Abstract
The modified Blalock–Taussig shunt is a synthetic shunt between the subclavian and pulmonary artery, used in the treatment of congenital cyanotic heart diseases with pulmonary hypoperfusion. Delayed complications include progressive failure of the shunt, serous fluid leak, and pseudoaneurysm formation. We report two different and rare mediastinal vascular complications following modified BT shunt surgery in this case report. The first one is a seroma, due to serous fluid leakage through the shunt graft, which is a relatively benign complication. The second one is a pseudoaneurysm, arising from the shunt, a frequently fatal complication. Generally, X-ray chest is used for screening in these patients. CT angiography plays a vital role in the diagnosis of both these conditions. Management in pseudoaneurysm should be aggressive, as timely intervention may be life saving, while in seroma the management is most often conservative occasionally requiring surgical intervention.
Keywords: Post BT shunt complications, Seroma, Pseudoaneurysm
1. Case 1
A 2-year-old male child, weighing 10 kg, presented with history of cyanotic spells since infancy, central cyanosis and failure to thrive. Echocardiography and cardiac catheterization study revealed Tetralogy of Fallot (TOF). The sizes of right pulmonary artery (PA), left PA and descending thoracic aorta (DTA) were 6.5 mm, 7 mm and 11 mm respectively. McGoon ratio was 1.23 Due to recurrent cyanotic spells he underwent emergency modified BT shunt (left side) using Gore-Tex graft (shunt size 4.5 mm). There were no periprocedural complications and the patient was discharged. Three years after the surgery, during routine evaluation for intracardiac repair, he was found to have an added opacity in the left upper mediastinum on chest X-ray (Fig. 1). CT angiography revealed a small hypodense collection of fluid attenuation surrounding the Gore-Tex graft (Fig. 2). No calcification, enhancement, septae, air foci or solid component was seen. Features were consistent with that of a postoperative seroma and the patient was conservatively managed for the same.
Fig. 1.
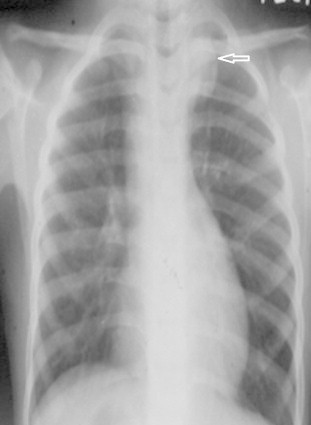
Frontal chest radiograph shows a well defined opacity in the upper mediastinum on the left side with well circumscribed lateral margins.
Fig. 2.
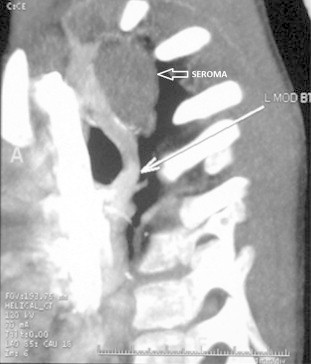
Axial contrast chest CT obtained in a helical mode. The sagittal oblique maximal intensity projection image shows a small hypodense lesion of fluid attenuation seen adjacent to the BT shunt. No calcification, enhancement, septae, air foci or solid component was seen. Features are consistent with a post operative seroma. Thin long arrow points BT shunt. Broader, short arrow points seroma
2. Case 2
A 2-year-old female child, weighing 12 kg, presented with history of central cyanosis since infancy and growth failure. Echocardiography and cardiac catheterization revealed double outlet right ventricle (DORV) with sub-aortic ventricular septal defect (VSD) and pulmonic stenosis (PS). The sizes of right PA, left PA and DTA were 7.5 mm, 7 mm and 13 mm respectively. Mc Goon ratio was 1.11. Patient underwent left sided modified BT shunt (size: 5 mm) and was discharged following an uneventful post operative course. Follow up echocardiography demonstrated a good left sided shunt function. Cyanosis had reduced and her growth had also improved. Five months after surgery the patient presented with history of hemoptysis. Chest X-ray revealed mediastinal opacification the left side which was confirmed by CT angiography to be a pseudoaneurysm arising from the subclavian end of the shunt (Figs. 3–5). Patient was scheduled for emergency aneurysmal repair, but she developed sudden massive hemoptysis which proved to be fatal.
Fig. 3.
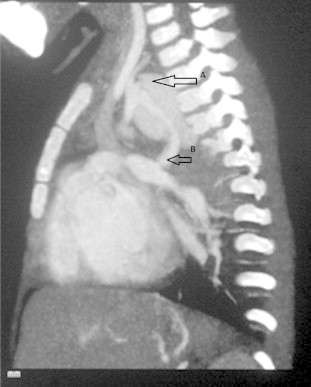
Axial chest CT. The sagittal oblique maximal intensity projection image shows a focal contrast outpouching in relation to the BT shunt. White arrow points PA.
Fig. 4.
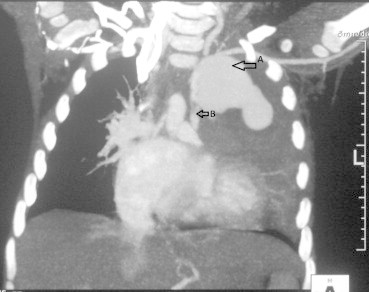
Axial chest CT – the coronal oblique maximal intensity projection image shows the pseudoaneurysm and its relation with the left subclavian artery. The periphery of this pseudoaneurysm shows thrombosis. White arrow points PA.
Fig. 5.
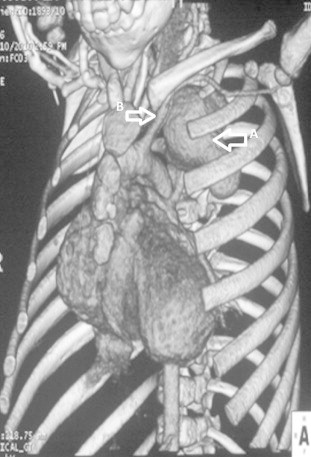
CT volume rendered image showing the size and extent of the pseudoaneurysm and its relation to the left subclavian artery. White arrow points PA.
3. Discussion
An expanded polytetrafluorethylene (PTFE) graft is used in modified Blalock–Taussig shunt which can be associated with various complications of differing severity. Early complications of this procedure include shunt occlusion, nerve damage at the time of operation, and hyperdynamic pulmonary blood flow which may require diuretics. Delayed complications include shunt failure due to growth of children, serous fluid leak, and pseudoaneurysm formation.1 One of the rare complications described after BT shunt was aneurysmal dilation of pulmonary artery which casts a mediastinal opacity in chest X-ray.2
The initial clue to these complications comes from chest X-ray. The differential diagnosis of mediastinal mass, in post BT shunt patients, include seroma, pseudoaneurysm, aneurysmal dilation of pulmonary artery, neuroenteric cyst, bronchogenic cyst, esophageal duplication cyst, epidermoid cyst, thymic and dermoid cysts. Partial absence of left pericardium can mimic a vascular mediastinal mass.2
Seroma following modified BT shunt is a rare complication with an incidence ranging from 4% to 20%.3,4 The age of presentation is from 9 days to 7 years and is slightly more common in females.5 The time taken for seroma formation is variable, can occur in the first post operative day to years later6 but commonly within 30 days of surgery.7,8 It is caused by plasma leakage through the graft, resulting in the formation of sterile, non-secretory fibrous pseudomembrane surrounding the graft.9 Outer layering of the Gore-Tex with silicone sheeting to facilitate subsequent takedown of the shunt can result in seroma formation but such techniques are rarely performed now-a-days.4 Heparin use may also predispose to development of seroma.
The most common presentation of seroma is with respiratory distress between 2 and 12 weeks of shunt surgery. Diagnostic modalities include Chest X-ray, thoracic ultrasonography, CT and MR imaging and catheter angiography is rarely required.10 USG can diagnose 73% of cases with reasonable accuracy and because of its portability and feasibility of bedside use, it is the initial imaging modality in suspected cases of perigraft seroma development.7 The general management is conservative.5 Topical application of fibrin glue, histoacryl tissue glue, collagen hemostat, approtinin, thrombin can be tried. Simple resection and aspiration of the cyst can be tried. Pleural wrapping of the PTFE graft can be done with parietal pleura.3 But these techniques may be associated with high recurrence rates, necessitating surgical replacement of the graft. Surgical management is required in cases of mediastinal or vascular compression.
The second case was an illustration of pseudoaneurysm following BT shunt. The mechanism of pseudoaneurysm post BT shunt could be infection leading to dehiscence of sutures or intra-operative damage to anastomotic site.11 A patent graft is not a prerequisite for the formation of anastomotic false aneurysm.12
The clinical manifestations of the pseudoaneurysm can range from being an incidental finding to causing massive hemoptysis,13,14 or mediastinal compression15 that might be fatal. Other causes of hemoptysis in post BT shunt patients include pulmonary hypertension, rupture of large collaterals, or secondary vascular changes. Some of the earlier case reports11 mention patients who were misdiagnosed as pneumonia or tuberculosis based on symptoms mimicking pulmonary disease and positive cultures. Timely diagnosis is crucial in the management of such patients and early surgery could be life saving.
4. Conclusion
Modified BT shunt is still being performed in our centre in patients with congenital cyanotic heart diseases with Tetralogy of Fallot with small pulmonary arteries and recurrent cyanotic spells, though intracardiac repair is the preferred procedure. Seroma is relatively benign and most often requires a conservative strategy, whereas pseudoaneurysm is often ominous and requires early intervention.
Conflicts of interest
All authors have none to declare.
References
- 1.Yuan S.M., Shinfeld A., Raanani E. The Blalock–Taussig shunt. J Cardiac Surg. 2009;24:101–108. doi: 10.1111/j.1540-8191.2008.00758.x. [DOI] [PubMed] [Google Scholar]
- 2.Reddy S., Kumar R. An uncommon cause of a vascular mass in the left lung in neonate: a case report with a brief review of literature. Pediatr Pulmonol. 2008;43:822–823. doi: 10.1002/ppul.20756. [DOI] [PubMed] [Google Scholar]
- 3.Ugurlu B.S., Sariosmanoglu O.N., Metin S.K., Hazan E., Oto O. Pleural flap for treating perigraft leak after a modified Blalock–Taussig shunt. Ann Thorac Surg. 2002;73:1638–1640. doi: 10.1016/s0003-4975(01)03365-3. [DOI] [PubMed] [Google Scholar]
- 4.LeBlanc J., Albus R., Williams W. Serous fluid leakage: a complication following the modified Blalock–Taussig shunt. J Thorac Cardiovasc Surg. 1984;88:259. [PubMed] [Google Scholar]
- 5.Sahoo M., Sahu M., Kale S., Saxena N. Serous fluid leakage following modified Blalock–Taussig operation using PTFE grafts. Indian Heart J. 2001;53:328–331. [PubMed] [Google Scholar]
- 6.Matsuyama K., Matsumoto M., Sugita T., Matsuo T. Slowly developing perigraft seroma after a modified Blalock–Taussig shunt. Pediatr Cardiol. 2003;24:412–414. doi: 10.1007/s00246-002-0373-3. [DOI] [PubMed] [Google Scholar]
- 7.van Rijn R.R., Berger R.M.F., Lequin M.H., Robben S.G.F. Development of a perigraft seroma around modified Blalock–Taussig shunts: imaging evaluation. Am J Roentgenol. 2002;178:629. doi: 10.2214/ajr.178.3.1780629. [DOI] [PubMed] [Google Scholar]
- 8.Rudd S.A., McAdams H.P., Cohen A.J., Midgley F.M. Mediastinal perigraft seroma: CT and MR imaging. J Thorac imaging. 1994;9:120. doi: 10.1097/00005382-199421000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Özkutlu S., Özbarlas N., Demircin M. Perigraft seroma diagnosed by echocardiography: a complication following Blalock–Taussig shunt. Int J Cardiol. 1992;36:244–246. doi: 10.1016/0167-5273(92)90018-x. [DOI] [PubMed] [Google Scholar]
- 10.Dogan O., Duman U., Ozkutlu S., Ersoy U. Diagnosis of perigraft seroma by use of different techniques in infants with respiratory distress after modified Blalock–Taussig shunt. Pediatr Cardiol. 2006;27:655–657. doi: 10.1007/s00246-005-8021-3. [DOI] [PubMed] [Google Scholar]
- 11.Coren M., Green C., Yates R., Bush A. Complications of modified Blalock–Taussig shunts mimicking pulmonary disease. Arch Dis Child. 1998;79:361. doi: 10.1136/adc.79.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parvathy U., Balakrishnan K., Ranjith M., Moorthy J. False aneurysm following modified Blalock–Taussig shunt. Pediatr Cardiol. 2002;23:178–181. doi: 10.1007/s00246-001-0043-x. [DOI] [PubMed] [Google Scholar]
- 13.Caffarena J., Llamas P., Otero-Coto E. False aneurysm of a palliative shunt producing massive hemoptysis. Chest. 1982;81:110. doi: 10.1378/chest.81.1.110. [DOI] [PubMed] [Google Scholar]
- 14.Sethia B., Pollock J.C. False aneurysm formation: a complication following the modified Blalock–Taussig shunt. Ann Thorac Surg. 1986;41:667–668. doi: 10.1016/s0003-4975(10)63085-8. [DOI] [PubMed] [Google Scholar]
- 15.Valliattu J., Jairaj P., Delamie T., Subramanyam R., Menon S., Vyas H. False aneurysm following modified Blalock–Taussig shunt. Thorax. 1994;49:383. doi: 10.1136/thx.49.4.383. [DOI] [PMC free article] [PubMed] [Google Scholar]


