Abstract
Background. Patients with multidrug-resistant (MDR) tuberculosis may have phenotypic heterogeneity in results of drug-susceptibility tests (DSTs). However, the impact of this on clinical outcomes among patients treated for MDR tuberculosis is unknown.
Methods. Phenotypic DST heterogeneity was defined as presence of at least 1 Mycobacterium tuberculosis isolate susceptible to rifampicin and isoniazid recovered <3 months after MDR tuberculosis treatment initiation from a patient with previous documented tuberculosis due to M. tuberculosis resistant to at least rifampicin and isoniazid. The primary outcome was defined as good (ie, cure or treatment completion) or poor (ie, treatment failure, treatment default, or death). A secondary outcome was time to culture conversion. Cox proportional hazard models were used to determine the association between phenotypic DST heterogeneity and outcomes.
Results. Phenotypic DST heterogeneity was identified in 33 of 475 patients (7%) with MDR tuberculosis. Poor outcome occurred in 126 patients (28%). Overall, patients with MDR tuberculosis who had phenotypic DST heterogeneity were at greater risk of poor outcome than those with MDR tuberculosis but no phenotypic DST heterogeneity (adjusted hazard ratio [aHR], 2.1; 95% confidence interval [CI], 1.2–3.6). Among HIV-infected patients with MDR tuberculosis, the adjusted hazard for a poor outcome for those with phenotypic DST heterogeneity was 2.4 (95% CI, 1.3–4.2) times that for those without phenotypic DST heterogeneity, whereas among HIV-negative patients with MDR tuberculosis, the adjusted hazard for those with phenotypic DST heterogeneity was 1.5 (95% CI, .5–4.3) times that for those without phenotypic DST heterogeneity. HIV-infected patients with MDR tuberculosis with phenotypic DST heterogeneity also had a longer time to culture conversion than with HIV-infected patients with MDR tuberculosis without phenotypic DST heterogeneity (aHR, 2.9; 95% CI, 1.4–6.0).
Conclusions. Phenotypic DST heterogeneity among persons with HIV infection who are being treated for MDR tuberculosis is associated with poor outcomes and longer times to culture conversion.
Keywords: Tuberculosis; multidrug-resistant tuberculosis; mixed infection, heteroresistance; treatment outcome; drug susceptibility testing
(See the editorial commentary by Khan and Behr on pages 1682–4.)
Previous studies have demonstrated that 10%–20% of individuals living in settings with a high burden of tuberculosis have infection with >1 Mycobacterium tuberculosis strain [1]. In some cases, patients can have heterogeneous results of drug-susceptibility tests (DSTs) during a single case of tuberculosis. Several mechanisms, including culture contamination, concurrent infection by >1 M. tuberculosis strain (ie, mixed infections), and heteroresistance within a single clonal M. tuberculosis population, could underlie the finding of heterogeneous drug susceptibility results in patients with tuberculosis [1–7]. Studies have demonstrated that patients with drug-susceptible tuberculosis can have poor treatment outcomes if they are also infected with a drug-resistant strain that is not detected and treated [1–4, 8]. Previous studies have suggested that patients with multidrug-resistant (MDR) tuberculosis (ie, tuberculosis due to strains with bacteriologically confirmed resistance to isoniazid and rifampicin) can be infected with multiple M. tuberculosis strains that may be drug susceptible [5–7]. However, the clinical impact of heterogeneous DST results among patients receiving treatment for MDR tuberculosis is unknown.
According to international tuberculosis treatment guidelines, the composition of appropriate treatment regimens should focus on treating the most resistant isolate, based on the DST results for all isolates collected from a patient during a single tuberculosis episode [9–12]. Thus, isoniazid and rifampicin are not recommended for treating MDR tuberculosis. However, isoniazid and rifampicin are 2 of the most effective drugs used to treat drug-susceptible tuberculosis [13, 14], and it is possible that their use in treatment regimens may improve the clinical outcomes of patients with MDR tuberculosis with concurrent infection by pansusceptible isolates. To begin to address this question, we hypothesized that the patients with concurrent infection with drug-susceptible and MDR M. tuberculosis isolates would have worse clinical outcomes than patients with MDR tuberculosis without concurrent infection with drug-susceptible M. tuberculosis.
METHODS
Study Design
This was a retrospective cohort study relating phenotypic heterogeneity in results of DSTs (hereafter, “phenotypic DST heterogeneity”) among patients with MDR tuberculosis and treatment outcomes. Phenotypic DST heterogeneity was defined as the detection of at least 1 M. tuberculosis isolate that was susceptible to isoniazid and rifampicin in cultures of specimens collected within 3 months of the MDR tuberculosis treatment initiation from a patient with culture-proven MDR tuberculosis. This study included all patients aged ≥21 years who were treated for culture-proven pulmonary MDR tuberculosis in Botswana from 1 January 2005 to 31 December 2011 [15, 16]. Patients with extensively drug-resistant (XDR) tuberculosis, as defined according to World Health Organization (WHO) criteria, were excluded [11].
Setting
This study was conducted in Botswana, a sub-Saharan African country with a human immunodeficiency virus (HIV) infection prevalence of 18% and a tuberculosis rate of 506 cases/100 000 population [12, 17]. In accordance with the national guidelines, all patients with MDR tuberculosis initiated a standardized MDR tuberculosis regimen while waiting for the DST results for second-line agents [9, 12]. The standardized MDR tuberculosis regimen was composed of amikacin, levofloxacin, ethionamide, cycloserine, and pyrazinamide. Individualized regimens were provided after the second-line DST results became available. The MDR tuberculosis treatment was administered daily at the observation clinic. Injectable antituberculosis drugs were administered once daily (7 days per week) and ceased 4 months after culture conversion. Patients were treated for a minimum of 18 months after culture conversion [12].
Laboratory Methods
From January 2005 to March 2010, sputum samples submitted to the National Laboratory of Botswana were processed using the N-acetyl cysteine–3% sodium hydroxide method and then cultured on Lowenstein-Jensen (LJ) solid medium. From April 2010 to December 2011, the Mycobacteria Growth Indicator Tube (MGIT 960) system was implemented for routine mycobacterial cultures [18]. DST was performed using the proportion method on LJ medium with the internationally recommended concentrations of antibiotics [11, 19].
Data Collection
Data were extracted from paper medical records and electronic databases at the tuberculosis clinics, the Botswana National Tuberculosis Program, and the Botswana National Tuberculosis Reference Laboratory. Data collected included patient demographic characteristics, microscopy-determined semiquantitative acid-fast bacilli (AFB) load at the time of diagnosis [11, 12], extrapulmonary involvement, HIV serostatus, and CD4+ T-cell count, along with the use of antiretroviral therapy (ART) for HIV-positive cases. All study participants (including those with evidence of concomitant extrapulmonary disease) had microbiological proof of pulmonary tuberculosis. Tuberculosis was classified as unilateral or bilateral and cavitary or noncavitary on the basis of chest radiograph findings. Patients with tuberculosis were also classified as having tuberculosis alone or pulmonary tuberculosis plus extrapulmonary involvement.
Outcome Variables
We used the WHO option 1 definition for treatment outcomes [11]. “Cure” was the outcome assigned to patients with MDR tuberculosis who completed treatment according to program protocol and had negative results of at least 5 consecutive cultures of samples collected at least 30 days apart in the final 12 months of treatment. “Treatment completion” was assigned to patients with MDR tuberculosis who completed treatment according to program protocol but did not meet the definition for cure because of a lack of bacteriological results. “Death” was assigned to patients with MDR tuberculosis who died for any reason during the course of treatment. “Treatment failure” was assigned to patients with ≥2 positive results among the 5 cultures performed in the final 12 months of therapy or patients with positive result of any of the final 3 cultures. “Default” was assigned to patients with MDR tuberculosis whose treatment was interrupted for ≥2 consecutive months for any reason without medical approval [11]. For our main analyses, clinical outcome was defined as good (ie, cure or completion of treatment) or poor (ie, treatment failure, treatment default, or death). The time to culture conversion was a secondary outcome for the time-to-event analyses. Culture conversion was defined as the presence of negative results for 2 consecutive cultures of specimens collected at least 30 days apart [11].
Statistical Analysis
Differences in baseline characteristics of exposed and unexposed patients were assessed using χ2 and t tests or Wilcoxon rank sum tests, as appropriate. In the survival analysis, individuals were followed from the date they initiated MDR tuberculosis treatment until they experienced a poor clinical outcome or were censored, which occurred if they continued to receive tuberculosis treatment at the end of the observation period, if they were lost to follow-up (ie, >9 months had passed since the last visit), or if the observation period ended (31 December 2011), whichever came first. For analyses assessing both clinical outcomes and culture conversion, we used Kaplan-Meier curves to compare times to event among patients with and those without phenotypic DST heterogeneity for the group overall, for those with HIV infection, and for those without HIV infection. Cox proportional hazard models were used to determine hazard ratios (HRs) and 95% confidence intervals (CIs) and to assess for confounding. Potential confounders included age, sex, prior tuberculosis history, baseline weight, HIV infection status, and the number of drugs to which the MDR M. tuberculosis isolate was susceptible at baseline. For analyses restricted to those infected with HIV, we also evaluated the CD4+ T-cell count and the use of ART at baseline as potential confounders. Potential confounders were considered actual confounders if their inclusion in the multivariable model changed the unadjusted HR by ≥10%. We also determined whether the severity of tuberculosis was worse in those with phenotypic DST heterogeneity, although adjustment for severity of disease could control for factors on the causal pathway between exposure and outcome if the exposure increases the risk of poor outcomes by worsening the severity of tuberculosis. Factors indicating severity of disease included semiquantitative bacillary load (by AFB microscopy, the findings of which were categorized as scanty, 1+, 2+, and 3+). We confirmed that the proportional hazards assumption was not violated, using log-log plots and Schoenfeld residuals.
We conducted 2 sensitivity analyses. The first restricted the outcome to death. The second addressed our use of a 3-month window to determine exposure. van Rie et al have demonstrated that drug-susceptible M. tuberculosis populations can reemerge in patients with MDR tuberculosis if they have decreased antibiotic selection pressure (eg, via default) [7]. Furthermore, in patients predominantly infected with MDR M. tuberculosis, a single sputum culture has relatively low sensitivity (approximately 60%) for detection of minority (eg, drug-susceptible) M. tuberculosis populations [6]. Therefore the 3-month treatment window was selected in an attempt to capture as many patients as possible (via multiple cultures) who had phenotypic DST heterogeneity at the start of therapy, while simultaneously limiting reverse causality between exposure and outcome. To evaluate the effect of our definition on the results, we performed an analysis limited to patients who remained culture positive after month 3. All data analysis was conducted using Stata, version 12 (Stata, College Station, TX).
Ethics
This study was approved by the Human Research Development Committee at the Botswana Ministry of Health and by the University of Pennsylvania Institutional Review Board (IRB). Additional review by the Centers for Disease Control and Prevention (CDC) IRB was not required because CDC investigators were determined not to be engaged in human subjects research as defined by relevant US government regulations.
RESULTS
Cohort and Study Population
During the 7-year study period, 539 patients received a diagnosis of MDR tuberculosis and were treated in Botswana. Of these patients, 483 (88.1%) had microbiologically confirmed MDR tuberculosis and were included in the analyses. Eight (1.7%) of 483 received a diagnosis of XDR tuberculosis and were excluded from the analysis.
The 475 patients with MDR tuberculosis accounted for 7025 person-months of follow-up after initiating treatment for MDR tuberculosis. Demographic and clinical characteristics of these patients are shown in Table 1. Thirty-three patients (7.0%), accounting for 504 person-months of follow-up time, had phenotypic DST heterogeneity. We did not find a significant difference between culture-positivity rates (68% vs 72%), contamination rates (3.6% vs 5.8%), or rates of infection involving phenotypic DST heterogeneity between the 2 periods in which our laboratory used LJ medium (13/198 [6.6%]) or MGIT medium (20/277 [7.2%]). There were no significant differences in the following characteristics between patients with and those without phenotypic DST heterogeneity: semiquantitative bacillary burden on sputum AFB microscopy at baseline, prevalence of extrapulmonary involvement, or number of effective drugs used to treat MDR tuberculosis (Table 1).
Table 1.
Clinical and Microbiological Characteristics of Patients With Multidrug-Resistant (MDR) Tuberculosis, by Presence or Absence of Phenotypic Heterogeneity in Results of Drug-Susceptibility Testing
| Characteristic | Phenotypic Heterogeneity (n = 33) | No Phenotypic Heterogeneity (n = 442) | P |
|---|---|---|---|
| Sex | |||
| Male | 18 (54.6) | 256 (57.9) | .71 |
| Female | 15 (45.5) | 186 (42.1) | |
| Age | |||
| 21–40 y | 18 (54.6) | 237 (53.6) | .918 |
| >40 y | 15 (45.5) | 205 (46.4) | |
| Treatment failure category | |||
| New MDR tuberculosis without prior tuberculosis | 0 | 27 (6.1) | .066 |
| Previous tuberculosis treatment with first-line drugs only | 30 (90.9) | 398 (90.1) | |
| Previous tuberculosis treatment with second-line drugs | 3 (3.9) | 17 (9.1) | |
| Semiquantitative bacillary load | |||
| Negative | 7 (21.2) | 80 (18.1) | .258 |
| Scanty or 1+ | 14 (42.4) | 179 (40.5) | |
| 2+ or 3+ | 12 (36.4) | 183 (41.4) | |
| Follow up duration, mo | 36 (8–22) | 36 (6–23) | .735 |
| Appropriateness of MDR tuberculosis regimen | |||
| Effective drugs, no. | 4 | 4 | |
| Interval of treatment with ≤3 effective drugs, mo | 3 (2–4) | 3 (2–4) | |
| Localization of tuberculosis | |||
| Pulmonary only | 29 (87.8) | 390 (88.2) | .440 |
| Pulmonary and extrapulmonary | 4 (12.2) | 52 (8.8) | |
| Extension of pulmonary disease | |||
| Unilateral | 22 (66.6) | 275 (62.2) | .611 |
| Bilateral | 11 (33.3) | 167 (37.8) | |
| Cavitation | |||
| Cavitary | 14 (46.7) | 239 (54.1) | .431 |
| Not cavitary | 16 (53.3) | 203 (45.9) | |
| Amikacin use | |||
| Dose, mg/kg | 17.2 (15.4–18.9) | 16.7 (15.3–18.9) | .238 |
| Treatment duration, mo | 8 (6–10) | 7 (5–9) | .946 |
| Outcome | |||
| Cured | 6 (18.2) | 93 (21.0) | <.001 |
| Completed | 6 (18.2) | 133 (29.9) | |
| Dead | 9 (27.3) | 85 (19.2) | |
| Failure/default | 10 (33.3) | 21 (6.4) | |
| On treatment | 2 (6.0) | 110 (25.0) | |
| Composite outcome | |||
| Good | 14 (42.4) | 336 (76.0) | <.001 |
| Poor | 19 (57.6) | 106 (24.0) | |
| Culture conversion | |||
| Absent | 17 (56.7) | 145 (43.9) | .180 |
| Present | 13 (43.3) | 185 (56.1) | |
| HIV infection status | |||
| Infected | 22 (66.6) | 306 (69.2) | .760 |
| Uninfected | 11 (33.3) | 136 (30.8) | |
| HIV treatment | |||
| No ART | 2 (9.1) | 32 (10.5) | .836 |
| ART | 20 (90.9) | 274 (89.5) | |
| CD4+ T-cell count, cells/mm3a | |||
| >350 | 11 (50.0) | 158 (51.6) | .236 |
| 200–349 | 4 (18.2) | 58 (18.9) | |
| <200 | 7 (31.8) | 90 (29.4) | |
| Median | 239 (149–406) | 249 (143–368) | .992 |
| Time to culture conversion, mo | 8 (5–11) | 5 (3–7) | <.01 |
Data are no. (%) of patients or median value (interquartile range).
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; HR, hazard ratio.
a Data are for HIV-infected patients only.
Among all patients, 126 of 475 (26.3%) had poor clinical outcomes during follow-up: 94 (19.8%) died during treatment, and 31 (6.5%) experienced treatment failure or default. There was no difference in the proportions of HIV-infected and HIV-uninfected persons with phenotypic DST heterogeneity (22 of 328 [6.7%] vs 11 of 147 [7.5%]; P = .75). Within the HIV-infected subgroup, phenotypic DST heterogeneity was not associated with the CD4+ T-cell count (median, 239 cells/µL [interquartile range {IQR}, 149–406] vs 249 cells/µL [IQR, 143–368]; P = .76). Of the 22 HIV-infected patients and 11 HIV-uninfected patients with phenotypic DST heterogeneity, 10 (45.5%) and 6 (54.5%), respectively, had conversion to negative culture results. Drug-susceptible M. tuberculosis isolates were the last ones cultured from 6 of the 10 HIV-infected patients (6.0%) and 3 of the 6 HIV-uninfected patients (50.0%) with phenotypic DST heterogeneity; these patients all tested culture-negative eventually.
Overall, 19 of 33 patients (57.6%) with phenotypic DST heterogeneity had poor outcomes during follow-up, compared with 106 of 442 patients (24.0%) without phenotypic DST heterogeneity. Deaths, treatment failure, or treatment default were more common in the patients with phenotypic DST heterogeneity (Table 1). Of 22 patients with phenotypic DST heterogeneity coinfected with HIV, 8 (36.4%) and 7 (31.8%) died or had treatment failure, respectively, compared with 63 (20.6%) and 12 (3.9%), respectively, of 306 patients without the exposure (P < .001). Of the 19 patients with phenotypic DST heterogeneity who had poor outcomes, 5 (26.3%) had those poor outcomes while having drug-susceptible M. tuberculosis in their last positive culture. Similarly, 7 of the 24 patients (27.3%) with phenotypic DST heterogeneity who had good outcomes experienced these outcomes while having a susceptible M. tuberculosis isolate in their last positive culture. Figure 1 presents the Kaplan-Meier curves for the group overall and by HIV infection status. As can be seen by the stratified Kaplan-Meier curves, the relationship between exposure and outcome was significant in patients infected with HIV but not in those without HIV infection.
Figure 1.
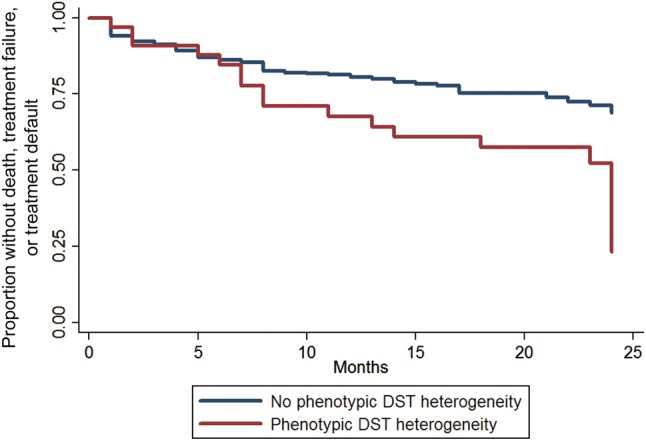
Kaplan-Meyer curves for poor outcome, by phenotypic heterogeneity in results of drug-susceptibility tests (hereafter, “phenotypic DST heterogeneity”).
The presence of phenotypic DST heterogeneity was associated with a shorter time to event overall, but the effect was statistically significant only among patients infected with HIV. The unadjusted HRs for those without HIV infection and those with HIV infection were 1.5 (95% CI, .5–4.3) and 2.6 (95% CI, 1.5–4.6), respectively. Among patients infected with HIV, no ART and lower CD4+ T-cell counts were associated with an increased risk of poor outcomes (Table 2). The association between concurrent infection with susceptible and MDR isolates and poor outcomes among patients infected with HIV persisted after adjustment for age, sex, prior history of tuberculosis, smear microscopy findings at the time of diagnosis, CD4+ T-cell counts, and ART (Table 2 and Figures 1 and 2). The trend toward this association remained present, yet not statistically significant, when the main outcome was restricted to persons who died (Supplementary Table 1 and Figure 1A and 1B). There were 325 patients who remained culture positive after month 3. In these patients, the unadjusted HRs were 1.5 (95% CI, .5–4.2) and 3.4 (95% CI, 1.8–6.4) for those without HIV infection and those with HIV infection, respectively.
Table 2.
Cox Proportional Hazard Models to Determine Risk Factors for Poor Outcomes Among Patients With Multidrug-Resistant (MDR) Tuberculosis, Overall and by Human Immunodeficiency Virus (HIV) Infection Status
| Factor | All Patients, HR (95% CI) | HIV Infected, HR (95% CI) | HIV Uninfected, HR (95% CI) |
|---|---|---|---|
| Phenotypic heterogeneity in DST results | 2.09 (1.20–3.61) | 2.37 (1.32–4.24) | 1.46 (.48–4.39) |
| Age, y | |||
| 21–40 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| >40 | 1.13 (.74–1.73) | 0.89 (.55–1.42) | 2.45 (1.22–4.96) |
| Sex | |||
| Female | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Male | 0.82 (.53–1.25) | 1.31 (.82–2.07) | 0.99 (.48–2.03) |
| Weight at baseline | 0.97 (.94–.99) | 0.97 (.94–.99) | 0.96 (.93–.99) |
| HIV infection status | |||
| Not infected | 1.00 (reference) | … | … |
| Infected, CD4+ T-cell count > 350 cells/mm3 | 2.98 (1.33–6.66) | 1.00 (reference) | … |
| Infected, CD4+ T-cell count 200–350 cells/mm3 | 6.51 (3.02–14.05) | 1.72 (.95–3.11) | … |
| Infected, CD4+ T-cell count < 200 cells/mm3 | 8.49 (4.55–15.85) | 1.91 (1.12–3.24) | … |
| Current ART receipt | 0.26 (.15–0.45) | 0.14 (.08–.24) | … |
| Treatment failure category | |||
| New MDR tuberculosis without prior tuberculosis | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| New MDR tuberculosis with prior use of first-line drugs | 2.11 (.75–5.90) | 2.28 (.70–7.41) | 2.69 (.33–21.59) |
| MDR tuberculosis with prior use of second-line drugs only | 2.46 (.69–8.70) | 3.02 (.74–12.32) | 4.75 (.44–51.59) |
| Smear microscopy finding at baseline | |||
| Negative | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Scanty or 1+ | 1.47 (1.02–1.92) | 1.68 (1.01–2.83) | 2.08 (.87–4.95) |
| 2+ or 3+ | 1.75 (1.05–2.91) | 2.31 (1.26–3.83) | 2.18 (.66–5.45) |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; DST, drug-susceptibility test; HIV, human immunodeficiency virus; HR, hazard ratio.
Figure 2.
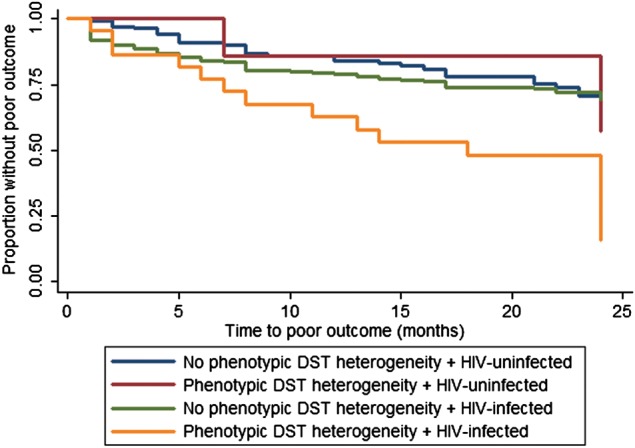
Kaplan-Meyer curves for poor outcome, by phenotypic heterogeneity in results of drug-susceptibility tests (hereafter, “phenotypic DST heterogeneity”) and human immunodeficiency virus (HIV) infection status.
HIV-infected patients with concurrent infection due to susceptible and MDR isolates also had significantly longer times to sputum culture conversion (median, 12 months; IQR, 8–22 months), compared with HIV-infected patients without concurrent infection due to susceptible and MDR isolates (median, 5 months; IQR, 3–8 months; P < .01), HIV-uninfected patients with concurrent infection due to susceptible and MDR isolates (median, 6.5 months; IQR, 4–9 months; P < .01), and HIV-uninfected patients without concurrent infection due to susceptible and MDR isolates (median, 5 months; IQR, 3–8 months; P < .01 Table 3 ). Nearly half of all HIV-infected persons with MDR tuberculosis who had concurrent infection with susceptible and MDR isolates did not achieve sputum conversion, compared with 15% of patients without concurrent infection due to susceptible and MDR isolates (Figure 3 and Supplementary Figure 2A and 2B). Findings of the sensitivity analysis using data from patients whose times to culture conversion were >3 months showed no difference from findings of the analysis that included the entire sample (Supplementary Figure 2A and 2B), suggesting the lack of a significant bias introduced by the definition of our primary exposure.
Figure 3.
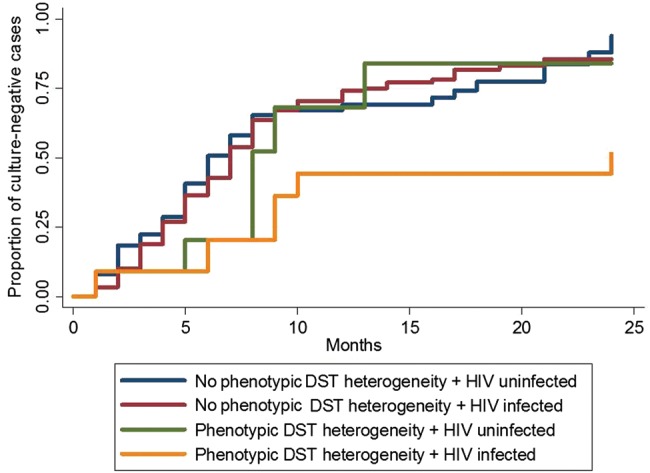
Kaplan-Meyer curves for time to achievement of a negative sputum culture result, by presence or absence of phenotypic heterogeneity in results of drug-susceptibility tests (hereafter, “phenotypic DST heterogeneity”) and human immunodeficiency virus (HIV) infection status.
DISCUSSION
We found a strong association between concurrent infection with isoniazid- and rifampicin-susceptible strains and poor clinical outcomes among patients coinfected with HIV and MDR M. tuberculosis. HIV-infected persons with phenotypic DST heterogeneity also remained culture positive for longer periods, which would be expected to increase the risk of tuberculosis transmission and to enhance the potential development of further resistance to second-line drugs. In contrast, we did not find an association between phenotypic DST heterogeneity and outcomes among patients without HIV infection. However, the small number of HIV-negative patients with phenotypic DST heterogeneity (n = 11) may have limited our ability to detect an effect within this group.
Table 3.
Cox Proportional Hazard Models to Determine Risk Factors for Time to Culture Conversion Among Patients With Multidrug-Resistant (MDR) Tuberculosis, Overall and by Human Immunodeficiency Virus (HIV) Infection Status
| Factor | All Patients, HR (95% CI) | HIV Infected, HR (95% CI) | HIV Uninfected, HR (95% CI) |
|---|---|---|---|
| Phenotypic heterogeneity in DST results | 1.91 (1.05–3.52) | 2.93 (1.43–5.99) | 1.21 (.47–3.09) |
| Age | |||
| 21–40 y | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| >40 y | 1.05 (.78–1.41) | 0.74 (.47–1.23) | 1.02 (.99–1.03) |
| Sex | |||
| Female | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Male | 1.10 (.82–1.49) | 1.10 (.77–1.56) | 0.91 (.54–1.54) |
| Weight at baseline | 0.97 (.70–1.33) | 0.67 (.38–1.17) | 0.97 (.68–1.37) |
| HIV infection status | |||
| Not infected | 1.00 (reference) | … | … |
| Infected, CD4+ T-cell count > 350 cells/mm3 | 0.61 (.28–1.32) | 1.00 (reference) | … |
| Infected, CD4+ T-cell count cells/mm3 | 0.37 (.16–.85) | 0.91 (.58–1.43) | … |
| Infected, CD4+ T-cell count < 200 cells/mm3 | 0.33 (.17–.77) | 0.59 (.39–.90) | … |
| Current ART receipt | 0.37 (.16–.85) | 0.26 (.03–.78) | … |
| Treatment failure category | |||
| New MDR tuberculosis without prior tuberculosis | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| MDR tuberculosis with prior use of first-line drugs | 0.71 (.42–1.20) | 2.50 (1.41–4.43) | 1.62 (.71–3.66) |
| MDR tuberculosis with prior use of second-line drugs | 0.53 (.20–1.42) | 2.98 (1.02–9.54) | 3.42 (1.53–6.11) |
| Smear microscopy finding at baseline | |||
| Negative | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Scanty or 1+ | 1.27 (.70–2.03) | 1.75 (1.05–2.91) | 1.9 (.97–1.01) |
| 2+ or 3+ | 2.46 (1.11–3.22) | 1.95 (1.06–2.89) | 2.03 (1.01–3. 98) |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; DST, drug-susceptibility test; HIV, human immunodeficiency virus; HR, hazard ratio.
Molecular-based studies have since demonstrated that tuberculosis may be caused by multiple strains within a patient [1, 2, 7, 8, 20–26]. Because drug-susceptible tuberculosis is still the most prevalent type of tuberculosis in most communities, patients with MDR tuberculosis may have concurrent disease caused by drug-susceptible M. tuberculosis isolates [7, 27]. The prevalence of phenotypic DST heterogeneity in our population was approximately 7%, regardless of HIV infection status. However, studies using molecular approaches have reported a prevalence of mixed M. tuberculosis infections as high as almost 60% among persons with pansusceptible M. tuberculosis in some tuberculosis-endemic settings [1, 7, 27]. Given the global burden of MDR tuberculosis (particularly among HIV-infected individuals), the resource-intensive nature of MDR tuberculosis treatment, and the potential impact of delayed culture conversion on transmission, identifying phenotypic DST heterogeneity from isolates with different drug-susceptibility profiles (as a potentially modifiable determinant of patient outcomes) might be essential for public health regardless of whether they represent mixed M. tuberculosis infections.
Our study does not distinguish between mixed DST results due to authentic mixed infection with independent strains carrying different DST patterns and heterogeneity of drug susceptibility within a population that may have originated from a single isolate. It will be important for future studies to distinguish the basis responsible for phenotypically mixed infections. Nevertheless, our results show the impact of phenotypically mixed infection on drug susceptibility and raise important questions regarding optimal management.
It is possible that phenotypic DST heterogeneity is simply a marker for more- severe tuberculosis or more-advanced immunodeficiency, although our results do not support this interpretation. By all measures, the clinical extent of disease was not different between patients with and those without phenotypic DST heterogeneity. In addition, the prevalence of phenotypic DST heterogeneity was similar between persons living with HIV and without HIV infection. Similarly, the prevalence of phenotypic DST heterogeneity among patients with HIV infection was similar, regardless of the level of immunosuppression. In addition, our multivariate analysis revealed that the association between phenotypic DST heterogeneity and poor outcomes among HIV-infected patients persisted, after adjustment for factors likely to impact outcomes, including the initial severity of disease, the appropriateness of the initial antituberculosis regimen, and prior tuberculosis treatment. Thus, although we cannot completely exclude the possibility that other, unidentified factors may lead to a greater likelihood of poor outcomes, our analysis suggests that phenotypic DST heterogeneity is an independent factor leading to poor outcomes.
The mechanism explaining how concurrent disease caused by both drug-susceptible and drug-resistant strains during MDR tuberculosis treatment may lead to worse outcomes remains to be defined [1]. One possibility is that in individuals with phenotypic DST heterogeneity, the failure to provide optimal treatment for even a minority of susceptible M. tuberculosis subpopulations can diminish treatment responses. However, although second-line drugs used for MDR tuberculosis are notoriously less effective than first-line drugs, this explanation would require that second-line drugs be less effective against drug-susceptible strains than against MDR strains. Recent data have suggested that drug-resistant M. tuberculosis may be associated with decreased fitness; therefore, isoniazid- and rifampin-susceptible (ie, wild-type) strains may be controlled less effectively than MDR isolates by second-line therapy [28]. It is possible that the clinical impact of suboptimal treatment for drug-susceptible isolates may only manifest in the setting of HIV-related immunosuppression. The association between phenotypic DST heterogeneity and poor outcomes was only present in HIV-infected patients, which supports one of 2 hypotheses: (1) more-virulent isolates may exhibit more-detrimental clinical effects in immunosuppressed patients [28, 29], or (2) M. tuberculosis isolates circulating and being transmitted among immunocompetent and immunosuppressed populations may carry different virulence factors or fitness [28–30]. However, even if either scenario was true, treating the susceptible and virulent isolates may still be recommended.
This study had several limitations. First, the presence of phenotypic DST heterogeneity in this observational study may have been related to increased disease severity in manners that we did not capture retrospectively. Because of the lack of M. tuberculosis genotyping data, we are unable to confirm the presence of mixed infection (ie, the presence of >1 M. tuberculosis genotype) among patients with concurrent disease caused by susceptible and MDR M. tuberculosis isolates, as opposed disease caused by a single clone that presented both a susceptible and resistant phenotype (eg, because of microevolution) [1, 4, 6, 31, 32]. To minimize misclassification caused by this potential limitation, we used conservative phenotypic definitions. Similarly, by narrowing the window for our definition of concurrent M. tuberculosis infections, based on extreme DST results (pansusceptible isolate that defined a mixed M. tuberculosis infection, we decreased the likelihood of misclassifying a reinfection (or superinfection during the course of treatment) as a mixed M. tuberculosis infection that was present from the moment of diagnosis. Although heterogeneity due to microevolution leading to the development of resistance within the host has been described in M. tuberculosis, to our knowledge this development has never led to the coexistence of clones with both pansusceptible and drug-resistant MDR phenotypes [1, 4, 6, 31, 32].
Our definitions of phenotypic DST heterogeneity were based exclusively on drug-susceptibility phenotype. This approach may have underestimated the proportion of patients coinfected with multiple M. tuberculosis strains [4, 32, 33]. In addition, by using the extreme phenotype of pansusceptible M. tuberculosis for our exposure of interest, by definition we did not address the impact of concurrent infections due to isolates that were susceptible to only one of the 2 first-line drugs. It also seems reasonable to assume that individuals with a higher bacterial load may have a higher likelihood of concurrent infection with susceptible and MDR M. tuberculosis isolates (especially if the mechanism involved emergence of resistance within the host), which could also be associated with poorer treatment outcomes. Although the semiquantitative bacillary load in sputum is a reasonable measure of the burden of disease, it is not perfect. However, to our knowledge, there is nothing to suggest that patients with concurrent infection with susceptible and MDR M. tuberculosis isolates have a higher burden of disease. Finally, it is possible that laboratory errors and contamination may have led to some degree of misclassification. However, this nondifferential misclassification would be expected to bias the results toward a null result and, therefore, is not a plausible explanation for the statistically significant findings. Additionally, we believe that this scenario is unlikely, because of previously reported data on laboratory proficiency and quality control [34, 35].
In summary, our data suggest that HIV-infected patients with MDR tuberculosis who are concurrently infected with drug-susceptible M. tuberculosis are at risk for poor clinical outcomes. Because current treatment paradigms do not aim to systematically identify and optimize the treatment for minority drug-susceptible isolates in MDR tuberculosis, larger studies are needed to define the scope of the problem and to evaluate the clinical impact of adding rifampin and/or isoniazid to the standard MDR tuberculosis regimen in this context. Furthermore, these data indicate that an isolate from a single sputum specimen from patients in areas where tuberculosis is highly endemic may not be representative of the total infecting bacillary population [7]. Thus, in areas where tuberculosis and HIV infection are endemic, efforts to accurately determine the composition of the infecting mycobacterial population could have positive clinical and public health effects.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Rosemarie Kappes and Drs Sarita Shah and Eugene McCray, for their constant guidance, support and input on the manuscript; the staff at the MDR tuberculosis clinics; the Penn Center for AIDS Research, for resources and support; the Botswana Ministry, for their constant support; and our patients, who made this study possible.
Drs Modongo and Zetola had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs Modongo, Zetola, Moonan, Ncube, and Sepako were involved in the study concept and design. Drs Modongo, Zetola, Ncube, Sepako, Machao, and Matlhagela acquired the data. Drs Modongo, Zetola, Bisson, Moonan, and Collman analyzed and interpreted the data. Drs Zetola and Modongo drafted the manuscript. Drs Modongo, Zetola, Bisson, Collman, Moonan, Ncube, Matlhagela, and Sepako critically revised the manuscript for important intellectual content. Drs Zetola and Moonan performed statistical analysis. Drs Zetola, Collman, and Ncube obtained funding. Drs Modongo, Matlhagela, and Sepako provided administrative, technical, or material support. Drs Zetola, Bisson, Collman, Moonan, and Ncube supervised the study.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the National Institutes of Health (grants R01AI097045 and P30AI45008 to the Penn Centre for AIDS Research).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Cohen T, van Helden PD, Wilson D, et al. Mixed-strain mycobacterium tuberculosis infections and the implications for tuberculosis treatment and control. Clin Microbiol Rev. 2012;25:708–19. doi: 10.1128/CMR.00021-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hingley-Wilson SM, Casey R, Connell D, et al. Undetected multidrug-resistant tuberculosis amplified by first-line therapy in mixed infection. Emerg Infect Dis. 2013;19:1138–41. doi: 10.3201/eid1907.130313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Deun A, Aung KJ, Bola V, et al. Rifampin drug resistance tests for tuberculosis: challenging the gold standard. J Clin Microbiol. 2013;51:2633–40. doi: 10.1128/JCM.00553-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia de Viedma D, Marin M, Ruiz Serrano MJ, Alcala L, Bouza E. Polyclonal and compartmentalized infection by Mycobacterium tuberculosis in patients with both respiratory and extrarespiratory involvement. J Infect Dis. 2003;187:695–9. doi: 10.1086/368368. [DOI] [PubMed] [Google Scholar]
- 5.Glynn JR, Yates MD, Crampin AC, et al. DNA fingerprint changes in tuberculosis: reinfection, evolution, or laboratory error? J Infect Dis. 2004;190:1158–66. doi: 10.1086/423144. [DOI] [PubMed] [Google Scholar]
- 6.Martin A, Herranz M, Ruiz Serrano MJ, Bouza E, Garcia de Viedma D. The clonal composition of Mycobacterium tuberculosis in clinical specimens could be modified by culture. Tuberculosis. 2010;90:201–7. doi: 10.1016/j.tube.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 7.van Rie A, Victor TC, Richardson M, et al. Reinfection and mixed infection cause changing Mycobacterium tuberculosis drug-resistance patterns. Am J Respir Crit Care Med. 2005;172:636–42. doi: 10.1164/rccm.200503-449OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Somoskovi A, Deggim V, Ciardo D, Bloemberg GV. Diagnostic implications of inconsistent results obtained with the Xpert MTB/Rif assay in detection of Mycobacterium tuberculosis isolates with an rpoB mutation associated with low-level rifampin resistance. J Clin Microbiol. 2013;51:3127–9. doi: 10.1128/JCM.01377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caminero JA, de March P. Statements of ATS, CDC, and IDSA on treatment of tuberculosis. Am J Respir Crit Care Med. 2004;169:316–7. doi: 10.1164/ajrccm.169.2.952. author reply 7. [DOI] [PubMed] [Google Scholar]
- 10.Caminero JA, Sotgiu G, Zumla A, Migliori GB. Best drug treatment for multidrug-resistant and extensively drug-resistant tuberculosis. Lancet Infect Dis. 2010;10:621–9. doi: 10.1016/S1473-3099(10)70139-0. [DOI] [PubMed] [Google Scholar]
- 11.Falzon D, Jaramillo E, Schunemann HJ, et al. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J. 2011;38:516–28. doi: 10.1183/09031936.00073611. [DOI] [PubMed] [Google Scholar]
- 12.Botswana Ministry of Health, Gaborone, Botswana: 2009. Botswana National Tuberculosis Programme report. [Google Scholar]
- 13.Jindani A, Dore CJ, Mitchison DA. Bactericidal and sterilizing activities of antituberculosis drugs during the first 14 days. Am J Respir Crit Care Med. 2003;167:1348–54. doi: 10.1164/rccm.200210-1125OC. [DOI] [PubMed] [Google Scholar]
- 14.Velayati AA, Farnia P, Mozafari M, et al. High prevelance of rifampin-monoresistant tuberculosis: a retrospective analysis among Iranian pulmonary tuberculosis patients. Am J Trop Med Hyg. 2013 doi: 10.4269/ajtmh.13-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zetola NM, Modongo C, Kip EC, Gross R, Bisson GP, Collman RG. Alcohol use and abuse among patients with multidrug-resistant tuberculosis in Botswana. Int J Tuberc Lung Dis. 2012;16:1529–34. doi: 10.5588/ijtld.12.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Modongo C, Zetola NM. Prevalence of hypothyroidism among MDR-TB patients in Botswana. Int J Tuberc Lung Dis. 2012;16:1561–2. doi: 10.5588/ijtld.12.0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ministry of Health of Botswana. BIAS 3 Report. 2008.
- 18.Cruciani M, Scarparo C, Malena M, Bosco O, Serpelloni G, Mengoli C. Meta-analysis of BACTEC MGIT 960 and BACTEC 460 TB, with or without solid media, for detection of mycobacteria. J Clin Microbiol. 2004;42:2321–5. doi: 10.1128/JCM.42.5.2321-2325.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madison B, Robinson-Dunn B, George I, et al. Multicenter evaluation of ethambutol susceptibility testing of mycobacterium tuberculosis by agar proportion and radiometric methods. J Clin Microbiol. 2002;40:3976–9. doi: 10.1128/JCM.40.11.3976-3979.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Rie A, Warren R, Richardson M, et al. Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment. N Engl J Med. 1999;341:1174–9. doi: 10.1056/NEJM199910143411602. [DOI] [PubMed] [Google Scholar]
- 21.Cohen T, Wilson D, Wallengren K, Samuel EY, Murray M. Mixed-strain Mycobacterium tuberculosis infections among patients dying in a hospital in KwaZulu-Natal, South Africa. J Clin Microbiol. 2011;49:385–8. doi: 10.1128/JCM.01378-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borgdorff MW, van Soolingen D. The re-emergence of tuberculosis: what have we learnt from molecular epidemiology? Am J Trop Med Hyg. 2014;90:99–105. doi: 10.1111/1469-0691.12253. [DOI] [PubMed] [Google Scholar]
- 23.Becerra MC, Appleton SC, Franke MF, et al. Tuberculosis burden in households of patients with multidrug-resistant and extensively drug-resistant tuberculosis: a retrospective cohort study. Lancet. 2011;377:147–52. doi: 10.1016/S0140-6736(10)61972-1. [DOI] [PubMed] [Google Scholar]
- 24.Caminero JA, Pena MJ, Campos-Herrero MI, et al. Exogenous reinfection with tuberculosis on a European island with a moderate incidence of disease. Am J Respir Crit Care Med. 2001;163:717–20. doi: 10.1164/ajrccm.163.3.2003070. [DOI] [PubMed] [Google Scholar]
- 25.Cohen T, Colijn C, Finklea B, Murray M. Exogenous re-infection and the dynamics of tuberculosis epidemics: local effects in a network model of transmission. J R Soc Interface. 2007;4:523–31. doi: 10.1098/rsif.2006.0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen T, Murray M, Abubakar I, et al. Multiple introductions of multidrug-resistant tuberculosis into households, Lima, Peru. Emerg Infect Dis. 2011;17:969–75. doi: 10.3201/eid1706.101471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huyen MN, Kremer K, Lan NT, et al. Mixed tuberculosis infections in rural South Vietnam. J Clin Microbiol. 2012;50:1586–92. doi: 10.1128/JCM.00434-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gagneux S, Long CD, Small PM, Van T, Schoolnik GK, Bohannan BJ. The competitive cost of antibiotic resistance in Mycobacterium tuberculosis. Science. 2006;312:1944–6. doi: 10.1126/science.1124410. [DOI] [PubMed] [Google Scholar]
- 29.Gagneux S, Small PM. Global phylogeography of Mycobacterium tuberculosis and implications for tuberculosis product development. Lancet Infect Dis. 2007;7:328–37. doi: 10.1016/S1473-3099(07)70108-1. [DOI] [PubMed] [Google Scholar]
- 30.Pillay M, Sturm AW. Evolution of the extensively drug-resistant F15/LAM4/KZN strain of Mycobacterium tuberculosis in KwaZulu-Natal, South Africa. Clin Infect Dis. 2007;45:1409–14. doi: 10.1086/522987. [DOI] [PubMed] [Google Scholar]
- 31.Al-Hajoj SA, Akkerman O, Parwati I, et al. Microevolution of Mycobacterium tuberculosis in a tuberculosis patient. J Clin Microbiol. 2010;48:3813–6. doi: 10.1128/JCM.00556-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Navarro Y, Herranz M, Perez-Lago L, et al. Systematic survey of clonal complexity in tuberculosis at a populational level and detailed characterization of the isolates involved. J Clin Microbiol. 2011;49:4131–7. doi: 10.1128/JCM.05203-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perez-Lago L, Herranz M, Lirola MM, Bouza E, Garcia de Viedma D. Characterization of microevolution events in Mycobacterium tuberculosis strains involved in recent transmission clusters. J Clin Microbiol. 2011;49:3771–6. doi: 10.1128/JCM.01285-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sibanda T, Tedla Z, Nyirenda S, et al. Anti-tuberculosis treatment outcomes in HIV-infected adults exposed to isoniazid preventive therapy in Botswana. Int J Tuberc Lung Dis. 2013;17:178–85. doi: 10.5588/ijtld.12.0314. [DOI] [PubMed] [Google Scholar]
- 35.Samandari T, Agizew TB, Nyirenda S, et al. 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:1588–98. doi: 10.1016/S0140-6736(11)60204-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


