Abstract
Objective
To study the clinical profile, diagnostic methods and management in patients with symptomatic pulmonary embolism (PE).
Methods
Retrospective assessment of clinical features and management of patients presenting with symptomatic pulmonary embolism from January 2005 to March 2012.
Results
35 patients who were newly diagnosed to have pulmonary embolism with a mean age of 52.1 years were included in the study. Dyspnea (91.4%) and syncope (22.8%) were the predominant symptoms. Echocardiography was done in all patients. 30 patients (85.7%) had pulmonary arterial hypertension, 31 patients (88.5%) had evidence of RV dysfunction and 4 patients (16.7%) had evidence of thrombus in PA, RV. Out of 35 patients, 34 patients (97.14%) showed positive d-dimer reports. Among 35 patients, 24 (68.5%) had positive troponin values. V/Q scan was done in 14 patients (40%) and CT pulmonary angiogram (CTPA) was done in 24 patients (68.5%.). Thrombolysis was done is 24 patients (68.5%). All patients received low molecular weight heparin followed by warfarin. Of the 35 patients, 34 (97.1%) were discharged and were under regular follow up for 6 months and one patient died during the hospital stay.
Conclusion
Pulmonary embolism is a common problem and can be easily diagnosed provided it is clinically suspected. Early diagnosis and aggressive management is the key to successful outcome.
Keywords: Pulmonary embolism, Spiral CTPA, Anticoagulation, Thrombolysis, Venous thromboembolism
1. Introduction
Pulmonary embolism is a common and potentially lethal condition. Most patients who succumb to pulmonary embolism do so within the first few hours of the event. Ten percent of PE is fatal in the first hour.1 Mortality rate of diagnosed and treated pulmonary embolism ranges from 3 to 8%, but increases to about 30% in untreated pulmonary embolism.1 Most of the deaths occur when the diagnosis is delayed or never made. Despite diagnostic advances, delays in pulmonary embolism diagnosis are common and represent an important issue.2
A pulmonary embolism is also characterized as central or peripheral, depending on the location or the arterial branch involved. Central vascular zones include the main pulmonary artery, the left and right main pulmonary arteries, the anterior trunk, the right and left interlobar arteries, and lobar arteries. A pulmonary embolus is characterized as massive when it involves main pulmonary arteries or when it results in hemodynamic compromise. Peripheral vascular zones include the segmental and subsegmental arteries.
The most important conceptual advance regarding pulmonary embolism over the last several decades has been the realization that pulmonary embolism is not a disease; rather, pulmonary embolism is a complication of venous thromboembolism. Pulmonary embolism is present in 60–80% of patients with DVT and more than half these patients are asymptomatic.3
Clinical signs and symptoms for pulmonary embolism are nonspecific; therefore, patients suspected of having pulmonary embolism because of unexplained dyspnea, tachypnea, or chest pain or the presence of risk factors for pulmonary embolism must undergo diagnostic tests until the diagnosis is ascertained or eliminated or an alternative diagnosis is confirmed. Further, routine laboratory findings are nonspecific and are not helpful in pulmonary embolism, although they may suggest another diagnosis. However with the advent of spiral CT pulmonary angiogram (CTPA), there is now an increased recognition of this entity in India. In spite of rapid advances in the diagnosis and management of PE, it is still unreported from India. Most of the reports are limited to autopsy reports and short case series.4 In this study, we describe our experience with the diagnosis and management of patients with symptomatic PE.
2. Materials and methods
A retrospective study of patients admitted with acute pulmonary thromboembolism at our institute from January 2005 to March 2012 were selected. All patients diagnosed as acute pulmonary embolism who had no pre-existing cardiac or pulmonary disease with no previous history of pulmonary embolism were included in the study. Their clinical presentation, investigation and management were analyzed. A patient was diagnosed to have pulmonary embolism if there is evidence of thrombus in CT pulmonary angiogram or a high probability ventilation perfusion scan.
Patients with pulmonary embolism were classified as massive if there was evidence of hemodynamic compromise (defined as systolic BP <90 mmHg) and as submassive if there was right ventricular dysfunction on echocardiography with no hemodynamic compromise. Patients without any evidence of these features were labeled as minor pulmonary embolism cases.
d-dimer testing was done using enzyme linked fluorescent assay. The normal value is 0–500 ng/ml. Any value greater than 500 ng/ml is considered positive. Troponin I was done using electrochemiluminescence method and a value greater than 0.03 was considered abnormal. Echocardiogram was done using Philips IE5 machine. Pulmonary arterial pressure was calculated by TR jet velocity using Bernoulli's method (p = 4v2) and RV function was assessed by eyeball method and also by Tricuspid Annular Plane Systolic Excursion (TAPSE). TAPSE less than 16 mm indicates RV systolic dysfunction. Pulmonary hypertension was categorized as mild, moderate, or severe based on pulmonary artery systolic pressures (mild: 40–45 mmHg, moderate: 46–60 mmHg, or severe >60 mmHg). Ventilation perfusion scan was done using intravenous injection of radioactive technetium macro aggregated albumin (Tc 99m MAA). CT pulmonary angiogram was done using 64 slice CT scanner (SOMATOM sensation 64). Apart from routine blood counts, hematological profile, all patients had a protein C, S values, antithrombin III, antiphospholipid antibody and homocysteine levels. Patients who were eligible for thrombolytic therapy were lysed using streptokinase bolus of 2.5 lakh units followed by an infusion of 1 lakh units per hour for a period of 24–48 h. Rest of the patients were anticoagulated with low molecular weight heparin. The patients were clinically followed up for a period of 6 months.
3. Results
35 patients who were newly diagnosed to have acute pulmonary embolism were included in the study. Their mean age was 52.1 years. Among 35 patients, 22 (62.8%) were males and 13 (37.2%) were females (Table 2).
Table 2.
Clinical features and diagnostic methods In patients with PE.
| Clinical features and diagnostic methods | No N-35 |
|
|---|---|---|
| Dyspnea | 32 (91.4%) | |
| Syncope | 8 (22.8%) | |
| Chest pain | 6 (17.1%) | |
| Fever | 4 (11.4%) | |
| Cough | 4 (11.4%) | |
| ECG abnormalities | ||
|
32 (91.4%) | |
|
23 (65.7%) | |
|
19 (34.2%) | |
|
7 (20%) | |
| Chest X-ray abnormalities | ||
|
30 (85.7%) | |
|
2 (5.7%) | |
|
2 (5.7%) | |
|
1 (2.8%) | |
| Echocardiography | ||
|
30 (85.7%) | |
|
31 (88.5%) | |
|
4 (16.7%) | |
| V/Q scan | 14 (40%) | |
|
11 (78.5%) | |
|
3 (21.5%) | |
| CT pulmonary angiogram | 24 (68.5%) | |
|
20 (83.3%) | |
|
4 (16.7%) | |
| Venous Doppler lower limbs | ||
| Evidence of DVT | 28 (80%) | |
| d-Dimer | Positive | 34 (97.1%) |
| Toponin I | Positive | 24 (68.5%) |
Out of 35 patients, 8 patients had a history of immobilization for a minimum of 2 weeks, 2 patients had carcinoma, one patient had confirmed HIV disease and one patient had protein C, protein S deficiency (Table 1).
Table 1.
Predisposing factors in patients with pulmonary embolism.
| Predisposing factors | No of patients n-35 |
|---|---|
| Immobilization history | 8 (22.8%) |
| Malignancy | 2 (5.7%) |
| Protein C, protein S deficiency | 1 (2.8%) |
| HIV disease | 1 (2.8%) |
The most common clinical presentation is dyspnea (91.4%) followed by syncope (22.8%). The other symptoms being chest pain (17.1%), fever (11.4%) and cough (11.4%) (Table 2). The mean duration of symptom onset to hospitalization was 6.2 days.
The most common finding in ECG is sinus tachycardia (91.4%) followed by RV strain pattern (65.7%), S1Q3T3 pattern (34.2%) and RBBB (20%) (Table 2).
Thirty patients had a normal chest radiograph. Of the remaining five patients, two had wedge shaped opacity suggestive of pulmonary infarct, 2 had lung collapse and one had pleural effusion.
Out of 35 patients, 34 patients (97.14%) showed positive d-dimer reports. Among 35 patients, 24 (68.5%) had positive troponin values. Venous Doppler of lower limbs was done in all patients. 28 patients (80%) had evidence of DVT in lower limbs (Table 2).
Echocardiography was done in all patients. 31 patients (88.5%) had evidence of RV dysfunction and 30 patients (85.7%) had pulmonary arterial hypertension, 22 patients had severe PAH and 8 patients had moderate PAH at presentation. Thrombus was visualized in pulmonary arteries in 3 patients and right ventricle in one patient (Table 2).
Ventilation Perfusion (V/Q) scan was done in 14 patients (40%) and CT pulmonary angiogram was done in 24 patients (68.5%). Out of 14 patients who underwent V/Q scan showed perfusion defects (Fig. 1) suggestive of pulmonary embolism in 11 patients. Among 24 patients who underwent CTPA, 20 patients had thrombus located in the main, and lobar arteries (Fig. 2). The remaining 4 patients (16.7%) had thrombus seen in subsegmental vessels.
Fig. 1.
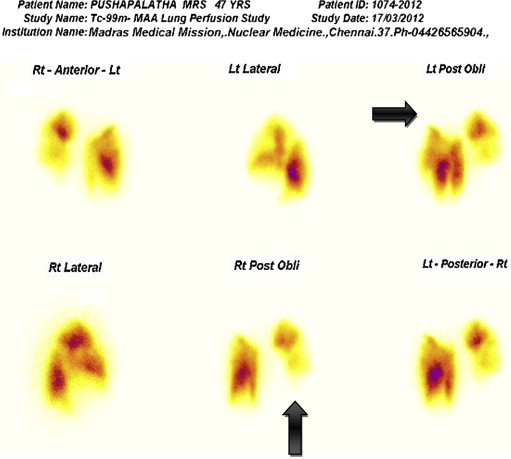
Ventilation perfusion scan showing perfusion defects in lungs (marked with arrows).
Fig. 2.
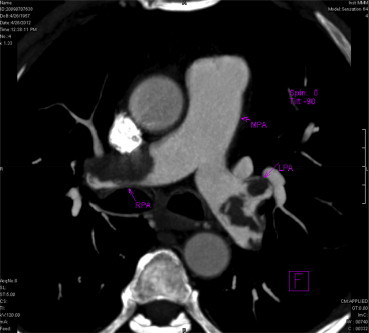
CT Pulmonary Angiogram showing embolus in right and left main pulmonary arteries.
Massive pulmonary embolism was diagnosed in 10 patients (28.5%), submassive pulmonary embolism in 21 patients (60%) and rest of 4 patients (11.5%) had minor pulmonary embolism. Out of 31 patients in whom thrombolysis was indicated, 24 patients (77.4%) were thrombolyzed. In the remaining 7 patients, thrombolysis was deferred in 4 patients due to absolute contraindication for thrombolysis and 3 patients had delayed presentation (>3 weeks) from symptom onset. All patients were thrombolyzed with streptokinase. The mean duration of thrombolysis was 24 h. One patient underwent catheter directed thrombolysis. Out of 35 patients one patient died due to bleeding post thrombolysis. All patients who survived were treated with LMWH and oral anticoagulants.
At 6 months of follow up, out of 34 patients, 7 patients had recurrent pulmonary thromboembolism in spite of adequate anticoagulation, 2 patients had persistent pulmonary arterial hypertension suggestive of chronic pulmonary thromboembolism and surgical pulmonary endarterectomy was done in these 2 patients. Their follow up at one month showed normal pulmonary artery pressures. 5 patients underwent IVC filter implantation.
Out of 34 patients, 8 patients who had a provoked PE was anticoagulated for a period of 6 months and rest of patients were anticoagulated based on American College of Chest Physician (ACCP) guidelines.5
4. Discussion
Pulmonary embolism presents with a wide clinical spectrum, from asymptomatic disease to life threatening massive PE that causes hypotension and cardiogenic shock. The clinical presentation and the investigations including electrocardiography, chest radiography, and analysis of arterial blood gases cannot be relied on to confirm or rule out PE because of lack of adequate specificity.6
Risk factors for venous thromboembolic disease and pulmonary embolism are well characterized in the literature. Risk factors are present in almost 96% of patients with confirmed venous thromboembolic disease.7 The major modifiable risk factors for PE were obesity, cigarette smoking and hypertension. The most common risk factors in hospitalized patients were major surgery, cancer, congestive cardiac failure, chronic obstructive pulmonary disease, chronic kidney disease especially nephrotic syndrome. The most common thrombophiliac risk factors for venous thromboembolism are factor V Leiden mutation, protein C, protein S deficiency, prothrombin gene mutation, antithrombin III deficiency and antiphospholipid antibody syndrome.8 In our series only 34.2% of patients had a definite risk factor.
It is believed that diagnosis of pulmonary embolism is more difficult than treatment. Clinical suspicion of this disease is of paramount importance in guiding diagnostic testing. Firstly, the patient's age is consistently a statistically significant univariate predictor for pulmonary embolism. The frequency of pulmonary embolism is increased in elderly patients.9 On the other hand, the patient's gender does not appear to be predictive. Dyspnea, syncope or cyanosis indicate massive pulmonary embolism.10,11 The lack of typical clinical manifestations of massive pulmonary embolism might be related to the insidious onset and progressive development of thromboembolism.
Pleuritic chest pain usually signifies that the embolism is located in the distal pulmonary arterial system, near the pleural lining. Unexplained dyspnea and chest pain are the most frequent symptoms, and sudden onset dyspnea and pleuritic chest pain are the most typical. In our study dyspnea is the most common symptom followed by syncope and chest pain.
The ECG in addition to clinical acumen, can be essential in directing the physician towards the diagnosis. While no isolated ECG abnormality is definitively associated with PE, certain constellations of ECG abnormalities have been shown to be reasonably specific. Several of the more frequently described associations include: normal ECG, sinus tachycardia, complete and incomplete RBBB, axis changes, transition zone shift, low voltage complexes, ST segment and T-wave changes, S1Q3T3 pattern, P-pulmonale and atrial arrhythmias.12,13 The most common ECG finding in our series in sinus tachycardia followed by ST–T wave changes in anterior leads, S1Q3T3 pattern and RBBB.
Stein et al12 found that the most common chest X-ray finding was atelectasis or parenchymal abnormality. It is a fact, however, that one cannot depend on chest x-ray for the diagnosis of pulmonary embolism. Although the chest radiograph cannot be used to diagnose or exclude PE, it contributes to the non-invasive diagnostic assessment of PE through the exclusion of disease processes that may mimic PE.
d-dimer is produced by the breakdown of cross-linked fibrin by the fibrinolytic system. d-dimer levels are elevated in acute thromboembolism and result from the lysis of cross-linked fibrin within the thrombus. d-dimer levels are, however, elevated in other conditions and thus are not pathognomonic for thromboembolic disease. d-dimer testing has been reported to have a sensitivity ranging from almost 80–100 percent.14,15 However, such high sensitivity often comes at the cost of low specificity (high false-positive rates). In the study of Hammond and Hassan,15 retrospective analysis of a sequential series of 376 patients revealed that no patient with d-dimer of <275 ng/ml was diagnosed with pulmonary embolism, irrespective of clinical probability. Egermayer et al16 showed that a negative d-dimer, a paO2 of ≥80 mmHg and a respiratory rate less than 20, also had a negative predictive value of 100% in patients with suspected pulmonary embolism. The results of a d-dimer test, irrespective of the sensitivity of the assay, cannot be interpreted in isolation. In our study d dimer was positive in 97.1% of patients.
Serum troponin levels I and T are sensitive but nonspecific markers of cardiac myocardial inflammation or injury. They are elevated in acute coronary artery occlusion and myocardial cellular necrosis from any cause, including scorpion sting toxins. The stretching of the right ventricle from pressure overload found with massive or submassive PE may cause the release of troponins. Right ventricular dysfunction or elevated troponin levels in an otherwise hemodynamically stable PE patient predict short-term adverse outcome.17 In our study troponin values were positive in 68.5 percent of patients.
Since the origin of the thrombus is mostly from deep veins of the legs, Doppler ultrasound of lower limb veins is a useful investigation in the diagnosis of PE. Doppler ultrasonography is positive in 10–20 percent of all patients without leg symptoms or signs who undergo evaluation and in approximately 50 percent of patients with proven embolism.18 Therefore, the possibility of embolism cannot be ruled out on the basis of negative results on ultrasonography. The sensitivity and specificity of this examination vary by vein, the best results are obtained for symptomatic proximal lower limb thrombosis showing a 96% sensitivity19 when compared to distal lower limb thrombosis which has a sensitivity of 73%. Doppler ultrasound has its value in situations where there is a high clinical probability of PE and the patient has no past history of Venous thromboembolism. In our study, 28 patients (80%) had evidence of DVT in venous Doppler.
The common echocardiographic feature of PE are dilated pulmonary artery, dilated right atrium, right ventricular hypokinesis, right ventricular enlargement, reduced left ventricular size, McConnell's sign, Flattening of the intraventricular septum or paradoxical septal motion, direct visualization of thrombus in the right heart or pulmonary artery and distention of the inferior vena cava with loss of normal respiratory variation.20 Echocardiography is a useful tool in identifying high risk patients such as those with right ventricular dysfunction, free floating thrombus and persistent pulmonary hypertension. All our patients underwent echocardiography. 88.5% had evidence of RV dysfunction and 85.7% had pulmonary arterial hypertension in our study.
Perfusion scan has been used for almost three decades for the diagnosis of PE and is a valuable tool when the results are definitive. But approximately 30–70% of scans are non-diagnostic and the clinician is left in a diagnostic dilemma of uncertainty.21 Further, it is insensitive in patients with pre-existing lung diseases, especially the chronic obstructive lung disease.22 The use of spiral CTPA is a major advancement in the diagnosis of PE. The sensitivity and specificity for detection of pulmonary embolus by CTPA at the main, lobar and segmental levels are greater than 90% with accuracy decreasing when isolated subsegmental vessels are involved.21 In our study, perfusion scans were performed in 14 patients and CTPA contributed to diagnosis in 24 patients. Perfusion scans were of high probability in 11 patients (78.5%) and non-contributory in the remaining three patients. CTPA confirmed the diagnosis in these three patients.
The ACCP guidelines recommend thrombolytic therapy be used in pulmonary embolism patients with evidence of hemodynamic compromise, except in the face of major contraindications due to bleeding risks (grade 2C). Thrombolytic therapy is suggested in selected high risk patients who do not have hypotension and are at low risk for bleeding (grade 2C). These high risk patients include patients with poor perfusion of vital organs, low blood oxygen level, abnormal serum cardiac enzymes, or abnormal right heart function on echo. A short infusion time of 2 h for systemic thrombolytics is suggested, rather than a longer infusion (grade 2C).5 Tissue plasminogen activator (tPA) has a short infusion time and has been recommended as the best agent for this reason. Although most studies demonstrate the superiority of thrombolytic therapy with respect to the resolution of radiographic and hemodynamic abnormalities within the first 24 h, this advantage disappears 7 days after treatment. Controlled clinical trials have not demonstrated benefits in terms of reduced mortality rates or earlier resolution of symptoms when currently compared with heparin. Until randomized clinical trials demonstrate a clear morbidity or mortality benefit, the role of thrombolytic therapy in the management of acute pulmonary embolism will remain controversial. The currently accepted indications for thrombolytic therapy include hemodynamic instability or right ventricular dysfunction demonstrated on ECHO. Current guidelines for patients with acute non massive pulmonary embolism recommend LMWH over UFH (grade 1A). We had thrombolyzed 24 (77.4%) out of the 35 patients with streptokinase with a mean duration of 24 h. Out of 24 patients thrombolyzed one patient died due to massive GI bleed and rest of the patients showed significant clinical improvement and was discharged.
A patient with a first episode of pulmonary embolism occurring in the setting of reversible risk factors, should receive warfarin therapy for 3–6 months. The current ACCP guidelines recommend that all patients with unprovoked pulmonary embolism should undergo a risk-to-benefit evaluation to determine if long-term therapy is needed (grade 1C). Long term treatment is recommended for these patients who do not have risk factors for bleeding and in whom accurate anticoagulant monitoring is possible (grade 1A).5 Patients who have pulmonary embolism and pre existing irreversible risk factors, such as deficiency of antithrombin III, protein S and C, factor V Leiden mutation, or the presence of antiphospholipid antibodies, should be placed on long-term anticoagulation.
5. Conclusion
PE is a clinical problem which is often under recognized and under diagnosed world over. A high index of suspicion is necessary to consider the diagnosis. The increasing availability of newer imaging modalities are likely to improve its diagnosis When diagnostic techniques are not immediately available, empiric treatment should be initiated anyway. Early recognition and aggressive and appropriate therapy improves the outcome in this potentially fatal condition.
Conflicts of interest
All authors have none to declare.
References
- 1.Kearon C. Natural history of venous thromboembolism. Circulation. 2003;107:I-22–I-30. doi: 10.1161/01.CIR.0000078464.82671.78. [DOI] [PubMed] [Google Scholar]
- 2.Ozsu S., Oztuna F., Bulbul Y. The role of risk factors in delayed diagnosis of pulmonary embolism. Am J Emerg Med. Jan 2011;29:26–32. doi: 10.1016/j.ajem.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Tapson V.F. Acute pulmonary embolism. N Engl J Med. Mar 6 2008;358:1037–1052. doi: 10.1056/NEJMra072753. [DOI] [PubMed] [Google Scholar]
- 4.Gowrinath K., Reddy G.K. CT appearances in patient with pulmonary thromboembolism and infarction. Indian J Chest Dis Allied Sci. 2003;45:116–120. [PubMed] [Google Scholar]
- 5.Kearon C. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e195S–e226S. doi: 10.1378/chest.11-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fedullo P.F., Tapson V.F. The evaluation of suspected pulmonary embolism. N Engl J Med. 2003;349:1247–1256. doi: 10.1056/NEJMcp035442. [DOI] [PubMed] [Google Scholar]
- 7.Ánderson F.A., Wheeler H.B. Venous thromboembolism. Risk factors and prophylaxis. Clin Chest Med. 1995;16:235–251. [PubMed] [Google Scholar]
- 8.Goldhaber Samuel Z. Risk factors of venous thromboembolism. J Am Coll Cardiol. 2010;56:1–7. doi: 10.1016/j.jacc.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 9.Anderson F.A., Wheeler Brownwell H., Goldberg J., Hosmer D.W. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. Arch Intern Med. 1991;151:933–938. [PubMed] [Google Scholar]
- 10.Agarwal R., Gulati M., Mittal B.R., Jindal S.K. Clinical profile, diagnosis and management of patients presenting with symptomatic pulmonary embolism. Indian J Chest Dis Allied Sci. 2006;48:111–115. [PubMed] [Google Scholar]
- 11.Stein Paul D., Henry Jerald W. Clinical characteristics of patients with acute pulmonary embolism stratified according to their presenting syndromes. Chest. OCTOBER, 1997;112 doi: 10.1378/chest.112.4.974. [DOI] [PubMed] [Google Scholar]
- 12.Stein P.D., Terrin M.L., Hales C.A., Palvesky H.I. Clinical, laboratory, roentgenographic and electrocardiographic findings in patients with acute pulmonary embolism and no preexisting cardiac or pulmonary disease. Chest. 1991;100:598–607. doi: 10.1378/chest.100.3.598. [DOI] [PubMed] [Google Scholar]
- 13.Sinha N., Yalamanchili K., Sukhija R., Aronow W.S. Role of 12-lead electrocardiogram in diagnosing pulmonary embolism. Cardiol Rev. 2005;13:46–49. doi: 10.1097/01.crd.0000134647.55135.4a. [DOI] [PubMed] [Google Scholar]
- 14.Bounameaux H., de Moerloose P., Perrier A., Reber G. Plasma measurement of d-dimmer as diagnostic aid in suspected venous thromboembolism: an overview. Thromb Headmost. 1994;71:1–6. [PubMed] [Google Scholar]
- 15.Hammond C.J., Hassan T.B. Screening for pulmonary embolism with a d-dimmer assay: do still need to assess clinical probability. J R Soc Med. 2005;98:54–58. doi: 10.1258/jrsm.98.2.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egermayer P., Town G.I., Turner J.G., Heaton D.C., Mee A.L., Beard M.E. Usefulness of d-dimer, blood gas, and respiratory rate measurements for excluding pulmonary embolism. Thorax. 1998 Oct;53:830–834. doi: 10.1136/thx.53.10.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becattini Cecilia, Vedovati Maria Cristina, Agnelli iancarlo. Prognostic value of troponins in acute pulmonary embolism a meta-analysis. Circulation. 2007;116:427–433. doi: 10.1161/CIRCULATIONAHA.106.680421. [DOI] [PubMed] [Google Scholar]
- 18.Moser K.M., Fedullo P.F., LitteJohn J.K., Crawford R. Frequent asymptomatic pulmonary embolism in patients with deep venous thrombosis. JAMA. 1994;271:223–225. [PubMed] [Google Scholar]
- 19.Kearon C., Julian J.A., Newman T.E., Ginsberg J.S. Noninvasive diagnosis of deep venous thrombosis. McMaster Diagnostic Imaging Practice Guidelines Initiative. Ann Intern Med. 1998;128:663–677. doi: 10.7326/0003-4819-128-8-199804150-00011. [DOI] [PubMed] [Google Scholar]
- 20.Goldhaber S.Z. Echocardiography in the management of pulmonary embolism. Arch Intern Med. 2002;136:691–700. doi: 10.7326/0003-4819-136-9-200205070-00012. [DOI] [PubMed] [Google Scholar]
- 21.Padley S.P.G. Lung scintigraphy vs spiral CT in the assessment of pulmonary emboli. Br J Radiol. 2002;75:5–8. doi: 10.1259/bjr.75.889.750005. [DOI] [PubMed] [Google Scholar]
- 22.Hartmann I.J., Hagen P.J., Melissant C.F., Postmus P.E., Prisis M.H. Diagnosing acute pulmonary embolism: effect of chronic obstructive pulmonary disease on performance of d-dimer testing, perfusion scintigraphy, spiral CT angiography and conventional angiography. ANTELOPE study group: advances in new technologies evaluating the localization of pulmonary embolism. Am J Respir Crit Care Med. 2000;162:2232–2237. doi: 10.1164/ajrccm.162.6.2006030. [DOI] [PubMed] [Google Scholar]


