysis of the Ghana vitamin A supplementation trial. Am J Clin Nut. 2009;90:629–39. doi: 10.3945/ajcn.2009.27477. [DOI] [PubMed] [Google Scholar]
- 34.Benn CS, Rodrigues A, Yazdanbakhsh M, et al. The effect of high-dose vitamin A supplementation administered with BCG vaccine at birth may be modified by subsequent DTP vaccination. Vaccine. 2009;27:2891–8. doi: 10.1016/j.vaccine.2009.02.080. [DOI] [PubMed] [Google Scholar]
- 35.Benn CS, Diness BR, Roth A, et al. Effect of 50,000 IU vitamin A given with BCG vaccine on mortality in infants in Guinea-Bissau: randomised placebo controlled trial. BMJ. 2008;336:1416–20. doi: 10.1136/bmj.39542.509444.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benn CS, Fisker A, Napirna BM, et al. Vitamin A supplementation and BCG vaccination at birth in low birthweight neonates: two by two factorial randomised controlled trial. BMJ. 2010;340:c1101. doi: 10.1136/bmj.c1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fisker AB, Aaby P, Rodrigues A, Frydenberg M, Bibby BM, Benn CB. Vitamin A supplementation at birth might prime the response to subsequent vitamin A supplements in girls. Three year follow-up of a randomized trial. PLoS One. 2011;6:e23265. doi: 10.1371/journal.pone.0023265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaheen SO, Aaby P, Hall AJ, et al. Cell mediated immunity after measles in Guinea-Bissau: historical cohort study. BMJ. 1996;313:969–74. doi: 10.1136/bmj.313.7063.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lisse I, Aaby P, Whittle H, Jensen H, Engelman M, Christensen LB. T-lymphocyte subsets in West Africa children: Impact of age, sex, and season. J Pediatr. 1997;130:77–85. doi: 10.1016/s0022-3476(97)70313-5. [DOI] [PubMed] [Google Scholar]
- 40.Martins C, Garly ML, Bale C, et al. Measles antibodies responses after Edmonston-Zagreb standard-titre measles vaccination at 4½ and 9 months, only 9 months, or 9 and 18 months of age. J Infect Dis. In press [Google Scholar]
- 41.Sørup S, Benn CS, Krause T, Aaby P, Ravn H. Reduced risk of hospital admissions due to infectious diseases after MMR-vaccination: a Danish nationwide cohort study. JAMA. In press. [Google Scholar]
- 42.Sørup S, Benn CS, Stensballe LG, Aaby P, Ravn H. Reduced risk of hospital admissions due to respiratory syncytial virus after MMR-vaccination: a Danish nationwide cohort study. Clin Infect Dis. In press. [Google Scholar]
- 43.Aaby P, Martins CL, Garly ML, Rodrigues A, Benn CS, Whittle HC. The optimal age of measles immunization in low-income countries: A secondary analysis of the assumptions underlying the current policy. BMJ Open. 2012;2:e0007. doi: 10.1136/bmjopen-2011-000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.World Health Organization. Working Group non-specific effects of vaccines: terms of reference. Available at: http://www.who.int/entity/immunization/sage/SAGE_non-specific_effects_vaccines_WG_jan_2013.pdf .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


