Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) infection remains a serious hazard to global health despite increases in public education and development of innovative treatment strategies. Use of immune modulatory therapy to combat infection is gaining interest as a novel treatment alternative. Lactoferrin (LF), an iron binding protein with multiple immune modulating properties, has the potential to modify the course of systemic MRSA infection. Specifically, LF is capable of limiting deleterious inflammatory responses while promoting development of antigen specific T-cell activity. The efficacy of a novel recombinant mouse LF (rmLF) to protect against MRSA infection was examined in a mouse peritonitis model. BALB/c mice were infected with a lethal dose of MRSA and treated at 2 hours post-infection with rmLF. The effects of rmLF on MRSA-infected primary monocytes and granulocytes were analyzed for inflammatory mediator production. The rmLF treated mice demonstrated only modest increase in survival by more than 24hrs, albeit with reduced bacteremia. Serum cytokines, IL-17 and IL-6, were significantly reduced post challenge in the rmLF treated mice. Treatment with rmLF led to a minor decrease in IL-1β, and a slight increase in TNF-α production. Preliminary investigation towards human clinical relevance was accomplished using human blood derived monocytes and granulocytes infected with MRSA and treated with a homologous recombinant human LF (rhLF). Treatment with (rhLF) led to increased production of IFN-γ and IL-2. The human cell studies also showed a concurrent decrease in TNF-α, IL-6, IL-1β, IL-12p40, and IL-10. The study reports the first investigation into the efficacy of a novel recombinant mouse lactoferrin (LF) therapy in a mouse model of MRSA peritonitis. Overall, these results indicate the rmLF and rhLF have a high degree of overlap to modify inflammatory responses, although differences in activities were observed indicating existence of mechanisms between the two heterologous recombinant molecules.
Keywords: Lactoferrin, MRSA, Staphylococcus aureus (MRSA), immune modulation
Introduction
The ever-increasing capability of bacteria to acquire antibiotic resistance out-paces development of new antibiotics; an adjunct effort may therefore include agents that modulate host immunity. This is a concept well accepted in vaccinology, which focuses on targeted generation of specific and effective immune responses. Staphylococcus aureus is estimated to inhabit 25–35% of the world’s population, with 1–50% carrying strains exhibiting methicillin resistance (1, 2). Cases of hospital acquired methicillin-resistant S. aureus (MRSA) infections have risen from 36% in 1992 to 64% in 2003 (3), with a mortality rate of 20% in the United States (4). Recently, incidence of hospital/health-care associated MRSA infections are declining, and community associated MRSA infection has remained unchanged or slightly increased (5). Efforts to control MRSA infection include a variety of programs, including public health awareness and improving diagnostics. The most widely used and preferred therapeutic agent for treatment of MRSA is vancomycin. However, emergence of vancomycin resistance (6), coupled with waning efficacy of vancomycin on strains with high MIC values (7, 8), indicate a need for development of novel treatment options for therapeutic intervention of MRSA infections.
MRSA presents an interesting challenge in development of effective adjunct immunotherapy due to contribution of the overwhelming activation of lymphocytes triggered by superantigens (9–11). However, even in the presence of T cell dysregulation, immune therapy can still be considered an important and effective strategy for disease therapy (12). This concept is highlighted in a recent report of an immunostimulatory peptide based on the C-terminal region of C5a, a complement split product, as a successful treatment for community-acquired MRSA skin infections (13).
Lactoferrin (LF), an iron binding protein that is secreted to mucosal surfaces and present in secondary granules of neutrophils, is a unique immune modulator that contributes to innate immune function during infectious assault (14). LF affects activities of numerous leukocytes that may affect defense against MRSA, including decreased macrophage production of inflammatory cytokines (15), increased T-cell specific cytokine (IL-12) in the presence of TLR agonist (16), increased natural killer cell activity (17), increased expression of antigen presentation and co-stimulatory molecules in macrophages and dendritic cells, and increased dendritic cell and macrophage stimulation of antigen specific T-cells (18, 19). Additionally, in mouse models of induced sepsis, bovine LF reduced serum inflammatory cytokines TNF-α and IL-6 which correlated with host survival (18, 20). These studies demonstrate that LF has unique immune modulatory properties that render it capable of suppressing excessive inflammatory responses.
Previous studies examined LF as an immune modulator to prevent development of septic condition in animal models utilized bovine milk-derived lactoferrin (bLF). The bLF is only 70% homologous with human and mouse LFs (21), which raises concern on the applicability for use of heterologous LF in mouse or human model systems. Recently, we developed a CHO-based expression protocols for the production of both mouse and human recombinant LFs using a high expressing and stable cell line (DG44) (22). These novel recombinant LF proteins, with a mammalian type glycosylation patterns, can serve as useful tools to investigate LF in models of infection, while eliminating immunogenicity issues related to antigenic recognition of heterologous epitopes. The studies reported here are the first investigations into the efficacy of a novel recombinant mouse LF (rmLF) therapy in a mouse (homologous) model of MRSA peritonitis. Using comparable in vitro models, the translational effectiveness of the recombinant human LF (rhLF) as a potential clinical therapeutic against MRSA was also investigated.
Materials and Methods
Lactoferrin, MRSA, and MRSA peritonitis
Recombinant human and mouse LFs produced in CHO stable cell lines were provided by PharmaReview Corporation (Houston, TX) (22, 23). LFs were produced under endotoxin free serum conditions, and further affinity purified to remove traces of endotoxin (<0.1EU/mg in end product). Methicillin-resistant Staphylococcus aureus (MRSA) Mu50 strain (ATCC) with intermediate vancomycin resistance was grown in BBL™ Muller-Hinton (MH) (Becton, Dickinson and Company) broth supplemented with 4µg/mL of Vancomycin (according to Sigma protocols) at 37°C and rotating at 225rpm. MRSA in log-phase was resuspended in 1x Dulbecco’s Phosphate Buffered Saline (PBS) and intraperitoneal (IP) injected into BALB/c mice (6–10 weeks old) (Harlan Labs) at 5–9×108 colony forming units (CFU)/300µL/mouse. At 2 hours post-infection, mice were given intravenously (IV) either 100µL/mouse 1xPBS or 1mg/mL rmLF. Mice were sacrificed at 1 hour post-LF treatment. Whole blood samples were used to determine circulating bacterial load, and serum was assessed for cytokines by analysis using a multiplex bead array (Invitrogen). Animal studies were conducted under the approval of the UTHHSC Institutional Review Board, document AWP 10–124.
Blood bacterial load
Whole blood collected from MRSA-infected mice was serially diluted and plated onto MH agar plates supplemented with 4µg/mL of Vancomycin. Colonies were enumerated after 48hrs incubation at 37°C.
Serum cytokine/chemokine analysis
Serum was isolated from whole blood collected at 1hr post-LF treatment from MRSA-infected mice by centrifuging at 8000rpm for 10 mins. The serum was clarified by centrifuge at 10,000 rpm for 10 minutes. The clarified serum was analyzed using the multiplex bead array (Invitrogen Mouse cytokine 20-plex panel) following manufacturer’s instructions.
In vitro analysis of MRSA-infected splenocytes
Total splenocytes were obtained from naïve BALB/c mice (6–10 weeks old) as previously described (16). Splenocytes (2×106 cells/mL) were cultured in DMEM with 10% FBS and exposed to MRSA (MOI 1:10 or 1:1 MRSA:cells) for 1hr. rmouseLF was added in increasing concentrations (10, 100 µg/mL). Supernatants were collected 6hrs post-LF treatment and analyzed by ELISA. Low production of cytokines is observed at <3hrs post-infection, and overnight cultures are overgrown with MRSA. This time point ensured that supernatants were collected at a time point where cells are properly stimulated and viable.
Mouse blood monocytes and granulocytes
Whole blood from naïve BALB/c mice were overlaid onto a discontinuous gradient of Histopaque 1119 and 1083 (Sigma) and spun at 700G for 30mins. Monocyte (89–93% purity) and granulocyte (97–99% purity) (24, 25) layers were collected, remaining RBC lysed with ACK buffer (BioWhittaker), and washed 2x with 1xPBS (spun at 1500rpm for 10 mins). Monocytes and granulocytes were cultured at 2×105 – 5×105 cells/mL in DMEM with 10% FBS at 37°C with 5% CO2. Cells were exposed to MRSA (MOI 1:10 MRSA:cells) for 1hr followed by addition of rm LF (100µg/mL). Supernatants were collected at 6hrs post-LF treatment and analyzed by ELISA.
Human blood monocytes and granulocytes
Human buffy coat from healthy donors were purchased from the Gulf Coast Regional Blood Bank in Houston, TX. Buffy coat was overlaid onto Histopaque 1077 and 1119 (Sigma) and spun at 700G for 30mins. Monocytes and granulocyte layers were collected and treated as mentioned above. Monoytes and granulocytes were cultured at 2×106 cells/mL in DMEM with 10% FBS at 37°C with 5% CO2. Cells were exposed to MRSA (MOI 1:10 MRSA:cells) for 1hr followed by addition of rhLF (10–100µg/mL). Supernatants were collected at 6hrs post-LF treatment and analyzed by ELISA.
ELISA
Mouse and human DuoSets for analysis of TNF-α, IL-6, IL-1β, IL-12p40, IL-10, IL-8, IFN-γ, IL-2, IL-17, and TGF-β1 was conducted following manufacturer’s instructions, as previously reported (16). Lower limits of detection were 16–32 pg/ml. Samples were assessed in duplicate or triplicate, and experiments were repeated for confirmation of results.
Statistics
In vivo mouse experiments contained 5–8 subjects, and the experiment was repeated at least twice. In vitro experiments were conducted in duplicate and repeated 3–6 times. Survival graph is representative, and analyzed by GraphPad Prizm software. Analysis of mouse CFU and cytokine data was determined for relative significance using the Student’s T-test or OneWay ANOVA with post-hoc Tukey test. All other graphs show individual subjects from experimental repetitions.
Human cell cytokine data shown is compiled from 3 individual donors, and each donor pooled cells represent an experiment that was repeated twice. Cytokine measurements were done in duplicate or triplicate. Human cytokine data was analyzed by OneWay ANOVA, repeated measures, and post-hoc Tukey.
Results
Limited protection against lethal MRSA challenge using recombinant mouse LF
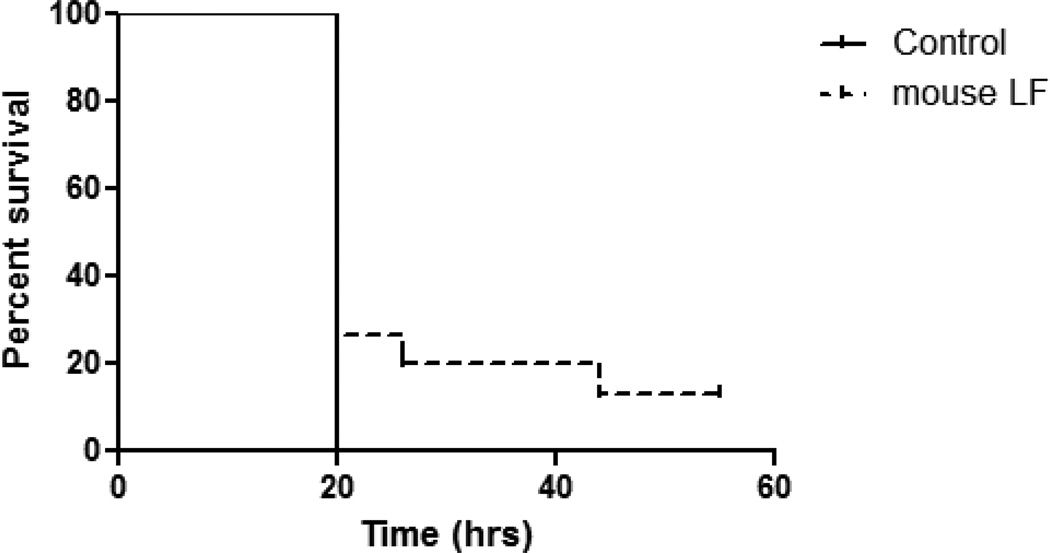
Using a mouse peritonitis model, MRSA at a LD100 level was IP introduced into 6 week old BALB/c mice. Mice were subsequently treated at 2hrs post-infection with one IV injection of rmLF (100µg/mouse). The dose of rm LF was set according to previous studies using bovine milk-derived LF (18). Survival of animals was monitored through 3 days, at which all remaining survivors were sacrificed. The control mice receiving mock treatment (PBS vehicle) succumbed to infection within 20 hours post-delivery of MRSA. In contrast, a single IV treatment with rmLF modestly extended host survival (Fig 1), with the difference in survival between the non-treated and LF treated group being statistically significant (p=0.029).
Figure 1. Modest change in survival post treatment with rmLF after lethal challenge with MRSA.
BALB/c mice were infected IP with MRSA (LD100) were treated at 2 hours post-infection with rmLF (100µg/mouse). Survival was monitored post-infection. Control (non-treated) mice succumbed to MRSA infection within 24 hours. Mice treated with rmLF demonstrated increased survival. Data representative of two experimental repeats with 8 mice per experiment.
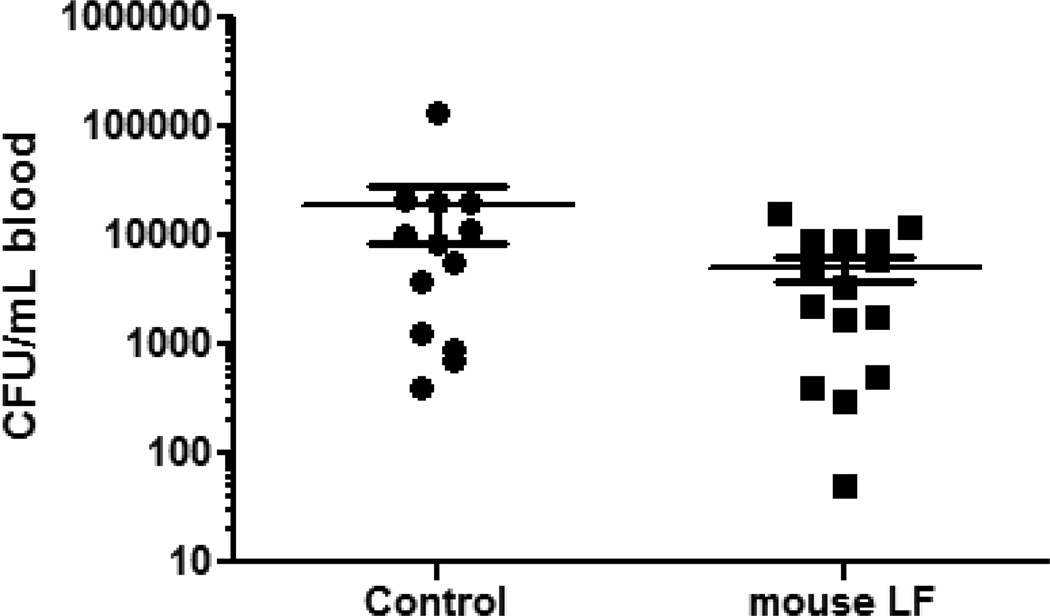
Mice were further assessed for bacterial burden in serum post infectious challenge with MRSA. The rationale is whether protection of the host against MRSA peritonitis induced mortality is associated with a decrease in overall blood bacteria number. Mice were sacrificed at 1hr post-treatment with rmLF or after sham treatment with PBS. Whole blood was collected for bacterial load analysis. The early time point was chosen due to our previous observations with bovine LF, as bacterial burden increases at later time points do not correlate with host death (18). At 1hr post-treatment with rmLF, there was a trend of decrease in overall MRSA numbers (5120 +/− 4950 CFU/mL) in whole blood (Fig 2) compared to the PBS treated control group (18700 +/− 36680 CFU/mL); the reduction in bacterial burden due to the rm LF was not significant.
Figure 2. Bacteremia of mice infected with MRSA.
BALB/c mice were infected with MRSA (LD100) and treated with 100µg/mouse rmLF at 2 hours post-infection. Blood was collected 1 hour later and colony forming units (CFU) were enumerated. Mice treated with rmLF showed a slight, but non-significant, decrease compared to non-treated controls. Data graphed as mean +/− standard deviation, log scale. Data representative of three experimental repeats with 4–5 mice per experiment.
Modulation of host inflammatory signals by mouse LF
Sepsis, whether induced by non-microbial insult (e.g. trauma) or bacterial infection, can progress via unregulated immune responses into severe sepsis or septic shock. LF is known to modulate inflammatory responses during acute infection primarily by limiting production of pro-inflammatory mediators. MRSA triggers immediate innate cellular activation events, followed by production of factors that trigger non-specific lymphocytic activation. To assess the potential of LF to modulate immediate immune function, serum factors were analyzed at 1hr post-treatment with rmLF, or with control PBS, using a multiplex bead assay.
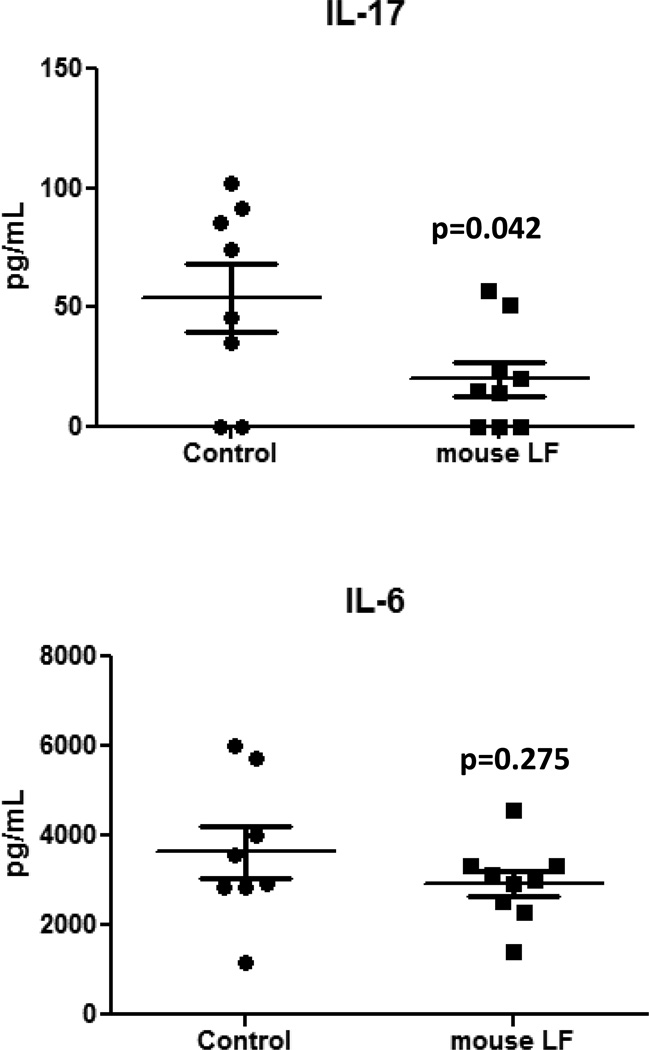
Treatment with the rmLF significantly decreased circulating IL-17 (p<0.05) compared to the treated controls (Fig 3); a non-significant decrease in IL-6 was also observed. However, no significant differences were observed in circulating factors between groups for all other cytokine/chemokine analyzed: IFN-γ, IL-2, IL-5, IP-10, IL-12p40, IL-10, TNF-α, IL-1β, IL-1α, KC, MCP-1, MIP-1α, and MIG.
Figure 3. IL-17 and IL-6 production from MRSA-infected granulocytes after rmLF treatment.
MRSA-infected mice given rmLF as described in Figures 1 and 2 demonstrated significant decrease in IL-17, but not IL-6. Data representative of three experimental repeats with at least 5 mice per experiment; rmLF alone did not induce responses above background levels. N=8 depicted with mean +/− standard deviation.
Mouse LF modulates cytokine production from MRSA-infected cells
Cell populations affected by LF immediately post MRSA challenge were examine in an in vitro infection assays. Total splenocyte populations obtained from naïve BALB/c mice were infected in vitro with MRSA. Two hrs post-infection, these cells were treated with increasing concentrations of rmLF. At 6hrs post-treatment, supernatants were analyzed for cytokine production.
Significant changes in TNF-α and IL-1β were observed in the MRSA-infected splenocyte group which was treated with rmLF (Table I). All concentrations of rmLF significantly (p<0.05) decreased production of IL-1β, compared to MRSA-infected splenocytes without rmLF. Treatment with 100µg/mL of mouse LF also increased TNF-α, compared to the MRSA-infected splenocytes treated with a lower dose (10µg/mL) or compared to those without LF treatment. No production of IL-6 was observed (data not shown). Little to no cytokine production was observed in splenocytes not infected with MRSA, with or without addition of rmLF.
Table I.
Modulation of pro-inflammatory cytokine production from MRSA-infected mouse splenocytes treated with rmLF.
| Mouse LF (µg/mL) | |||
|---|---|---|---|
| 0 | 10 | 100 | |
| TNF-α (pg/mL) | 145 +/− 66 | 147 +/− 64 | 177 +/− 76 |
| IL-1β (pg/mL) | 234 +/− 115 | 206 +/− 97 | 196 +/− 97 |
Total splenocytes from BALB/c mice were infected with MRSA (MOI 1:10) and treated with or without rmLF after 2 hours. Supernatants were collected at 6 hours and analyzed for cytokine production by ELISA. Data represented as mean +/− standard deviation. Gray cells represent significant differences compared to no mouse LF controls (p<0.05).
Blood cells were subdivided into monocytes and granulocytes to examine if a directed cell population was preferentially affected. Supernatants were examined for cytokine production at 6hrs post-treatment with rmLF (100µg/mL). Of interest, the MRSA-infected granulocytes were identified as the population that demonstrated a significant increase in TNF-α production, whereas this was not evident in the monocyte population (p<0.001). Analysis of IL-1β showed no differences in production with mouse LF treatment in either the monocyte or granulocyte populations (Table II). No production of IL-6 was observed (data not shown).
Table II.
TNF-α production from MRSA infected granulocytes after rmouseLF treatment.
| Mouse LF (µg/mL) | |||
|---|---|---|---|
| TNF-α (pg/106 cells) | 0 | 100 | |
| Monocytes | 114 +/− 67 | 145 +/− 46 | |
| Granulocytes | 88 +/− 83 | 247 +/− 107 | |
| IL-1β (pg/106 cells) | |||
| Monocytes | 257 +/− 65 | 247 +/− 69 | |
| Granulocytes | 384 +/− 161 | 421 +/− 60 | |
Monocytes and granulocyte populations isolated from mouse whole blood were infected with MRSA (MOI 1:10) and treated at 2 hours post-infection with or without rmouseLF (100µg/mL). Cytokines were analyzed at 6 hours post-treatment. Addition of rmouseLF enhanced TNF-α production of MRSA infected granulocyte populations. ***=p<0.001
Recombinant human lactoferrin differentially modulates MRSA-infected human monocyte and granulocyte populations
To determine if the immune modulatory effects of rmLF demonstrated in the mouse MRSA sepsis model would be predictive to human infection, MRSA-infected human monocyte and granulocyte populations were further assessed in vitro. Cells were obtained from healthy donor buffy coats, infected with MRSA, and treated with increasing concentrations of rhLF at 2hrs post-infection. Supernatants were collected at 6hrs post-treatment and analyzed for presence of IL-4, IL-17, IL-12p40, IL-10, TNF-α, IL-6, IL-1β, and IL-8.
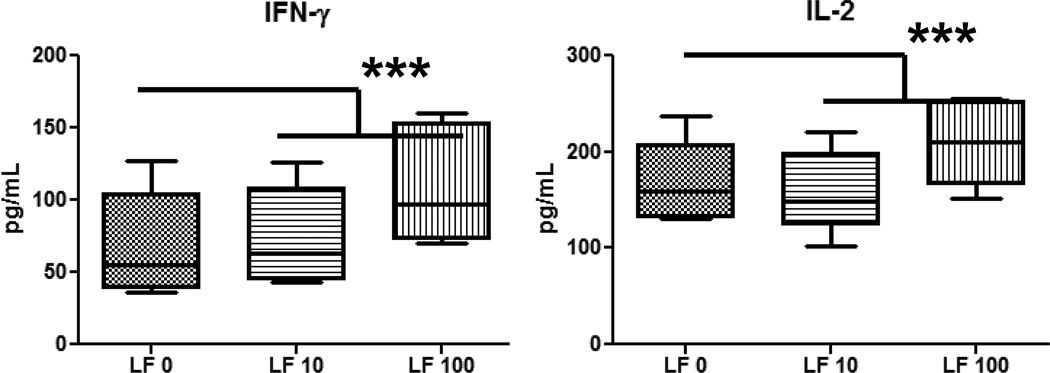
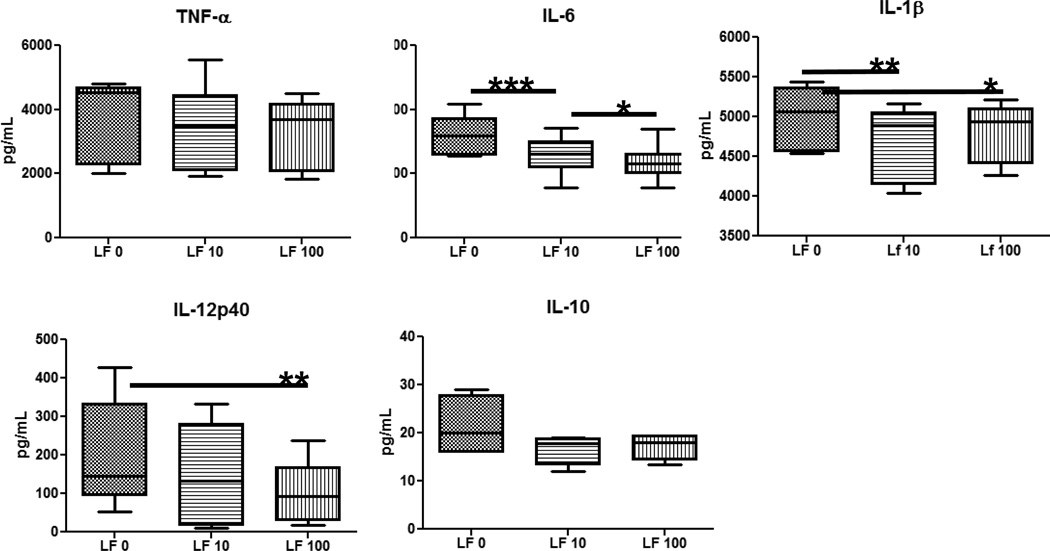
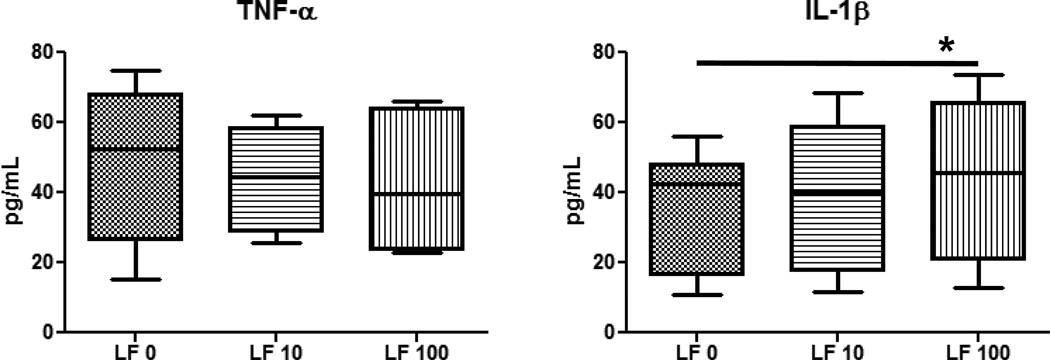
Overall, MRSA stimulated high concentration of cytokines/chemokines from monocytes and granulocytes. MRSA-infected monocytes cultured with rhLF showed a significant increase in T-cell cytokines, IFN-γ and IL-2 (Fig 4) and decreased production of inflammatory factors, including IL-6, IL-1β, and IL-12p40 (Fig 5). No changes were observed with rhLF in production of TNF-α, IL-10, IL-8, IL-17 or IL-4 (data not shown). The granulocytic population from the healthy donors was more limited in response. Granulocytes infected with MRSA produced limited amounts of TNF-α and IL-1β. In contrast to monocytes, MRSA-infected granulocytes treated with rhLF demonstrated an increase in inflammatory factor, IL-1β (Fig 6). No differences were observed with regard to production of TNF-α and IL-8 (data not shown). Granulocytes did not produce any IL-6 or IL-10 during MRSA infection (data not shown).
Figure 4. Change in T-cell derived cytokines in MRSA-infected human PBMCs upon treatment with rhLF.
Human blood monocytes isolated from healthy human buffy coats were infected with MRSA (MOI 1:10) and treated with rhLF at 2 hours post-infected were analyzed for cytokine production at 6 hours post-treatment. Addition of rhLF at 100µg/mL increased MRSA-infected PBMC production of IFN-γ and IL-2. rhLF alone did not induce responses above background levels. Data represented as box and whiskers with the box expressing mean, minimum, and maximum, and whiskers as standard deviation. ***=p<0.001
Figure 5. Reduced pro-inflammatory cytokine production from MRSA-infected human PBMCs treated with recombinant human LF.
Human blood monocytes isolated from healthy human buffy coats were infected with MRSA (MOI 1:10) and treated with rhLF at 2 hours postinfected were analyzed for cytokine production at 6 hours post-treatment. Treatment with rhumanLF decreased levels of IL-6, IL-1β, and IL-12p40. A non-significant decrease was also observed in IL-10. *=p<0.05; **=p<0.01; ***=p<0.001
Figure 6. Production of pro-inflammatory mediators from MRSA-infected granulocytes after treatment with rhLF.
Granulocytes isolated from human buffy coats were infected with MRSA (MOI 1:10) and treated with rhLF at 2 hours post-infection. Supernatants were collected and analyzed by ELISA at 6 hours post-treatment. While there was no change in TNF-α, addition of rhLF led to an increase in IL-1β production from MRSA-infected granulocytes. Data represented as box and whiskers with the box expressing mean, minimum, and maximum, and whiskers as standard deviation. *=p<0.05
Discussion
The experiments outlined here examine the feasibility to employ LF as a novel method to combat MRSA infection, functioning as an immune modulator. S. aureus is a causative agent of life-threatening pneumonia and sepsis (26); the increased worldwide incidence of methicillinresistant S. aureus (MRSA) (27) demonstrates the need to develop more effective therapeutic treatment. Immune modulators as adjunct therapeutics may be a novel way to compliment primary antibiotic activity by altering immune status of the infected host. This is the first report of using CHO-derived recombinant mouse or human LF for this purpose. Overall, while addition of the LF modestly enhanced host survival upon infectious challenge, the response was limited. The response seen was consistent with modulations to inflammation occurring during infection. Preliminary comparisons in vitro with a rhLF indicated that the human counterpart shared many properties with the mouse molecule, although exhibited differences in activities on comparative cellular populations were uncovered.
The concept to use LF for treatment of sepsis is not unique, given the protein’s ability to limit bacterial growth, and to suppress microbial-induced inflammation in a variety of animal models (20, 28–35). Our previous studies using bovine LF in the mouse model of MRSA challenge showed similar (modest) improvements in host survival concurrent with decreased serum cytokines (18). We fully expected to see improved survival when using the homologous recombinant in the mouse, however, this was not proven in our model system. In humans, several investigators have now reported that oral bovine LF is effective as a prophylactic to prevent onset of sepsis in high risk infants (36–39). Bovine LF has been shown to protect gut integrity in mouse models of LPS endotoxemia (32) or gram negative bacteremia (40). However, the conclusion of these studies suggests a mechanism tied to protection of gut integrity to prevent translocation of intestinal-residing bacteria, rather than straight improvement due to decreased bacterial load or modification of cytokine storm post infection.
The novel recombinant mouse and human LFs in this report are made from CHO cells with glycosylation patterns resembling LF secreted by mammalian epithelial cells. Other reported recombinants, such as those produced in Aspergillus oryzae, may not render glycosylation pattern fully compatible with human counterpart molecules (41). Both the rmLF and rhLF demonstrated a decrease in inflammatory cytokine production from monocytes and increased cytokine production from granulocytes during MRSA infection, indicating that autologous LF effects share many activities between species. The in vivo mouse model showed that treatment with rmLF decreased circulating IL-6 with 100µg/mouse dose, which represents a dose considerably lower compared to previously published reports using the bovine LF (20, 32, 42–44). This decrease in serum IL-6 is theoretically important for host survival in the mouse sepsis model, as high levels of IL-6 in the early sepsis phase may be a predictor of mortality (45). In addition to lower serum IL-6, treatment with rmLF also decreased circulating IL-17. The implication of this finding is less clear, as the role of T-cell (CD4+) helper-17 cells which produces the majority of IL-17 has not been well studied for systemic MRSA infection. While IL-17 is protective in MRSA cutaneous infections, systemic MRSA infection (as modeled in this report) is not affected by IL-17 deficiency (46, 47). It’s not suprising to find MRSA stimulated blood monocytes produce T-cell cytokines as peripheral blood monocyte layer by Histopaque separation includes limited numbers of lymphocytes.
These experiments are the first to report on the activities of a homologous rmLF on mouse models of infectious disease, as a foundation to predict effect of recombinant human LF in the clinic. The same rmLF was also investigated by Zimecki, et al., for induction of myelopoiesis in mouse bone marrow cells (23). Despite similar trends caused by LF to decrease inflammation, there are clear differences in in vitro response to MRSA infection between mouse and human primary monocytes and granulocytes. For one, mouse granulocytes demonstrated a different cytokine pattern upon MRSA infection than human granulocytes. Therefore, in humans, the MRSA induced acute inflammation may be primarily attributed to stimulated monocytes. The studies reported here strongly suggest that rhLF can decrease inflammatory responses, albeit modestly, in innate cells due to MRSA infection.
Systemic MRSA infections result in increases of pro-inflammatory mediators TNF-α and IL-6, as well as T-cell derived cytokine IFN-γ (48, 49). Part of this responses has been attributed to virulence factor secretion (50). The ability of LF to control early (innate) inflammation is crucial, as strong systemic inflammation (TNF-α, IL-6, IL-1β, and IL-8) is a threat to host survival (51–53). Interestingly, the effect of rhLF to decrease pro-inflammatory responses from MRSA-infected monocytes is associated with a concurrent increase in T-cell cytokines, specifically IFN-γ and IL-2. Protection and resolution against MRSA is dependent on IFN-γ production, which is linked to enhancement of reactive oxygen species (ie. NO) in neutrophils (54–56). The ability of rhLF to down-regulate pro-inflammatory mediators without decreasing IFN-γ, may serve as an indication that rhLF treatment will suppress inflammation but preserve the beneficial IFN-γ induced NO production to combat MRSA infection.
This report presents a potential for development of lactoferrin as a supportive treatment for MRSA through modulation of host immunity. Lactoferrin limits MRSA induced inflammation without compromising effective immunity. These results also demonstrated that use of autologous LF to examine effects in the mouse model provides similar immune modulatory outcomes to that of human LF in an in vitro model using human derived monocytes and granulocytes. Extension of these findings to rhLF suggests the potential for its use as an effective adjunct therapeutic for MRSA systemic infection in humans. Thus, although some differences between molecules exist, the utilization of the recombinant mouse LF may serve as a pre-clinical proof-of-concept for human LF therapy in patients.
Highlights.
-
►
Novel recombinant human and mouse lactoferrins (LF) were examined as modulatory agents during MRSA infection in mice. Modest protection was elicited by recombinant mouse LF (rmLF).
-
►
Serum cytokines, IL-17 and IL-6, were significantly reduced post MRSA challenge in the rmLF treated mice, with changes also seen in IL-1β, and a slight increase in TNF-α production.
-
►
Human blood derived monocytes and granulocytes infected with MRSA and treated with a homologous recombinant human LF (rhLF) led to increased production of IFN-γ and IL-2. The human cell studies also showed a concurrent decrease in TNF-α, IL-6, IL-1β, IL-12p40, and IL-10.
-
►
Overall, these results indicate the rmLF and rhLF have a high degree of overlap to modify inflammatory responses, although differences in activities were observed.
Acknowledgements
We acknowledge PharmaReview, Corp. (Houston, TX) for the kind gifts of recombinant lactoferrins. These studies were completed in part through NIH support, in accordance with guidelines to produce recombinant human lactoferrin (R42-AI051050-05) and to facilitate investigations of lactoferrin to modulate bacterial sepsis (1R41GM079810-03A).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grundmann H, Aanensen DM, van den Wijngaard CC, Spratt BG, Harmsen D, Friedrich AW. Geographic distribution of Staphylococcus aureus causing invasive infections in Europe: a molecular-epidemiological analysis. PLoS Med. 2010 Jan;7(1):e1000215. doi: 10.1371/journal.pmed.1000215. PubMed PMID: 20084094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005 Dec;5(12):751–762. doi: 10.1016/S1473-3099(05)70295-4. PubMed PMID: 16310147. [DOI] [PubMed] [Google Scholar]
- 3.Klevens RM, Edwards JR, Tenover FC, McDonald LC, Horan T, Gaynes R. Changes in the epidemiology of methicillin-resistant Staphylococcus aureus in intensive care units in US hospitals: 1992–2003. Clin Infect Dis. 2006 Feb 1;42(3):389–391. doi: 10.1086/499367. PubMed PMID: 16392087. [DOI] [PubMed] [Google Scholar]
- 4.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. Jama. 2007 Oct 17;298(15):1763–1771. doi: 10.1001/jama.298.15.1763. PubMed PMID: 17940231. [DOI] [PubMed] [Google Scholar]
- 5.Perencevich EN, Diekema DJ. Decline in invasive MRSA infection: where to go from here? Jama. 2010 Aug 11;304(6):687–689. doi: 10.1001/jama.2010.1125. PubMed PMID: 20699464. [DOI] [PubMed] [Google Scholar]
- 6.Chang S, Sievert DM, Hageman JC, Boulton ML, Tenover FC, Downes FP, et al. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N Engl J Med. 2003 Apr;Mar;348(14):1342–1347. doi: 10.1056/NEJMoa025025. PubMed PMID: 12672861. [DOI] [PubMed] [Google Scholar]
- 7.Moise PA, Sakoulas G, Forrest A, Schentag JJ. Vancomycin in vitro bactericidal activity and its relationship to efficacy in clearance of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2007 Jul;51(7):2582–2586. doi: 10.1128/AAC.00939-06. PubMed PMID: 17452488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC, Jr, Eliopoulos GM. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol. 2004 Jun;42(6):2398–2402. doi: 10.1128/JCM.42.6.2398-2402.2004. PubMed PMID: 15184410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster TJ. Immune evasion by staphylococci. Nat Rev Microbiol. 2005 Dec;3(12):948–958. doi: 10.1038/nrmicro1289. PubMed PMID: 16322743. [DOI] [PubMed] [Google Scholar]
- 10.Holtfreter S, Broker BM. Staphylococcal superantigens: do they play a role in sepsis? Arch Immunol Ther Exp (Warsz) 2005 Jan-Feb;53(1):13–27. PubMed PMID: 15761373. [PubMed] [Google Scholar]
- 11.McCormick JK, Yarwood JM, Schlievert PM. Toxic shock syndrome and bacterial superantigens: an update. Annu Rev Microbiol. 2001;55:77–104. doi: 10.1146/annurev.micro.55.1.77. PubMed PMID: 11544350. [DOI] [PubMed] [Google Scholar]
- 12.Hancock RE, Nijnik A, Philpott DJ. Modulating immunity as a therapy for bacterial infections. Nat Rev Microbiol. 2012 Apr;10(4):243–254. doi: 10.1038/nrmicro2745. PubMed PMID: 22421877. [DOI] [PubMed] [Google Scholar]
- 13.Sheen TR, Cavaco CK, Ebrahimi CM, Thoman ML, Sanderson SD, Morgan EL, et al. Control of methicillin resistant Staphylococcus aureus infection utilizing a novel immunostimulatory peptide. Vaccine. 2011 Dec 9;30(1):9–13. doi: 10.1016/j.vaccine.2011.10.054. PubMed PMID: 22044742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kruzel ML, Actor JK, Boldogh I, Zimecki M. Lactoferrin in health and disease. Postepy Hig Med Dosw (Online) 2007;61:261–267. PubMed PMID: 17507874. [PubMed] [Google Scholar]
- 15.Actor JK, Hwang SA, Olsen M, Zimecki M, Hunter RL, Jr, Kruzel ML. Lactoferrin immunomodulation of DTH response in mice. Int Immunopharmacol. 2002 Mar;2(4):475–486. doi: 10.1016/s1567-5769(01)00189-8. PubMed PMID: 11962727. [DOI] [PubMed] [Google Scholar]
- 16.Hwang SA, Wilk KM, Bangale YA, Kruzel ML, Actor JK. Lactoferrin modulation of IL-12 and IL-10 response from activated murine leukocytes. Med Microbiol Immunol. 2007 Sep;196(3):171–180. doi: 10.1007/s00430-007-0041-6. PubMed PMID: 17377816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Damiens E, Mazurier J, el Yazidi I, Masson M, Duthille I, Spik G, et al. Effects of human lactoferrin on NK cell cytotoxicity against haematopoietic and epithelial tumour cells. Biochim Biophys Acta. 1998 Apr 24;1402(3):277–27. doi: 10.1016/s0167-4889(98)00013-5. PubMed PMID: 9606986. [DOI] [PubMed] [Google Scholar]
- 18.Hwang SA, Actor JK. Lactoferrin modulation of BCG-infected dendritic cell functions. Int Immunol. 2009 Oct;21(10):1185–1197. doi: 10.1093/intimm/dxp084. PubMed PMID: 19692539. Pubmed Central PMCID: 2750247. Epub 200908/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang SA, Kruzel ML, Actor JK. Influence of bovine lactoferrin on expression of presentation molecules on BCG-infected bone marrow derived macrophages. Biochimie. 2009 Jan;91(1):76–85. doi: 10.1016/j.biochi.2008.04.008. PubMed PMID: 18486627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kruzel ML, Harari Y, Mailman D, Actor JK, Zimecki M. Differential effects of prophylactic, concurrent and therapeutic lactoferrin treatment on LPS-induced inflammatory responses in mice. Clin Exp Immunol. 2002 Oct;130(1):25–31. doi: 10.1046/j.1365-2249.2002.01956.x. PubMed PMID: 12296849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SJ, Yu DY, Pak KW, Jeong S, Kim SW, Lee KK. Structure of the human lactoferrin gene and its chromosomal localization. Mol Cells. 1998 Dec 31;8(6):663–668. PubMed PMID: 9895117. [PubMed] [Google Scholar]
- 22.Kruzel ML, Actor JK, Zimecki M, Wise J, Płoszaj P, Mirza S, et al. Novel recombinant human lactoferrin: Differential activation of oxidative stress related gene expression. Journal of Biotechnology. 2013;168:666–675. doi: 10.1016/j.jbiotec.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zimecki M, Artym J, Kocieba M, Kaleta-Kuratewicz K, Kuropka P, Kuryszko J, et al. Homologous Lactoferrin Triggers Mobilization of the Myelocytic Lineage of Bone Marrow in Experimental Mice. Stem Cells Dev. Aug 24; doi: 10.1089/scd.2013.0242. PubMed PMID: 23879888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.English D, Andersen BR. Single-step separation of red blood cells. Granulocytes and mononuclear leukocytes on discontinuous density gradients of Ficoll-Hypaque. J Immunol Methods. 1974 Aug;5(3):249–252. doi: 10.1016/0022-1759(74)90109-4. PubMed PMID: 4427075. [DOI] [PubMed] [Google Scholar]
- 25.Feldman DL, Mogelesky TC. Use of Histopaque for isolating mononuclear cells from rabbit blood. J Immunol Methods. 1987 Sep 24;102(2):243–249. doi: 10.1016/0022-1759(87)90083-4. PubMed PMID: 3655375. [DOI] [PubMed] [Google Scholar]
- 26.Liu GY. Molecular pathogenesis of Staphylococcus aureus infection. Pediatr Res. 2009 May;65(5 Pt 2):71R–77R. doi: 10.1203/PDR.0b013e31819dc44d. PubMed PMID: 19190527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seybold U, Kourbatova EV, Johnson JG, Halvosa SJ, Wang YF, King MD, et al. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin Infect Dis. 2006 Mar 1;42(5):647–656. doi: 10.1086/499815. PubMed PMID: 16447110. [DOI] [PubMed] [Google Scholar]
- 28.Welsh KJ, Hwang SA, Boyd S, Kruzel ML, Hunter RL, Actor JK. Influence of oral lactoferrin on Mycobacterium tuberculosis induced immunopathology. Tuberculosis (Edinb) 2011 Dec;91(Suppl 1):105–113. doi: 10.1016/j.tube.2011.10.019. PubMed PMID: 22138562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kruzel ML, Bacsi A, Choudhury B, Sur S, Boldogh I. Lactoferrin decreases pollen antigen-induced allergic airway inflammation in a murine model of asthma. Immunology. 2006 Oct;119(2):159–166. doi: 10.1111/j.1365-2567.2006.02417.x. PubMed PMID: 16800860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doursout MF, Horton H, Hoang L, Liang Y, Hwang SA, Boyd S, et al. Lactoferrin moderates LPS-induced hypotensive response and gut injury in rats. Int Immunopharmacol. 2013 Feb;15(2):227–231. doi: 10.1016/j.intimp.2012.12.009. PubMed PMID: 23267765. [DOI] [PubMed] [Google Scholar]
- 31.Fischer R, Debbabi H, Dubarry M, Boyaka P, Tome D. Regulation of physiological and pathological Th1 and Th2 responses by lactoferrin. Biochem Cell Biol. 2006 Jun;84(3):303–311. doi: 10.1139/o06-058. PubMed PMID: 16936801. [DOI] [PubMed] [Google Scholar]
- 32.Kruzel ML, Harari Y, Chen CY, Castro GA. Lactoferrin protects gut mucosal integrity during endotoxemia induced by lipopolysaccharide in mice. Inflammation. 2000 Feb;24(1):33–44. doi: 10.1023/a:1006935908960. PubMed PMID: 10704062. [DOI] [PubMed] [Google Scholar]
- 33.Welsh KJ, Hwang SA, Hunter RL, Kruzel ML, Actor JK. Lactoferrin modulation of mycobacterial cord factor trehalose 6-6'-dimycolate induced granulomatous response. Transl Res. 2010 Oct;156(4):207–215. doi: 10.1016/j.trsl.2010.06.001. PubMed PMID: 20875896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leon-Sicairos N, Martinez-Pardo L, Sanchez-Hernandez B, de la Garza M, Carrero JC. Oral lactoferrin treatment resolves amoebic intracecal infection in C3H/HeJ mice. Biochem Cell Biol. 2012 Jun;90(3):435–441. doi: 10.1139/o2012-008. PubMed PMID: 22452668. [DOI] [PubMed] [Google Scholar]
- 35.Legrand D. Lactoferrin, a key molecule in immune and inflammatory processes. Biochem Cell Biol. 2012 Jun;90(3):252–268. doi: 10.1139/o11-056. PubMed PMID: 22136726. [DOI] [PubMed] [Google Scholar]
- 36.ELFIN Trial Investigators Group T. Lactoferrin immunoprophylaxis for very preterm infants. Arch Dis Child Fetal Neonatal Ed. 2013 Jan;98(1):F2–F4. doi: 10.1136/archdischild-2011-301273. PubMed PMID: 22894857. [DOI] [PubMed] [Google Scholar]
- 37.Manzoni P, De Luca D, Stronati M, Jacqz-Aigrain E, Ruffinazzi G, Luparia M, et al. Prevention of nosocomial infections in neonatal intensive care units. Am J Perinatol. 2013 Feb;30(2):81–88. doi: 10.1055/s-0032-1333131. PubMed PMID: 23292914. [DOI] [PubMed] [Google Scholar]
- 38.Ochoa TJ, Pezo A, Cruz K, Chea-Woo E, Cleary TG. Clinical studies of lactoferrin in children. Biochem Cell Biol. 2012 Jun;90(3):457–467. doi: 10.1139/o11-087. PubMed PMID: 22380791. [DOI] [PubMed] [Google Scholar]
- 39.Stronati M, Bollani L, Maragliano R, Ruffinazzi G, Manzoni P, Borghesi A. Neonatal sepsis: new preventive strategies. Minerva Pediatr. 2013 Febbraio;65(1):103–110. PubMed PMID: 23422580. [PubMed] [Google Scholar]
- 40.Zimecki M, Artym J, Chodaczek G, Kocieba M, Kruzel ML. Protective effects of lactoferrin in Escherichia coli-induced bacteremia in mice: relationship to reduced serum TNF alpha level and increased turnover of neutrophils. Inflamm Res. 2004 Jul;53(7):292–296. doi: 10.1007/s00011-004-1257-1. PubMed PMID: 15241563. [DOI] [PubMed] [Google Scholar]
- 41.Ward PP, Piddington CS, Cunningham GA, Zhou X, Wyatt RD, Conneely OM. A system for production of commercial quantities of human lactoferrin: a broad spectrum natural antibiotic. Biotechnology (N Y) 1995 May;13(5):498–503. doi: 10.1038/nbt0595-498. PubMed PMID: 9634791. [DOI] [PubMed] [Google Scholar]
- 42.Artym J, Zimecki M, Kruzel ML. Enhanced clearance of Escherichia coli and Staphylococcus aureus in mice treated with cyclophosphamide and lactoferrin. Int Immunopharmacol. 2004 Sep;4(9):1149–1157. doi: 10.1016/j.intimp.2004.05.002. PubMed PMID: 15251111. [DOI] [PubMed] [Google Scholar]
- 43.Zimecki M, Dawiskiba J, Zawirska B, Krawczyk Z, Kruzel M. Bovine lactoferrin decreases histopathological changes in the liver and regulates cytokine production by splenocytes of obstructive jaundiced rats. Inflamm Res. 2003 Jun;52(7):305–310. doi: 10.1007/s00011-003-1178-4. PubMed PMID: 12861396. [DOI] [PubMed] [Google Scholar]
- 44.Zimecki M, Wlaszczyk A, Wojciechowski R, Dawiskiba J, Kruzel M. Lactoferrin regulates the immune responses in post-surgical patients. Arch Immunol Ther Exp (Warsz) 2001;49(4):325–333. PubMed PMID: 11726036. [PubMed] [Google Scholar]
- 45.Xiao H, Siddiqui J, Remick DG. Mechanisms of mortality in early and late sepsis. Infect Immun. 2006 Sep;74(9):5227–5235. doi: 10.1128/IAI.01220-05. PubMed PMID: 16926416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krishna S, Miller LS. Innate and adaptive immune responses against Staphylococcus aureus skin infections. Semin Immunopathol. 2012 Mar;34(2):261–280. doi: 10.1007/s00281-011-0292-6. PubMed PMID: 22057887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, et al. Differential roles of interleukin-17A and-17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009 Jan 16;30(1):108–119. doi: 10.1016/j.immuni.2008.11.009. PubMed PMID: 19144317. [DOI] [PubMed] [Google Scholar]
- 48.Nakane A, Okamoto M, Asano M, Kohanawa M, Minagawa T. Endogenous gamma interferon, tumor necrosis factor, and interleukin-6 in Staphylococcus aureus infection in mice. Infect Immun. 1995 Apr;63(4):1165–1172. doi: 10.1128/iai.63.4.1165-1172.1995. PubMed PMID: 7890367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sasaki S, Miura T, Nishikawa S, Yamada K, Hirasue M, Nakane A. Protective role of nitric oxide in Staphylococcus aureus infection in mice. Infect Immun. 1998 Mar;66(3):1017–1022. doi: 10.1128/iai.66.3.1017-1022.1998. PubMed PMID: 9488390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watkins RL, Pallister KB, Voyich JM. The SaeR/S gene regulatory system induces a proinflammatory cytokine response during Staphylococcus aureus infection. PLoS One. 2011;6(5):e19939. doi: 10.1371/journal.pone.0019939. PubMed PMID: 21603642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boontham P, Chandran P, Rowlands B, Eremin O. Surgical sepsis: dysregulation of immune function and therapeutic implications. Surgeon. 2003 Aug;1(4):187–206. doi: 10.1016/s1479-666x(03)80018-5. PubMed PMID: 15570763. [DOI] [PubMed] [Google Scholar]
- 52.Darville T, Giroir B, Jacobs R. The systemic inflammatory response syndrome (SIRS): immunology and potential immunotherapy. Infection. 1993 Sep-Oct;21(5):279–290. doi: 10.1007/BF01712446. PubMed PMID: 8300243. [DOI] [PubMed] [Google Scholar]
- 53.Fang M, Dai H, Yu G, Gong F. Gene delivery of SOCS3 protects mice from lethal endotoxic shock. Cell Mol Immunol. 2005 Oct;2(5):373–377. PubMed PMID: 16368064. [PubMed] [Google Scholar]
- 54.Ahlin A, Larfars G, Elinder G, Palmblad J, Gyllenhammar H. Gamma interferon treatment of patients with chronic granulomatous disease is associated with augmented production of nitric oxide by polymorphonuclear neutrophils. Clin Diagn Lab Immunol. 1999 May;6(3):420–424. doi: 10.1128/cdli.6.3.420-424.1999. PubMed PMID: 10225847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ezekowitz RA, Orkin SH, Newburger PE. Recombinant interferon gamma augments phagocyte superoxide production and X-chronic granulomatous disease gene expression in Xlinked variant chronic granulomatous disease. J Clin Invest. 1987 Oct;80(4):1009–1016. doi: 10.1172/JCI113153. PubMed PMID: 2821069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao YX, Tarkowski A. Impact of interferon-gamma receptor deficiency on experimental Staphylococcus aureus septicemia and arthritis. J Immunol. 1995 Dec 15;155(12):5736–5742. [PubMed] [Google Scholar]