Abstract
Background. With wild poliovirus nearing eradication, preventing circulating vaccine-derived poliovirus (cVDPV) by understanding oral polio vaccine (OPV) community circulation is increasingly important. Mexico, where OPV is given only during biannual national immunization weeks (NIWs) but where children receive inactivated polio vaccine (IPV) as part of their primary regimen, provides a natural setting to study OPV community circulation.
Methods. In total, 216 children and household contacts in Veracruz, Mexico, were enrolled, and monthly stool samples and questionnaires collected for 1 year; 2501 stool samples underwent RNA extraction, reverse transcription, and real-time polymerase chain reaction (PCR) to detect OPV serotypes 1, 2, and 3.
Results. OPV was detected up to 7 months after an NIW, but not at 8 months. In total, 35% of samples collected from children vaccinated the prior month, but only 4% of other samples, contained OPV. Although each serotype was detected in similar proportions among OPV strains shed as a result of direct vaccination, 87% of OPV acquired through community spread was serotype 2 (P < .0001).
Conclusions. Serotype 2 circulates longer and is transmitted more readily than serotypes 1 or 3 after NIWs in a Mexican community primarily vaccinated with IPV. This may be part of the reason why most isolated cVDPV has been serotype 2.
Keywords: polio, oral polio vaccine, inactivated polio vaccine, VDPV, Mexico
Global wild poliovirus eradication may soon be achieved. Since 1988, when the World Health Organization proposed a plan to eradicate poliomyelitis, the number of reported annual global cases has dropped from 350 000 to 223 in 2012 [1]. Only 3 countries remain endemic with uninterrupted transmission, and wild poliovirus serotype 2 has been eradicated for over a decade. The primary tool in the global eradication campaign has been oral polio vaccine (OPV), an inexpensive live attenuated vaccine that is easy to administer and promotes community immunity by spread from vaccinated children to community contacts through the fecal-oral route. However, despite its efficacy, OPV carries risks that will complicate the eradication effort.
OPV consists of RNA viruses that can mutate during replication into forms capable of causing paralytic disease. Because about one out of 500 000 vaccinees develop vaccine-associated paralytic poliomyelitis (VAPP) from their first OPV dose, the United States has only used the more expensive inactivated polio vaccine (IPV), a killed virus vaccine that cannot cause poliomyelitis, since 2000 [2, 3]. Of more concern, with prolonged replication of typically more than one year, the viruses in OPV can mutate into vaccine-derived polioviruses (VDPV), which are 1%–15% divergent from parent OPV strains (0.6%–15% for serotype 2) [4]. VDPV can develop after persistent OPV virus replication in the intestinal tracts of individuals with primary humoral immunodeficiencies (iVDPV; 65 cases identified to date) or from continued person-to-person circulation in undervaccinated communities (cVDPV) [4]. From 2000 until September 2013, 23 separate cVDPV outbreaks in 20 different countries have been identified [5]. The one study, conducted in Nigeria, which compared outbreaks of cVDPV and wild poliovirus, suggests that cVDPV outbreaks have an attack rate and disease severity similar to outbreaks from wild poliovirus [6].
Because of the risks of poliomyelitis due to OPV, one of the components of the new “Polio Eradication and Endgame Strategic Plan 2013–2018” includes initiating at least 1 dose of IPV for children in countries that currently only use OPV and then phasing out OPV, starting by transitioning from trivalent OPV to bivalent OPV, which contains only serotypes 1 and 3 [7]. Unknown factors that could affect this new plan include the duration and pattern of OPV-derived virus circulation in a community vaccinated with IPV. To help fill those knowledge gaps, we conducted a study looking at the circulation patterns of OPV and OPV-derived strains in Mexico, a country that introduced IPV into its regular childhood vaccination schedule in 2007 but continues to give OPV twice a year during national immunization weeks (NIW).
MATERIALS AND METHODS
Study Design and Population
This was a prospective, longitudinal, observational study in 2 urban municipalities (Orizaba and Rio Blanco) and 2 rural municipalities (Rafael Delgado and Tlilapan) in Veracruz, Mexico, with a total population of 183 855 inhabitants. Of these, 14 573 (7.9%) were 5 years old or younger at the time of the study [8]. IPV, as a component of a pentavalent vaccine, has been given to children in Mexico at 2, 4, 6, and 18 months as part of the routine childhood immunization schedule since August of 2007. In addition, children 5 years old or younger who have received at least 2 doses of IPV are offered trivalent OPV during biannual national immunization weeks. Around the study period, there was 1 NIW 3 months before study enrollment (29 May to 4 June 2010), 1 NIW about 6 months after enrollment (15 to 19 February 2011), and 1 NIW about 9 months after enrollment (28 May to 3 June 2011). The study protocol was approved by the Ethics, Biosafety, and Research Committees of the Mexican National Institute of Public Health, by the Public Health Center of Orizaba, Veracruz, Mexico, by the Stanford University Institutional Review Board, and by the Eastern Virginia Medical School (EVMS) Institutional Review Board.
For this observational study, 72 children from distinct households plus 2 household contacts per child, evenly distributed between the 4 municipalities, were enrolled between 25 August and 22 September 2010. Inclusion criteria for the enrolled children included age ≤30 months and having received or planning on receiving IPV as their primary polio vaccination regimen. Exclusion criteria for the enrolled children included international travel in the last 8 months, a household contact already enrolled in the study, an unknown vaccination history, or no household contacts willing to provide monthly stool samples. Household contacts could be any age and have any vaccination history but had to reside in the same household as the enrolled child; each household was allowed to choose which 2 household contacts would participate. The study was advertised at local health clinics, and families who expressed an interest in participating were referred to the study team. The first 18 children from each municipality who were referred to the study team and who fit the inclusion and exclusion criteria were enrolled in the study.
After written informed consent was obtained from the parents and the household contacts (or the parents of the household contacts if children), participation included monthly study visits over the 12-month study period. At each visit, stool was collected from the enrolled child and the 2 household contacts, and questionnaires were completed that included details on health, vaccinations, travel, and demographics. Vaccination history for children was confirmed by checking immunization cards; immunization cards were not usually available for adult household contacts, although, per Mexican vaccination policy, none of them should have been vaccinated against polio since they were young children. Of note, given the timing of the national immunization weeks, monthly stool collections corresponded to 3, 4, 5, 6, 7, and 8 months after the May 2010 NIW, 0.5, 1.5, and 2.5 months after the February 2011 NIW, and 0.5, 1.5, and 2.5 months after the May 2011 NIW.
Sewage was also collected from 4 arroyos (creeks) draining sewage from each of the 4 municipalities at monthly intervals; the analysis of the sewage has already been published elsewhere [9].
Stool Sample Analysis
On the day of collection, stool samples were kept in a cooler until brought to the lab, aliquoted into cryovials, and placed in a −80°C freezer. Samples were stored at −80°C except when shipped on dry ice to Dr Maldonado's laboratory at Stanford, and when a portion of the samples were later shipped on dry ice to Dr Troy's laboratory at EVMS. At Stanford and EVMS, stool underwent RNA extraction, reverse transcription, and real-time polymerase chain reaction (PCR) to look for vaccine poliovirus serotypes-1, 2, and 3 and their revertant forms according to previously published methods with the following exception [10]. At EVMS, a 96 well ABI PRISM 7700 Real Time PCR Instrument was used (Applied Biosystems, Foster City, CA), with a threshold of 0.099 ΔRn for detecting fluorescence. In total, 53 samples were analyzed in duplicate at both Stanford and EVMS to confirm consistency of results between the 2 laboratories.
Categorization of Method of OPV Acquisition
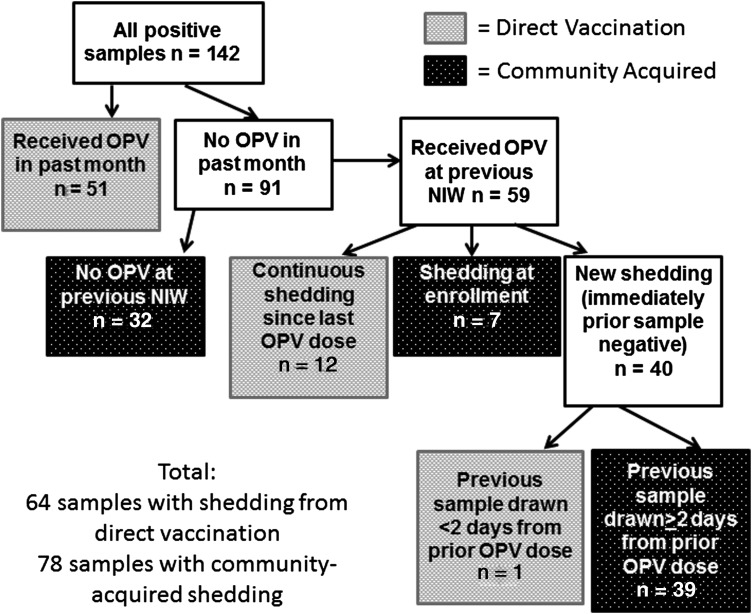
OPV strains detected in stool were assumed to result from direct vaccination if the subject had received OPV in the prior month or had been continuously shedding the same serotype since last receiving OPV. In the one case where the subject had received OPV in an NIW, had a negative stool sample 1 day later, and had a positive stool sample the next month, the OPV strains in the positive sample were also assumed to be a result of direct vaccination (as 1 day would likely be too short for OPV to travel to the stool). Detected OPV strains were assumed to come from community acquisition if subjects had not received OPV in the prior NIW, had received OPV in the prior NIW but had a negative stool sample between the NIW and the positive sample that was taken ≥2 days after the NIW, or were shedding at enrollment (which occurred 3 months after the prior NIW; the longest persistent shedding in the study, seen in 2 of the 216 subjects, was through 2.5 months after an NIW).
Among the community-acquired OPV strains detected from household contacts, household transmission was assumed if the OPV strain correlated with the same serotype shed that month or the prior month by either the enrolled child or the other household contact. In the few examples where 2 household contacts shed in the same month without the enrolled child shedding that month or the prior month, only 1 of the 2 samples was counted as household transmission.
Statistical Analysis
Categorical data were compared using a 2-tailed Fisher exact test. A P value of ≤ .05 was considered statistically significant. Analysis was performed in SAS 9.1 (Cary, NC).
RESULTS
We enrolled 216 subjects (72 children and 144 of their household contacts). One child and 2 household contacts withdrew in month 3, and 2 children and 4 household contacts withdrew in month 5, so that 207 subjects completed the study. We analyzed all 2511 stool samples collected, of which 10 were removed from the final analysis (8 because the stool sample was collected too close to another stool sample from the same subject, and 2 because the samples were mislabeled), for 2501 samples included in the final analysis. The demographics of our study subjects are described in Table 1.
Table 1.
Demographics of Study Subjects
| Enrolled Children | Household Contacts | |
|---|---|---|
| N | 72 | 144 |
| Mean age at enrollment (range) in years | 1.4 (0.3–2.5) | 22.5 (0.2–84.5) |
| Age distribution at Enrollment: | ||
| <6 y old | 72 (100%) | 13 (9%) |
| 6–10 y old | 0 (0%) | 33 (23%) |
| 11–20 y old | 0 (0%) | 22 (15%) |
| 21–30 y old | 0 (0%) | 42 (29%) |
| 31–40 y old | 0 (0%) | 21 (15%) |
| >40 y old | 0 (0%) | 13 (9%) |
| Gender: % female | 37 (51%) | 99 (69%) |
| Received OPV in May 2010 NIW | 35 (49%) | 4 (3%) |
| Received OPV in February 2011 NIWa | 47 (68%) | 6 (4%) |
| Received OPV in May 2011 NIWa | 46 (67%) | 7 (5%) |
| Received all recommended IPV doses for age at enrollment | 53 (74%) | NA |
| Mean prior IPV doses at enrollment | 2.9 | NA |
| International travel in the last 8 mo | 0 (0%) | 0 (0%) |
| Known Immunosuppression | 0 (0%) | 0 (0%) |
| Rural (vs urban) residence | 36 (50%) | 72 (50%) |
| Residence with dirt floor | 8 (11%) | 16 (11%) |
| Residence without indoor plumbing | 34 (47%) | 68 (47%) |
| Mean no. of children in household | 2.3 | 2.3 |
| Mean no. of children in household <6 y old | 1.8 | 1.8 |
Abbreviations: IPV, inactivated polio vaccine; NA, not applicable; NIW, National Immunization Weeks; OPV, oral polio vaccine.
a For the February and May 2011 NIWs, n = 69 for the enrolled children, and n = 138 for the household contacts.
Overall, 6% (142/2501) of the stool samples contained detectable OPV strains. This consisted of 35% (51/145) of stool samples containing OPV from subjects vaccinated with OPV in the prior month, and 4% (91/2356) of stool samples containing OPV from subjects not vaccinated with OPV in the prior month. OPV strain detection was highest immediately after an NIW and continued through 6 months after an NIW in the enrolled children and 7 months after an NIW in the household contacts, but was not detected 8 months after an NIW (Figure 1). The 3 serotypes of OPV were detected in similar percentages immediately after an NIW (Figure 2). However, serotype 2 was detected for 3 months longer than serotypes 1 and 3 and at significantly higher rates compared to serotype 1 at 1.5, 2.5, 3, and 4 months and compared to serotype 3 at 2.5 and 4 months after the NIW (P < .02 for all significant comparisons). For all serotypes, nonrevertant OPV strains (not containing the point mutation in the 5′ untranslated region associated with VAPP) were primarily only seen in the month after the NIW, and OPV strains detected after 1 month were predominantly revertant (containing the serotype-specific point mutation in the 5′ untranslated region associated with VAPP).
Figure 1.
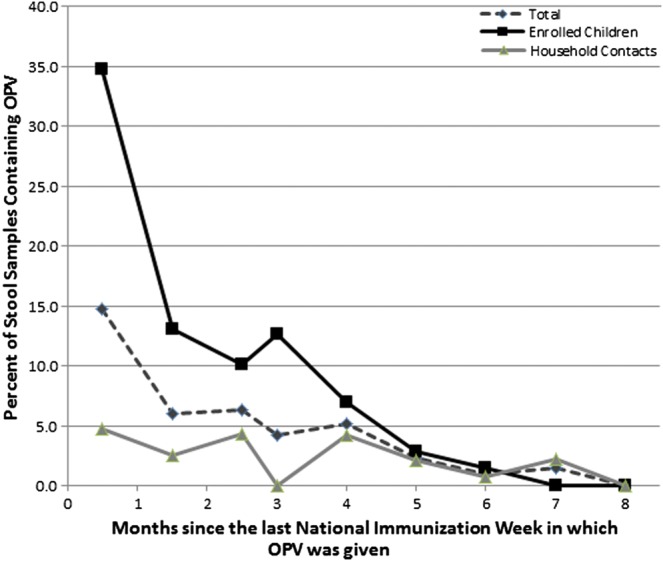
OPV circulation in Mexico following an NIW: enrolled children vs household contacts. This graph contains data for shedding after all 3 NIWs combined. The number of samples from enrolled children and household contacts respectively were as follows: 138 and 275 from 0.5 months, 138 and 276 from 1.5 months, 138 and 275 from 2.5 months, 71 and 141 from 3 months, 72 and 141 from 4 months, 71 and 142 from 5 months, 70 and 142 from 6 months, 69 and 138 from 7 months, and 68 and 136 from 8 months after an NIW. Abbreviations: NIW, National Immunization Weeks; OPV, oral polio vaccine.
Figure 2.
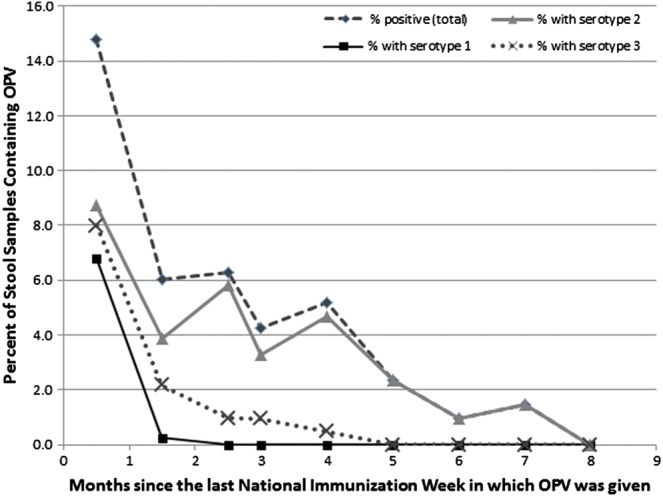
OPV circulation in Mexico following an NIW by serotype. This graph contains data on shedding of specific OPV serotypes from all 3 NIWs combined. Of note, 30 samples contained more than 1 serotype, so the sum of the frequency of the 3 serotypes sometimes exceeds the total percentage of stools containing OPV strains. Abbreviations: NIW, National Immunization Weeks; OPV, oral polio vaccine.
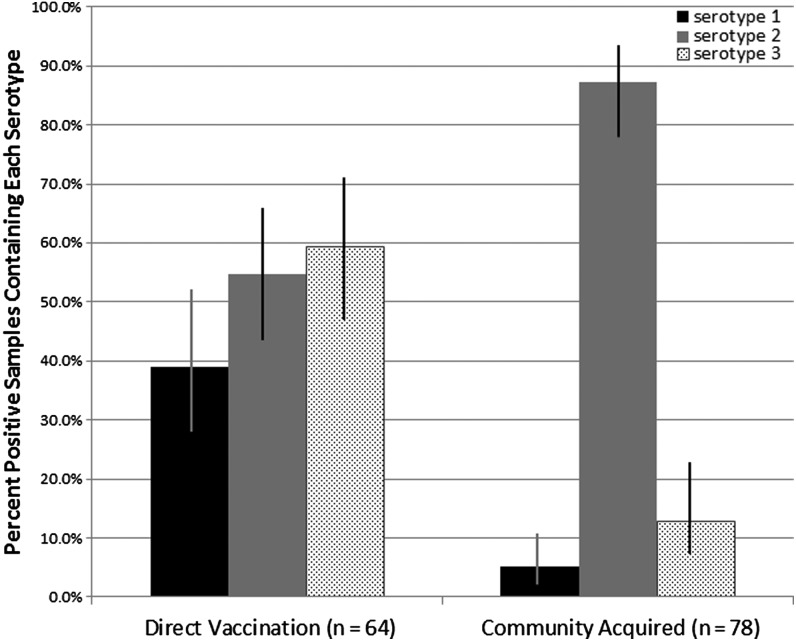
In order to determine if the prevalence of the 3 OPV serotypes differed by method of OPV acquisition, we divided the 142 positive stool samples into 2 groups as described in the methods section: the direct vaccination group, in which the OPV strain was likely acquired as a result of direct vaccination, and the community-acquired group, in which the OPV strain was likely acquired from someone shedding OPV in the community (Figure 3). Although direct vaccination resulted in shedding of similar proportions of the 3 serotypes, community-acquired OPV strains were significantly more likely to be serotype 2 (P < .0001; Figure 4, Table 2). Multiple serotypes in the same sample were significantly more likely to be detected as a result of direct vaccination than of community acquisition (P < .0001; Table 2). There was no association identified between mode of OPV acquisition (direct vaccination vs community acquisition) and high vs low PCR cycle threshold number (data not shown), a measure inversely related to OPV virus concentration in the stool [11].
Figure 3.
Algorithm for determining whether OPV strains were acquired through direct vaccination vs community spread. Abbreviation: OPV, oral polio vaccine.
Figure 4.
Serotypes seen in positive samples by mode of acquisition. The 95% confidence intervals of each proportion were calculated using the modified Wald method. Of note, 30 samples contained multiple serotypes, so the percentages do not add up to 100%.
Table 2.
Type and Number of Serotypes Shed by Subjects Who Acquired OPV Virus Through Direct Vaccination vs Community Spread
| Direct Vaccination (n = 64) |
Community Acquisition (n = 78) |
||||||
|---|---|---|---|---|---|---|---|
| Serotype shed | n | % | 95% CIa | n | % | 95% C.I.a | P Value* |
| Any Type 1 | 25 | 39.1% | 28.0%–51.3% | 4 | 5.1% | 2.1%–11.2% | <.0001 |
| Any Type 2 | 35 | 54.7% | 42.6%–66.3% | 68 | 87.2% | 77.8%–93.1% | <.0001 |
| Any Type 3 | 38 | 59.4% | 47.1%–70.6% | 10 | 12.8% | 6.9%–22.2% | <.0001 |
| Shedding by No. of Serotypes | |||||||
| One serotype | 38 | 59.4% | 47.1%–70.6% | 74 | 94.9% | 87.2%–98.4% | <.0001 |
| Type 1 only | 9 | 14.1% | 7.4%–24.8% | 2 | 2.6% | .2%–9.4% | .0009 |
| Type 2 only | 12 | 18.8% | 10.1%–30.1% | 64 | 82.1% | 72.0%–89.1% | <.0001 |
| Type 3 only | 17 | 26.6% | 17.2%–38.6% | 8 | 10.3% | 5.0%–19.2% | <.0001 |
| Two serotypes | 18 | 28.1% | 18.5%–40.2% | 4 | 5.1% | 1.6%–12.8% | .0003 |
| Type 1 and 2 | 5 | 7.8% | 3.0%–17.4% | 2 | 2.6% | .2%–9.4% | .016 |
| Type 1 and 3 | 3 | 4.7% | 1.1%–13.4% | 0 | 0.0% | .0%–5.6% | .56 |
| Type 2 and 3 | 10 | 15.6% | 8.5%–26.6% | 2 | 2.6% | .2%–9.4% | .0004 |
| Three serotypes | 8 | 12.5% | 6.2%–23.0% | 0 | 0.0% | .0%–5.6% | .001 |
Abbreviation: OPV, oral polio vaccine.
a 95% confidence interval for a proportion calculated using the modified Wald method.
* Fisher exact test for differences between shedding from direct vaccination vs community acquisition.
Overall, 56 (58%) of the 97 positive samples from enrolled children were attributed to direct vaccination, whereas 41 (42%) were attributed to community acquisition. In contrast, 8 (18%) of the 45 positive samples from household contacts were attributed to direct vaccination, whereas 37 (82%) were attributed to community acquisition. Using the definition of household transmission described in the methods section, 25 of the 37 (68%) community acquired OPV viruses isolated from household contacts could be attributed to household transmission.
Shedding of the same OPV serotype by the same subject for more than 1 month was seen in 18 subjects: in 2 subjects for 3 consecutive time points immediately following direct vaccination, and in 16 subjects for 2 consecutive time points (in 9 subjects immediately after direct vaccination, and in 7 from community acquisition). Among those shedding for more than 1 month, 9 subjects shed serotype 2, 8 subjects shed serotype 3, and 1 subject shed both serotype 2 and 3 for 2 consecutive time points. No shedding was seen for more than 3 consecutive time points.
DISCUSSION
We present the results of real-time PCR analysis of 2501 stool samples, collected monthly in Mexico over a 1-year period from 72 young children and 144 of their household contacts, to determine the circulation patterns of OPV strains after national immunization weeks in a community where infants are now primarily vaccinated with IPV. OPV was detected up to 6 months after an NIW in the enrolled children and up to 7 months in their household contacts but was not detected at 8 months in either group. Although slightly longer than the 3–4 months reported in similar studies in New Zealand and Cuba, this is consistent with our previously reported sewage analysis from the same study and suggests that community OPV circulation may cease by 8 months after an NIW [9, 12, 13]. All 3 OPV serotypes were shed early by vaccinated children in roughly equivalent proportions, but over 85% of the community-acquired OPV among both children and adults was serotype 2, and serotype 2 circulated longer and at higher rates in the community after an NIW than serotypes 1 and 3. This suggests that serotype 2 is more transmissible than serotypes 1 and 3 in the Mexican community we studied and could be, in part, why >85% of cVDPV isolated from global outbreaks to date have been serotype 2 [5].
The increased fitness of serotype 2 compared to serotypes 1 and 3 has been known since OPV was first developed and is the reason behind the subsequent formulation changes in trivalent OPV [14]. Serotype 2 was reported to be more transmissible compared to the other serotypes in studies from Russia and the United States conducted between 1957 and 1960, which examined shedding in household contacts of recipients of older formulations of trivalent OPV [15–17]. Very early studies also showed that when the 3 serotypes were given together in equal amounts, serotype 2 was shed more than the other serotypes, and seroconversion was higher for serotype 2 than the other serotypes among vaccinees [18]. As a consequence, the formulation of trivalent OPV was “balanced” so that the ratio of serotypes 1, 2, and 3 was 10:1:3 in around 1963, and the minimum concentration of serotype 3 was further increased by recommendation of the World Health Organization Global Advisory Group in the early 1990s.
There have been limited studies on community circulation of OPV in the past several decades, especially since the introduction of newer vaccine formulations and the inclusion of IPV-vaccinated populations in developing settings. An American study in 1990, 11 years after the last case of wild poliovirus in the United States, suggested that administration of the 10:1:3 formulation may still result in increased transmissibility of serotype 2 compared to the other serotypes [19]. This study investigated immunity to each serotype among unvaccinated inner-city children in Houston and Detroit and found immunity to serotype 2 to be significantly higher than to the other 2 serotypes, presumably from increased secondary spread of that serotype from vaccinees. Sewage analyses conducted in New Zealand and Switzerland, after those countries switched to using IPV exclusively for routine infant immunization, also show a predominance of serotype 2 (13 serotype 2 strains vs 3 serotype 1 and 4 serotype 3 in Switzerland, and 36 serotype 2 vs 9 serotype 1 and 26 serotype 3 in New Zealand) [13, 20], similar to what we reported in our sewage analysis as well as in this analysis of shedding in household contacts [9]. However, sewage analyses from Cuba (where OPV is used exclusively), Argentina (in a province in which 3 of the 4 towns sampled exclusively used OPV and the fourth used both IPV and OPV), and in South Africa and Italy when those countries exclusively used OPV showed no predominance of serotype 2 [12, 21–23]. Given that IPV is known to confer inferior intestinal immunity than OPV [24], it is possible that serotype 2 exhibits higher levels of community circulation in communities that are either undervaccinated or primarily vaccinated with IPV but not in communities primarily vaccinated with OPV. However, direct comparisons of OPV and IPV regimens have not been conducted in contiguous regions or in those with similar sociodemographic conditions.
Our study also showed that two-thirds of OPV detected in stools of household contacts who were not shedding as a result of direct vaccination were likely attributable to acquisition from someone living in the same household. This was probably an underestimate, as we did not sample everyone living in each household, and we only collected stool samples once each month.
Our study has some limitations. Although we estimated how each subject acquired their OPV strain through their vaccination history and used a strict definition of direct vaccination vs community acquired shedding, we cannot know with 100% certainty that our estimations are correct, and we may have misclassified how some of the strains were acquired. However, we repeated the analysis with more stringent criteria, excluding samples from the community acquisition grouping from all children who had received OPV in the prior NIW and who may have had intermittent shedding from direct vaccination, with similar results (Supplementary Table 3). Second, our PCR assay is more sensitive for serotype 2 than for serotypes 1 and 3 [11]. However, this would not explain why we saw similar amounts of each serotype immediately after vaccination, and an overwhelming predominance of serotype 2 in subjects shedding due to community acquisition. Furthermore, the concentration of virus in samples containing OPV strains due to direct vaccination vs those due to community acquisition were not significantly different, as measured by the PCR cycle threshold numbers. Consequently, we believe the differential circulation patterns of the 3 serotypes that we report is likely to be real and not an artifact of our assay. Third, as women were more willing to participate as household contacts in this community, 69% of the participating household contacts were female. As women are also more likely to come into contact with their children's feces through changing diapers and household cleaning, this may have increased the proportion of household contacts found to be shedding OPV. Finally, the most robust method to identify chains of OPV transmission would be to sequence the OPV isolates we detected in order to determine genetic linkage; this project is currently underway.
In summary, we found that OPV strains continue to circulate for 7 months after an NIW in a Mexican community where infants are now primarily vaccinated with IPV, but no OPV strains were detected at 8 months, suggesting circulation may have ceased. Although all 3 serotypes are shed early by vaccinated children in roughly equivalent amounts, children and adults who likely acquired OPV through community spread predominantly shed serotype 2, and serotype 2 circulates longer and at a higher rate after an NIW than serotypes 1 and 3 in this same community. This suggests that serotype 2 is more transmissible after trivalent OPV administration in a community primarily vaccinated with IPV and could be part of the reason why the vast majority of isolated cVDPV has been serotype 2. These results support transitioning from trivalent to bivalent OPV after introduction of at least 1 dose of IPV globally, as proposed in the “Polio Eradication and Endgame Strategic Plan 2013–2018,” but suggest that the transition should occur in a coordinated fashion to minimize the risk of persistent OPV serotype 2 community circulation and cVDPV formation.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We would like to thank Nita Srinivas, Jean Guo, Jennifer Hawken, Anna Young, Katie Nelson, and Julia Janssen for their help analyzing samples; and Julie Kerry for sharing her laboratory space, equipment, and expertise. We would also like to thank the population, participants, and healthcare workers of the Orizaba Health Jurisdiction, Mexico, for their generous support and cooperation.
Financial Support. This study was supported by a grant from the World Health Organization (Protocol ID RPC378, title: “Post-eradication polio vaccination strategies: effect of routine IPV immunization on OPV, VAPP, and VDPV shedding in Mexico after NIDs,” PI: Yvonne Maldonado), by a National Institutes of Health Career Development Award (Protocol ID 5K23AI093678, title: “Oral Polio Vaccine Circulation in Mexico,” PI: Stephanie Troy), by the Bill and Melinda Gates Foundation, and by the Instituto Nacional de Salud Pública in Mexico.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Global Polio Eradication Initiative. Available at: http://www.polioeradication.org/Dataandmonitoring/Poliothisweek.aspx. Accessed 9 September 2013.
- 2.Minor P, Macadam A, Stone D, Almond J. Genetic basis of attenuation of the Sabin oral poliovirus vaccines. Biologicals. 1993;21:357–63. doi: 10.1006/biol.1993.1096. [DOI] [PubMed] [Google Scholar]
- 3.Troy S, Maldonado Y. Polioviruses. In: Long SS, Pickering LK, Prober CG, editors. Principles and practice of pediatric infectious diseases. 4th ed. China: Elsevier Inc.; 2012. [Google Scholar]
- 4.(CDC) CfDCaP. Update on vaccine-derived polioviruses - worldwide, april 2011-june 2012. MMWR Morb Mortal Wkly Rep. 2012;61:741–6. [PubMed] [Google Scholar]
- 5.Global Polio Eradication Initiative. Available at: http://www.polioeradication.org/Dataandmonitoring/Poliothisweek/Circulatingvaccinederivedpoliovirus.aspx. Accessed 20 November 2013.
- 6.Jenkins H, Aylward R, Gasasira A, et al. Implications of a circulating vaccine-derived poliovirus in Nigeria. N Engl J Med. 2010;362:2360–9. doi: 10.1056/NEJMoa0910074. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization (WHO) Polio Eradication & Endgame Strategic Plan 2013–2018: Executive Summary. Geneva, Switzerland: WHO Press; 2013. [Google Scholar]
- 8.INEGI (Instituto Nacional de Estadística G, e Informática) XIII Censo General de Población y Vivienda 2010. Mexico: 2011. [Google Scholar]
- 9.Troy SB, Ferreyra-Reyes L, Canizales-Quintero S, et al. Real-time polymerase chain reaction analysis of sewage samples to determine oral polio vaccine circulation duration and mutation after Mexican National Immunization Weeks. J Pediatric Infect Dis Soc. 2012;1:223–9. doi: 10.1093/jpids/pis062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Troy SB, Musingwini G, Halpern MS, et al. Vaccine poliovirus shedding and immune response to oral polio vaccine in HIV-infected and -uninfected Zimbabwean infants. J Infect Dis. 2013;208:672–8. doi: 10.1093/infdis/jit208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Troy SB, Ferreyra-Reyes L, Huang C, et al. Use of a novel real-time PCR assay to detect oral polio vaccine shedding and reversion in stool and sewage samples after a Mexican national immunization day. J Clin Microbiol. 2011;49:1777–83. doi: 10.1128/JCM.02524-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Más Lago P, Gary HJ, Pérez L, et al. Poliovirus detection in wastewater and stools following an immunization campaign in Havana, Cuba. Int J Epidemiol. 2003;32:772–7. doi: 10.1093/ije/dyg185. [DOI] [PubMed] [Google Scholar]
- 13.Huang Q, Greening G, Baker M, et al. Persistence of oral polio vaccine virus after its removal from the immunisation schedule in New Zealand. Lancet. 2005;366:394–6. doi: 10.1016/S0140-6736(05)66386-6. [DOI] [PubMed] [Google Scholar]
- 14.Grassly NC. The final stages of the global eradication of poliomyelitis. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120140. doi: 10.1098/rstb.2012.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fine PE, Carneiro IA. Transmissibility and persistence of oral polio vaccine viruses: implications for the global poliomyelitis eradication initiative. Am J Epidemiol. 1999;150:1001–21. doi: 10.1093/oxfordjournals.aje.a009924. [DOI] [PubMed] [Google Scholar]
- 16.Benyesh-Melnick M, Melnick JL, Rawls WE, et al. Studies of the immunogenicity, communicability and genetic stability of oral poliovaccine administered during the winter. Am J Epidemiol. 1967;86:112–36. doi: 10.1093/oxfordjournals.aje.a120717. [DOI] [PubMed] [Google Scholar]
- 17.Horstmann DM, Paul JR, Godenne-Mccrea M, et al. Immunization of preschool children with oral poliovirus vaccine (Sabin) JAMA. 1961;178:693–701. doi: 10.1001/jama.1961.03040460001001. [DOI] [PubMed] [Google Scholar]
- 18.Sutter R, Kew O, Cochi S, Aylward RB. Poliovirus vaccine-live. In: Paul O, Orenstein W, Plotkin S, editors. Vaccines. 6th ed. China: Elsevier/Saunders; 2013. pp. 598–645. [Google Scholar]
- 19.Chen RT, Hausinger S, Dajani AS, et al. Seroprevalence of antibody against poliovirus in inner-city preschool children: implications for vaccination policy in the United States. JAMA. 1996;275:1639–45. [PubMed] [Google Scholar]
- 20.Zurbriggen S, Tobler K, Abril C, et al. Isolation of sabin-like polioviruses from wastewater in a country using inactivated polio vaccine. Appl Environ Microbiol. 2008;74:5608–14. doi: 10.1128/AEM.02764-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueller JE, Bessaud M, Huang QS, et al. Environmental poliovirus surveillance during oral poliovirus vaccine and inactivated poliovirus vaccine use in Córdoba Province, Argentina. Appl Environ Microbiol. 2009;75:1395–401. doi: 10.1128/AEM.02201-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavlov DN. Poliovirus vaccine strains in sewage and river water in South Africa. Can J Microbiol. 2006;52:717–23. doi: 10.1139/w06-026. [DOI] [PubMed] [Google Scholar]
- 23.Patti AM, Santi AL, Fiore L, et al. Environmental surveillance of poliovirus in Italy: pilot study. Ann Ig. 2003;15:97–105. [PubMed] [Google Scholar]
- 24.Hird TR, Grassly NC. Systematic review of mucosal immunity induced by oral and inactivated poliovirus vaccines against virus shedding following oral poliovirus challenge. PLoS Pathog. 2012;8:e1002599. doi: 10.1371/journal.ppat.1002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.