Abstract
Objective
Right ventricular (RV) dysfunction in isolated severe mitral stenosis (MS) patients have prognostic significance. Study aim was to assess RV function in these subjects by strain and strain rate analysis, pre and post-balloon mitral valvuloplasty (BMV).
Methods
Twenty five patients with isolated severe MS in sinus rhythm were assessed for RV function by two dimensional (2D) longitudinal strain & strain rate imaging before and after BMV and compared with that from twelve healthy age matched controls.
Results
Patients with severe MS had significantly lower global RV systolic strain; segmental strain at basal, mid, apical septum and basal RV free wall; but similar strain at mid and apical RV free wall as compared to controls. The systolic strain rate was significantly lower only at mid septum. In addition, they had higher estimated pulmonary artery systolic pressure and RV myocardial performance index; lower tricuspid annular plane systolic excursion (TAPSE), peak systolic velocity at lateral tricuspid annulus, isovolumic acceleration and fractional area change (FAC). Global RV systolic strain as well as, segmental strain at basal, mid and apical septum showed a statistically significant rise after BMV. TAPSE and FAC also increased significantly post BMV.
Conclusions
RV systolic function is impaired in patients with severe MS and can be assessed by global and segmental RV strain before the appearance of clinical signs of systemic venous congestion. Impaired global and segmental RV strain values in these patients are primarily due to increased after load and improve after BMV with reduction in RV afterload.
Keywords: Mitral stenosis, Strain, Strain rate, Balloon mitral valvuloplasty, Right ventricular function
1. Introduction
Right ventricular (RV) function plays an important role in development of clinical symptoms and prognosis in patients with mitral stenosis (MS).1,2 This is primarily affected by hemodynamic effects on RV due to pulmonary hypertension (PH). RV dysfunction is not detected clinically until the development of clinical signs of systemic venous congestion.
RV functional assessment is difficult and not done routinely because of its complex anatomy and high load dependence. Many indices have been developed for quantifying RV function, among which strain and strain rate is relatively new. Myocardial strain is a measure of tissue deformation, which is expressed as a percentage change, whereas, strain rate is the rate of such deformation. Although till recently, tissue Doppler imaging (TDI) method was used for evaluation of RV function by strain and strain rate,3 this method is plagued by various limitations, such as angle dependence. Of late, two-dimensional (2D) speckle tracking method has been used to quantify strain and strain rate for assessment of global and regional myocardial function. This method measures myocardial movement and deformation without depending on the Doppler signals. Various studies have demonstrated the importance of 2D strain and strain rate analysis in assessing RV function in different disorders. However, there are only a few studies, which assessed 2D RV strain and strain rate in patients with isolated MS.4
Long-term improvement in RV function in patients with MS has been shown in different hemodynamic studies after percutaneous balloon mitral valvuloplasty (BMV).5,6 However, immediate effect of BMV on RV function was examined in only few studies.7 One of such study showed discordant result in terms of improvement in RV function post BMV, as measured by conventional echocardiographic parameters.8 The effect of BMV on RV by 2D longitudinal strain and strain rate in patients with severe MS has not been studied yet.
As the RV function has a prognostic importance in patients with MS, the present study intended to assess RV function as quantified by 2D longitudinal global and segmental RV strain, strain rate and other conventional parameters in patients with isolated severe MS, before and after BMV.
2. Methods
2.1. Population
Our study included adult patients older than 18 years who had isolated severe MS, in sinus rhythm, and were admitted for the BMV. Patients with atrial fibrillation, NYHA class I or IV symptoms, overt right heart failure, pregnancy, chronic obstructive pulmonary disease, other valvular lesions or undergoing emergency BMV were excluded from the study. Patients who developed more than mild mitral regurgitation after BMV were also excluded from the study. Twenty five patients, who met inclusion criteria, were assessed for RV strain and strain rate, and other conventional echocardiographic parameters of RV function. The effect of BMV on these parameters was also assessed. These parameters were also compared with that from twelve healthy age matched controls.
2.2. Echocardiographic measurements
Echocardiographic evaluation was done with 3.5 Hz probe using – iE 33 echocardiography machine, Philips Medical Systems, USA. All the measurements were performed according to guidelines for assessment of right heart set by American society of echocardiography (ASE).9 All examinations were recorded for off-line analysis. RV functional parameters, RV strain and strain rate were derived from modified apical 4 chamber view (A4C) – RV focused view. Echocardiographic measurements were done and recorded for five cardiac cycles and an average of three values was taken. All these measurements were repeated 24–48 hours after BMV. Frame rates used for 2D longitudinal strain analysis were 40–80 frames/s. The data were transferred to a software system (QLAB, Philips Systems) for off-line analysis. After defining the three points (RV apex, medial and lateral tricuspid annulus) the software automatically traced the endocardial border and epicardial border in modified A4C view. After adjusting tracking points manually, if required, and adjusting myocardial penetration, 2D longitudinal strain and strain rate curves for each segment were obtained. Peak negative longitudinal systolic strain and systolic strain rates were derived from these curves (Figs. 1 and 2). The myocardium of the interventricular septum (IVS) and the RV free wall both were subdivided into three segments each (apical, mid, and basal) that resulted in six segments. The longitudinal peak negative systolic strain and peak systolic strain rates were obtained for each segment. In addition global RV systolic strain was also obtained. The peak systolic strain was defined as a peak negative strain after pulmonary valve (PV) opening. The systolic strain rate was defined as the peak negative strain rate between PV opening and PV closure.
Fig. 1.
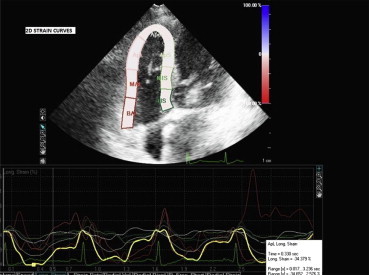
Graphic demonstration of 2D longitudinal strain curves for each segment of RV free wall and interventricular septum.
Fig. 2.
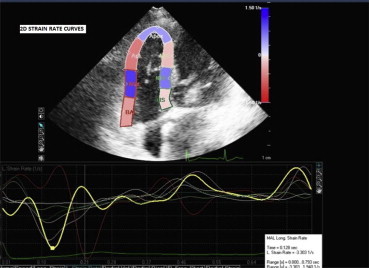
Graphic demonstration of 2D longitudinal strain rate curves for each segment of RV free wall and interventricular septum.
RV fractional area change (FAC), Tei index or RV myocardial performance index (MPI) and tricuspid annular plane systolic excursion (TAPSE) were also calculated as per ASE guidelines.9 Peak systolic velocity at the lateral tricuspid annulus (S’) was obtained from pulsed TDI and highest systolic velocity was recorded without averaging the Doppler envelope (Fig. 3). Isovolumic contraction time (IVCT) was measured from the duration of isovolumic velocity (IVV) measured by pulse wave TDI at the lateral tricuspid annulus. Isovolumic relaxation time (IVRT) was measured from the end of S’ to the onset of early diastolic lateral tricuspid annular velocity (E'). RV MPI was calculated by dividing isovolumic time (IVCT + IVRT) by ejection time (ET). Isovolumic acceleration (IVA) was defined as the peak IVV divided by activation time i.e. time to peak velocity. PA systolic pressure (PASP) was estimated from peak tricuspid regurgitation jet velocity (V) using modified Bernoulli equation (PASP = 4 V2 + estimated RA pressure).
Fig. 3.
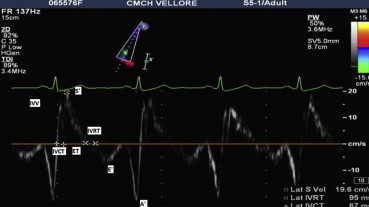
Tissue Doppler imaging at the lateral tricuspid annulus.
RA pressure was estimated from inferior vena cava (IVC) diameter and extent of inspiratory collapse. IVC diameter was measured at end of expiration in subcostal view; just proximal to the site of hepatic vein drainage. The change in diameter of the IVC with quite respiration and with sniff was measured. If IVC diameter was ≤2.1 cm and collapse with a sniff was >50%, RA pressure was taken as 3 mm Hg. Where as if the IVC diameter of >2.1 cm with <50% collapse with a sniff, RA pressure of 15 mm Hg was taken. In cases with IVC diameter ≤2.1 cm, but collapse with sniff <50% or IVC diameter >2.1 cm and collapse >50%, intermediate value of 8 mm Hg was taken.
2.3. Statistical analysis
All the variables were expressed as mean ± standard deviation (SD). Skewed variables were also expressed as median. For paired data, student's t-test was used to determine the significance of differences in RV function parameters pre- and post-BMV after testing for normal distribution. Kolmogorov–Smirnov test with lilliefors significance correction was used for testing for normality. For variables, which were not normally distributed, Wilcoxon signed rank test was used. The differences in means of the parameters between patients with MS and healthy subjects were analyzed using unpaired Student's t-test for normally distributed variables (after checking of homogeneity of variance assumption) and Mann–Whitney U test for skewed variables. Pearson correlation analysis was done to show the relationship between the variables in MS patients. A p-value of ≤0.05 was considered statistically significant. Interobserver and intraobserver variability of global, segmental RV strain and segmental RV strain rate were assessed by Bland–Altman analysis and intraclass correlation coefficient (ICC) respectively. Statistical analyses were done by using software SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).
3. Results
Study group consisted of 25 patients with isolated severe MS in sinus rhythm, who met the inclusion criteria and 12 healthy controls. Baseline variables are summarized in Table 1. We analyzed 96% of the RV segments in MS group and 92% of the RV segments in the control group for 2D longitudinal strain and strain rate. The rest of the segments were excluded because of suboptimal image quality. All the variables were normally distributed except for segmental systolic strain rates at the RV free wall before and after BMV. Tables 1 and 2 show the comparison between various echocardiographic parameters between MS patients and controls. The comparison between pre and post-BMV parameters are presented in Tables 3–6.
Table 1.
Echocardiographic parameters in patients with severe MS and controls.
| Variable | Controls (n = 12) mean ± SD (median) | Cases (n = 25) mean ± SD (median) | p value |
|---|---|---|---|
| Age (years) | 34.00 ± 4.63 | 34.48 ± 9.05 | 0.86 |
| Left atrial dimension in PLAX view (mm) | 31.45 ± 3.02 | 44.87 ± 5.50 | <0.001 |
| LVEF (%) | 64.36 ± 5.40 | 62.66 ± 5.59 | 0.38 |
| Estimated PA systolic pressure (mm Hg) | 21.00 ± 4.67 (19.5) | 48.64 ± 23.93 (45) | <0.001 |
| RV MPI | 0.50 ± 0.04 | 0.61 ± 0.13 | 0.001 |
| TAPSE (mm) | 27.95 ± 4.47 | 21.12 ± 4.47 | <0.001 |
| S' (cm/s) | 15.14 ± 3.12 | 11.98 ± 2.19 | 0.001 |
| FAC (%) | 56.37 ± 4.20 | 35.67 ± 14.83 | <0.001 |
| IVA (m/s2) | 3.44 ± 1.31 | 2.73 ± 0.76 | 0.004 |
LVEF – left ventricular ejection fraction; RV MPI – right ventricular myocardial performance index; TAPSE – tricuspid annular plane systolic excursion; S' – peak systolic velocity at lateral tricuspid annulus; FAC – fractional area change; IVA – isovolumic acceleration.
Table 2.
RV 2D longitudinal strain in patients with severe MS and controls.
| Peak systolic strain (%) | Controls (n = 12) mean ± SD | Cases (n = 25) mean ± SD | p value |
|---|---|---|---|
| Basal septum | −18.22 ± 9.65 | −11.63 ± 6.63 | 0.020 |
| Mid septum | −18.98 ± 3.25 | −9.31 ± 5.69 | <0.001 |
| Apical septum | −19.69 ± 6.38 | −13.75 ± 5.32 | 0.005 |
| Basal RV free wall | −32.57 ± 14.98 | −19.35 ± 14.10 | 0.012 |
| Mid RV free wall | −28.33 ± 17.86 | −17.84 ± 11.33 | 0.095 |
| Apical RV free wall | −9.17 ± 9.30 | −13.53 ± 11.33 | 0.27 |
| Global RV | −14.67 ± 3.95 | −9.07 ± 4.70 | 0.02 |
Table 3.
RV 2D longitudinal strain rate in patients with severe MS and controls.
| Strain rate (s−1) | Controls (n = 12) mean ± SD (median) | Cases (n = 25) mean ± SD (median) | p value |
|---|---|---|---|
| Basal septum systolic | −1.22 ± 0.55 (1.18) | −1.12 ± 0.76 (0.88) | 0.27 |
| Mid septum systolic | −1.12 ± 0.42 (1.05) | −1.04 ± 1.47 (0.67) | 0.043 |
| Apical septum systolic | −0.98 ± 0.31 (0.94) | −0.79 ± 0.38 (0.78) | 0.095 |
| Basal RV free wall systolic | −3.02 ± 1.97 (2.03) | −2.67 ± 1.87 (2.06) | 0.559 |
| Mid RV free wall systolic | −1.98 ± 0.76 (2.01) | −3.21 ± 2.99 (2.66) | 0.503 |
| Apical RV free wall systolic | −1.04 ± 0.70 (1.10) | −1.49 ± 0.96 (1.32) | 0.185 |
Table 4.
Comparison of echocardiographic parameters before and after BMV in patients with severe MS.
| Variable | Pre-BMV, mean ± SD | Post BMV, mean ± SD | p value |
|---|---|---|---|
| MVA by planimetry (cm2) | 0.81 ± 0.14 | 1.78 ± 0.22 | <0.001 |
| Peak MV gradient (mm Hg) | 22.75 ± 7.57 | 9.96 ± 3.36 | <0.001 |
| Mean MV gradient (mm Hg) | 12.72 ± 5.04 | 4.16 ± 1.28 | <0.001 |
| Left atrial dimension in PLAX view (mm) | 44.87 ± 5.50 | 40.91 ± 6.26 | <0.001 |
| LVEF (%) | 62.66 ± 5.59 | 63.81 ± 5.46 | 0.019 |
| Estimated PA systolic pressure (mm Hg) | 48.64 ± 23.93 | 36.88 ± 15.71 | 0.001 |
| RV MPI | 0.61 ± 0.13 | 0.58 ± 0.10 | 0.293 |
| TAPSE (mm) | 21.12 ± 5.00 | 23.10 ± 4.03 | 0.031 |
| S' (cm/s) | 11.98 ± 2.19 | 12.41 ± 2.07 | 0.242 |
| FAC (%) | 35.67 ± 14.83 | 47.78 ± 10.53 | <0.001 |
| IVA (m/s2) | 2.73 ± 0.76 | 2.89 ± 1.01 | 0.444 |
MVA – mitral valve area; LVEF – left ventricular ejection fraction; RV MPI – right ventricular myocardial performance index; TAPSE – tricuspid annular plane systolic excursion; S' – peak systolic velocity at lateral tricuspid annulus; FAC – fractional area change; IVA – isovolumic acceleration.
Table 5.
Comparison of 2D longitudinal strain in different RV segments before and after BMV in patients with severe MS.
| Peak systolic strain (%) | Pre-BMV (mean ± SD) | Post BMV (mean ± SD) | p value |
|---|---|---|---|
| Basal septum | −11.63 ± 6.63 | −14.92 ± 6.34 | 0.011 |
| Mid septum | −9.31 ± 5.69 | −13.09 ± 5.21 | 0.002 |
| Apical septum | −13.75 ± 5.32 | −16.37 ± 5.24 | 0.005 |
| Basal RV free wall | −19.35 ± 14.10 | −25.84 ± 17.06 | 0.062 |
| Mid RV free wall | −17.84 ± 11.33 | −20.00 ± 11.99 | 0.262 |
| Apical RV free wall | −13.53 ± 11.33 | −15.71 ± 13.89 | 0.419 |
| Global RV | −9.07 ± 4.70 | −11.24 ± 4.24 | 0.026 |
Table 6.
Comparison of 2D longitudinal strain rates in different RV segments before and after BMV in patients with severe MS.
| Strain rate (s−1) | Pre-BMV, mean ± SD (median) | Post-BMV, mean ± SD (median) | p value |
|---|---|---|---|
| Basal septum systolic | 1.12 ± 0.76 (0.88) | 1.15 ± 0.99 (0.93) | 0.609 |
| Mid septum systolic | 1.04 ± 1.47 (0.67) | 0.92 ± 0.47 (0.83) | 0.333 |
| Apical septum systolic | 0.79 ± 0.38 (0.78) | 0.88 ± 0.29 (0.84) | 0.326 |
| Basal RV free wall systolic | 2.67 ± 1.87 (2.06) | 3.15 ± 2.46 (2.74) | 0.412 |
| Mid RV free wall systolic | 3.21 ± 2.99 (2.66) | 2.59 ± 2.15 (1.87) | 0.609 |
| Apical RV free wall systolic | 1.49 ± 0.96 (1.32) | 1.36 ± 0.85 (1.30) | 0.638 |
Age and LV ejection fraction were similar in both the groups. Left atrial (LA) anteroposterior dimension in parasternal long axis (PLAX) view measured by M-mode echocardiography was greater in MS group (31.45 ± 3.02 vs. 34.48 ± 9.05 mm; p < 0.001). Patients had higher estimated PASP (48.64 ± 23.93 mm Hg vs. 21 ± 4.67 mm Hg; p < 0.001), they also had higher MPI (0.61 ± 0.13 vs. 0.50 ± 0.04; p = 0.001), lower TAPSE (21.12 ± 4.47 vs. 27.95 ± 4.47; p < 0.001), lower S' (11.98 ± 2.19 vs. 12.49 ± 2.07; p = 0.001), lower FAC (35.67 ± 14.83 vs. 47.78 ± 10.53; p < 0.001) and lower IVA (2.73 ± 0.76 vs. 2.89 ± 1.01; p = 0.004) compared to controls.
Patients with severe MS had significantly lower global RV strain (−14.67 ± 3.95 vs. −9.07 ± 4.70; p = 0.02), segmental strain at basal septum (−11.63 ± 6.63 vs. −18.22 ± 9.65; p = 0.02), mid septum (−9.31 ± 5.65 vs. −18.98 ± 3.25; p < 0.001), apical septum (−13.75 ± 5.32 vs. −19.69 ± 6.38; p = 0.005) and basal RV free wall (−19.35 ± 14.10 vs. −32.57 ± 14.98; p = 0.012). Whereas there was no significant difference in strain in the mid (−17.84 ± 11.33 vs. −28.33 ± 17.86; p = 0.095) and apical RV free wall (−13.53 ± 11.33 vs. −9.17 ± 9.30, p = 0.27) as compared to controls. There was no difference between strain rates in all the segments of RV as compared to controls except for systolic strain rate at mid septum (−1.12 ± 0.42 vs. −1.04 ± 1.47; p = 0. 047).
In a small subgroup of patients with severe PH (PASP> 60 mm Hg, n = 11), strain at the mid RV free wall was also significantly less compared to controls (−14.28 ± 6.34 vs. 28.33 ± 17.86; p = 0.02), in addition to reduced global RV strain and segmental strain at the basal RV free wall, basal, mid and apical IVS. No significant difference was observed in segmental strain rates in this subgroup compared to controls.
The mean mitral valve area (MVA) by planimetry in patients with MS was 0.81 ± 0.14 and significantly increased after BMV (1.78 ± 0.22; p < 0.001). The transmitral peak (22.75 ± 7.57 vs. 9.96 ± 3.36; p < 0.001) as well as mean gradient (12.72 ± 5.04 vs. 4.16 ± 1.28; p < 0.001) significantly decreased after BMV. There was also significant reduction in LA anteroposterior dimension (44.87 ± 5.50 vs. 40.91 ± 6.26; p < 0.001) and estimated PASP (48.64 ± 23.93, median-45 vs. 36.88 ± 15.71, median-35; p = 0.001) after BMV. There was a minor improvement noted in left ventricular ejection fraction (LVEF) post BMV (62.66 ± 5.59 vs. 63.81 ± 5.46; p = 0.019).
The peak systolic global RV strain (−9.07 ± 4.70 vs. −11.24 ± 4.24; p = 0.02), segmental strain at basal (−11.63 ± 6.63 vs. −14.92 ± 6.34; p = 0.011), mid (−9.31 ± 5.69 vs. −13.09 ± 5.21; p = 0.002) and apical septum (−13.75 ± 5.32 vs. −16.37 ± 5.24; p = 0.005) increased significantly post-BMV; whereas no significant difference was observed in strain at basal, mid and apical RV free wall and strain rates at the RV free wall and septal segments. In the subgroup of patients with severe PH (n = 11), reduced strain at mid and apical septum improved after BMV with no significant change in basal septal and RV free wall strain.
TAPSE (21.12 ± 5.00 vs. 23.10 ± 4.03; p = 0.031) and FAC (35.67 ± 14.83 vs. 47.78 ± 10.53; p < 0.001) showed a significant rise after BMV, whereas there was no significant reduction in RV MPI (0.61 ± 0.13 vs. 0.58 ± 0.10; p = 0.293), nonsignificant increase in S' (11.98 ± 2.19 vs. 12.41 ± 2.07; p = 0.242) and IVA (2.73 ± 0.76 vs. 2.89 ± 1.01; p = 0.444).
The intraobserver and interobserver variability of global, segmental RV strain and segmental RV strain rate were examined in ten patients selected randomly and results are presented in Table 7.
Table 7.
Results of Intra- and Inter-observer agreement.
| Intraobserver agreement |
Interobserver agreement |
|||
|---|---|---|---|---|
| Variables | ICC | 95% CI | Mean difference | 95% CI |
| RV global strain | 0.99 | 0.96–0.99 | −0.30 | 2.69;−3.29 |
| Basal septal strain | 0.94 | 0.79–0.98 | −1.46 | 6.57;−9.49 |
| Mid septal strain | 0.97 | 0.89–0.99 | −0.21 | 5.23;−5.65 |
| Apical septal strain | 0.91 | 0.71–0.97 | 0.21 | 7.38;−6.96 |
| Basal RV free wall strain | 0.98 | 0.93–0.99 | −0.13 | 10.35;−10.61 |
| Mid RV free wall strain | 0.96 | 0.92–0.99 | −0.15 | 4.53;−4.83 |
| Apical RV free wall strain | 0.99 | 0.97–0.99 | −0.28 | 4.87;−5.43 |
| Basal septum strain rate | 0.97 | 0.90–0.99 | −0.04 | 0.33;−0.41 |
| Mid septum strain rate | 0.87 | 0.57–0.96 | 0.01 | 0.28;−0.26 |
| Apical septum strain rate | 0.75 | 0.27–0.93 | −0.05 | 0.28;−0.38 |
| Basal RV free wall strain rate | 0.79 | 0.36–0.94 | 0.53 | 2.17;−1.11 |
| Mid RV free wall strain rate | 0.86 | 0.54–0.96 | −0.99 | 2.77;−4.76 |
| Apical RV free wall strain rate | 0.73 | 0.23–0.92 | −0.05 | 0.28;−0.38 |
RV – right ventricle; ICC – intra-class correlation coefficient; CI – confidence interval.
3.1. Correlation analysis
There was an inverse correlation between PASP and global RV systolic strain(r = −0.416, p = 0.02), basal (r = −0.389, p = 0.017), mid (r = −0.523, p = 0.001), apical (r = −0.337, p = 0.042) septal systolic strain, and basal RV free wall systolic strain (r = −0.44, p = 0.005). Whereas positive correlation was found between PASP and RV MPI (r = 0.372, p = 0.023). There was also an inverse correlation between PASP and TAPSE (r = 0.558, p < 0.001) and FAC (r = 0.69, p < 0.001).
4. Discussion
Our study showed that the RV systolic function is impaired in patients with severe MS as assessed by global and segmental RV strain. It correlates with earlier hemodynamic and clinical studies, which showed impaired RV function in MS patients.2,10-12 The cause of RV dysfunction is attributed to the increased RV afterload in these patients. Left atrial hypertension in these patients leads to chronic pulmonary venous congestion, which ultimately leads to PH. This is thought to be responsible for increased RV after load and subsequent RV dysfunction in these patients. However some authors had suggested that the direct rheumatic involvement of the RV with resultant myocyte necrosis, replacement fibrosis and calcification is the explanation of such depressed myocardial function.13,14
We assessed RV strain and strain rate in patients with severe MS by 2D speckle tracking method and compared it to that in controls. We found that peak systolic global RV strain and segmental strain at basal, mid & apical septum was lower than the same in controls. Also there was reduced strain at the basal RV free wall, whereas mid and apical RV free wall were not affected. This finding is in concordance with the findings by Ozdemir et al4 who showed lower global RV and IVS strain, but normal RV free wall strain. The reason for relative sparing of RV free wall is speculated by Ozdemir et al.4 They suggested that RV free wall contractility is not affected by rheumatic process and because septum is in continuity to mitral apparatus, it may be affected directly. Further moderate PH may not affect strain in all segments of RV free wall.4 In our study, subgroup analysis of patients with severe PH (estimated PASP >60 mm Hg; n = 11) showed reduced strain values in mid RV free wall also in addition to strain at basal RV free wall and septal segments. This suggests that different levels of PA pressure differently affect RV free wall and septum. In a recent study by Tanboga et al, 15 patients with moderate MS had lower global RV strain and global RV strain rate values and these values did not correlate with severity of MS. But this study consisted of only moderate MS patients and did not include patients with severe MS. This is in contradiction with finding of Ozedemir et al, 4 which showed similar global RV strain rate values in MS patients and controls. Our study did not measure the global RV strain rate, but showed patients with MS had lower segmental strain rate value at mid septum, whereas there was no significant difference at other segments.
A study by Nagel et al16 showed that RV ejection fraction is related to mean PA pressure, suggesting RV afterload to be the culprit for RV dysfunction rather than intrinsic contractile dysfunction. We also found an inverse correlation between PA systolic pressure and peak systolic global RV strain, segmental strain at basal, mid, apical septum and the basal RV free wall. The preserved systolic strain rate in most of the segments, which is relatively load independent further supports increased afterload being the primary cause of RV systolic dysfunction in these patients.
We also analyzed the effect of BMV on global and segmental RV strain, strain rate and other RV function parameters. There were significant rise in peak systolic global and segmental RV strain at basal, mid, distal septum, whereas no significant difference was observed in basal, mid and apical RV free wall systolic strain post BMV. Also there was no change in strain rates in any of these segments. This supports the observation by Weidemann et al17 that strain can be affected by loading conditions, whereas the strain rate more closely reflects contractility and is load independent. RV free wall strain did not show immediate improvement, which may be due to the effect of sustained pressure overload and might have shown improvement in long-term follow up. The greater improvement of IVS strain than RV free wall strain may be because of enhanced LV filling and LV contractility. In the subgroup of patients with severe PH (n = 11) there was improvement in strain at mid and apical septum, whereas the reduced strain at basal septum, basal and mid RV free wall strain did not demonstrate any significant improvement immediately post BMV. This further supports our observation that different RV segments are differently affected by different amount of RV afterload and their function may not improve shortly after BMV.
In addition, significant rise in FAC and TAPSE and decrease in PASP was noted post BMV, which indicates improved RV function. This finding is concordant with the observation by Burger et al12 who showed immediate improvement in RV ejection fraction post BMV. Such a rapid improvement of RV function can be explained only by a reduction in afterload by relieving left atrial hypertension and resultant decrease in PASP. In the current study RV MPI, S' or IVA showed no significant change after BMV. This lack of immediate improvement in RV MPI, S' or IVA may be due to effects of sustained pressure overload on the RV for prolonged periods and these might have shown improvement over longer follow up. This again is concordant with the observation by Mohan et al2 who showed that RV MPI shows no immediate change after BMV, but decrease in long term follow up. However in a study done by Drighil et al, 8 there was a decrease in RV MPI post-BMV. In the same study IVA was decreased after BMV, whereas in this study IVA showed no change. IVA and RV MPI is a relatively load independent parameter of the global RV function, which may not change immediately after BMV. There is change in afterload immediately post BMV, which results in improvement in most of the load dependent parameters.
Thus, in the patients with severe MS, RV performance is decreased predominantly due to increase in RV afterload, which improves after BMV, reflected by improved global RV systolic strain and segmental strain at basal, mid and apical septum without any change in strain rates. Alternatively there is a possibility that this immediate improvement in RV function is due to improved hemodynamics, better LV filling and RV emptying after BMV as only the load dependent parameters are improved. Since there is no immediate change in load independent parameters like RV MPI in previous studies2 and strain rates, S' and IVA as well as RV MPI in the current study, they suggest RV myocardial structural changes secondary to long standing increased pressure overload. Finally, significant improvement in these parameters in long term studies18 supports the gradual remodeling of the RV after BMV and the need for strict medical and secondary rheumatic chemoprophylaxis.
There was significant immediate reduction in LA anteroposterior dimension. This finding is concordant with a recently published study by Adavane et al, 19 who showed immediate decrease in LA volume after BMV in patients in sinus rhythm. The most probable explanation of this immediate reduction in LA size is decompression of LA and better emptying by releasing the mitral valve obstruction by the BMV.
We also found that there was minor improvement in LV ejection fraction. This correlated with the findings in an earlier study by Mohan et al.20 The exact reason for this immediate improvement is unclear, but improvement in the atrial contribution to LV filling, 21 improved myocardial contractility22 may be the possible explanations.
5. Study limitations
The number of patients was small, which is the main limitation of this study. To confirm these findings a study with larger sample size is required. Two dimensional strain analyses depend on adequate image quality, so suboptimal images were excluded from study, although this percentage was small.
6. Conclusion
RV systolic function is impaired in patients with severe MS and can be assessed by global and segmental RV strain before the appearance of clinical signs of systemic venous congestion. Impaired global and segmental RV strain values in these patients are primarily due to increased after load and improve after BMV with reduction in RV afterload.
Conflicts of interest
All authors have none to declare.
References
- 1.Sagie A., Freitas N., Padial L.R. Doppler echocardiographic assessment of long-term progression of mitral stenosis in 103 patients: valve area and right heart disease. J Am Coll Cardiol. 1996;28:472–479. doi: 10.1016/0735-1097(96)00153-2. [DOI] [PubMed] [Google Scholar]
- 2.Mohan J.C., Sengupta P.P., Arora R. Immediate and delayed effects of successful percutaneous transvenous mitral commissurotomy on global right ventricular function in patients with isolated mitral stenosis. Int J Cardiol. 1999;68:217–223. doi: 10.1016/s0167-5273(98)00358-1. [DOI] [PubMed] [Google Scholar]
- 3.Kukulski T., Hubbert L., Arnold M., Wranne B., Hatle L., Sutherland G.R. Normal regional right ventricular function and its change with age: a Doppler myocardial imaging study. J Am Soc Echocardiogr. 2000;13:194–204. doi: 10.1067/mje.2000.103106. [DOI] [PubMed] [Google Scholar]
- 4.Ozdemir A.O., Kaya C.T., Ozdol C. Two-dimensional longitudinal strain and strain rate imaging for assessing the right ventricular function in patients with mitral stenosis. Echocardiography. 2010;27:525–533. doi: 10.1111/j.1540-8175.2009.01078.x. [DOI] [PubMed] [Google Scholar]
- 5.Burger W., Brinkies C., Illert S., Teupe C., Kneissl G.D., Schrader R. Right ventricular function before and after percutaneous balloon mitral valvuloplasty. Int J Cardiol. 1997;58:7–15. doi: 10.1016/s0167-5273(96)02860-4. [DOI] [PubMed] [Google Scholar]
- 6.Hirata N., Sakakibara T., Shimazaki Y. Preoperative and postoperative right ventricular function during exercise in patients with mitral stenosis. J Thorac Cardiovasc Surg. 1992;104:1029–1034. [PubMed] [Google Scholar]
- 7.Burger W., Kneissl G.D., Kober G., Schrader R. Effect of balloon valvuloplasty for mitral stenosis on right ventricular function. Am J Cardiol. 1993;71:994–996. doi: 10.1016/0002-9149(93)90921-x. [DOI] [PubMed] [Google Scholar]
- 8.Drighil A., Bennis A., Mathewson J.W., Lancelotti P., Rocha P. Immediate impact of successful percutaneous mitral valve commissurotomy on right ventricular function. Eur J Echocardiogr. 2008;9:536–541. doi: 10.1093/ejechocard/jen126. [DOI] [PubMed] [Google Scholar]
- 9.Rudski L.G., Lai W.W., Afilalo J. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. quiz 86–8. [DOI] [PubMed] [Google Scholar]
- 10.Unterberg R., Konig S., Volker W., Pietsch-Breitfeld B., Karsch K.R. Function of the right ventricle in patients with mitral valve diseases. Z Kardiol. 1989;78:386–393. [PubMed] [Google Scholar]
- 11.Ozdemir K., Altunkeser B.B., Gok H., Icli A., Temizhan A. Analysis of the myocardial velocities in patients with mitral stenosis. J Am Soc Echocardiogr. 2002;15:1472–1478. doi: 10.1067/mje.2002.128645. [DOI] [PubMed] [Google Scholar]
- 12.Burger W., Illert S., Teupe C., Kneissl G.D., Kober G., Schrader R. Right ventricular function in patients with rheumatic mitral valve stenosis. Effect of balloon mitral valvuloplasty. Z Kardiol. 1993;82:545–551. [PubMed] [Google Scholar]
- 13.Harvey R.M., Ferrer I., Samet P. Mechanical and myocardial factors in rheumatic heart disease with mitral stenosis. Circulation. 1955;11:531–551. doi: 10.1161/01.cir.11.4.531. [DOI] [PubMed] [Google Scholar]
- 14.Malhotra V., Beohar P.C., Gondal R., Kaul U.A., Khanna S.K. An autopsy study of rheumatic heart disease. Part II. Associated findings. Jpn Heart J. 1987;28:7–14. doi: 10.1536/ihj.28.7. [DOI] [PubMed] [Google Scholar]
- 15.Tanboga I.H., Kurt M., Bilen E. Assessment of right ventricular mechanics in patients with mitral stenosis by two-dimensional deformation imaging. Echocardiography. 2012;29:956–961. doi: 10.1111/j.1540-8175.2012.01738.x. [DOI] [PubMed] [Google Scholar]
- 16.Nagel E., Stuber M., Hess O.M. Importance of the right ventricle in valvular heart disease. Eur Heart J. 1996;17:829–836. doi: 10.1093/oxfordjournals.eurheartj.a014963. [DOI] [PubMed] [Google Scholar]
- 17.Weidemann F., Jamal F., Sutherland G.R. Myocardial function defined by strain rate and strain during alterations in inotropic states and heart rate. Am J Physiol Heart Circ Physiol. 2002;283:H792–H799. doi: 10.1152/ajpheart.00025.2002. [DOI] [PubMed] [Google Scholar]
- 18.Hamdy I. Assessment of right ventricular systolic function in patients with successful percutaneous mitral valve commissurotomy: the role of three-dimensional echocardiography. Heart Mirror J. 2011;5:356–359. [Google Scholar]
- 19.Adavane S., Santhosh S., Karthikeyan S. Decrease in left atrium volume after successful balloon mitral valvuloplasty: an echocardiographic and hemodynamic study. Echocardiography. 2011;28:154–160. doi: 10.1111/j.1540-8175.2010.01300.x. [DOI] [PubMed] [Google Scholar]
- 20.Mohan J.C., Bhargava M., Agrawal R., Arora R. Effects of balloon mitral valvuloplasty on left ventricular muscle function. Int J Cardiol. 1995;49:17–24. doi: 10.1016/0167-5273(94)02272-k. [DOI] [PubMed] [Google Scholar]
- 21.Mohan J.C., Agrawal R., Arora R., Khalilullah M. Atrial contribution to left ventricular filling in mitral stenosis: effects of balloon mitral valvuloplasty. Indian Heart J. 1994;46:129–132. [PubMed] [Google Scholar]
- 22.Pan J.P., Chen C.Y., Hsu T.L., Wang S.P., Chiang B.N., Chang M.S. Response of left ventricular ejection performance following balloon valvuloplasty in patients with mitral stenosis. Zhonghua Yi Xue Za Zhi (Taipei) 1992;49:303–312. [PubMed] [Google Scholar]


