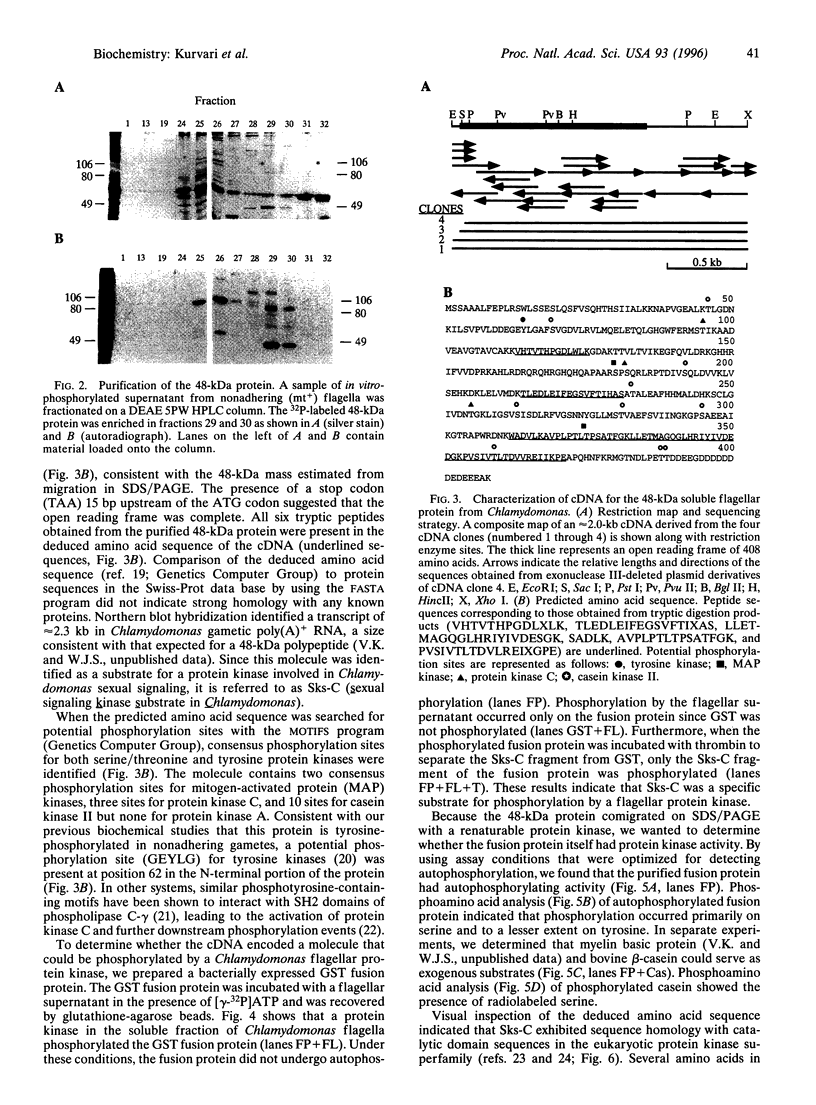
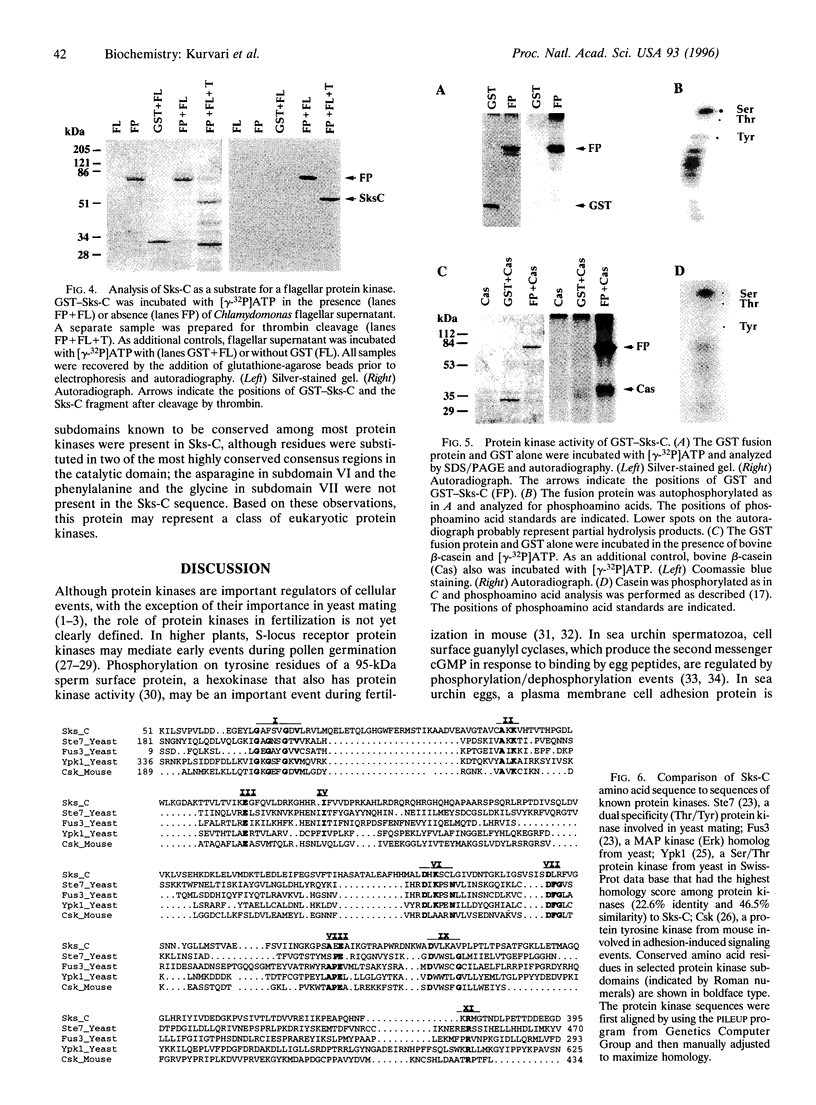
Abstract
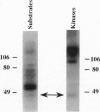
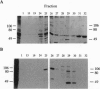
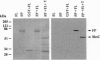
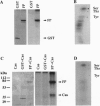
Fertilization in Chlamydomonas is initiated by adhesive interactions between gametes of opposite mating types through flagellar glycoproteins called agglutinins. Interactions between these cell adhesion molecules signal for the activation of adenylyl cyclase through an interplay of protein kinases and ultimately result in formation of a diploid zygote. One of the early events during adhesion-induced signal transduction is the rapid inactivation of a flagellar protein kinase that phosphorylates a 48-kDa protein in the flagella. We report the biochemical and molecular characterization of the 48-kDa protein. Experiments using a bacterially expressed fusion protein show that the 48-kDa protein is capable of autophosphorylation on serine and tyrosine and phosphorylation of bovine beta-casein on serine, confirming that the 48-kDa protein itself has protein kinase activity. This protein kinase exhibits limited homology to members of the eukaryotic protein kinase superfamily and may be an important element in a signaling pathway in fertilization.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abassi Y. A., Foltz K. R. Tyrosine phosphorylation of the egg receptor for sperm at fertilization. Dev Biol. 1994 Aug;164(2):430–443. doi: 10.1006/dbio.1994.1213. [DOI] [PubMed] [Google Scholar]
- Burks D. J., Carballada R., Moore H. D., Saling P. M. Interaction of a tyrosine kinase from human sperm with the zona pellucida at fertilization. Science. 1995 Jul 7;269(5220):83–86. doi: 10.1126/science.7541556. [DOI] [PubMed] [Google Scholar]
- Cairns B. R., Ramer S. W., Kornberg R. D. Order of action of components in the yeast pheromone response pathway revealed with a dominant allele of the STE11 kinase and the multiple phosphorylation of the STE7 kinase. Genes Dev. 1992 Jul;6(7):1305–1318. doi: 10.1101/gad.6.7.1305. [DOI] [PubMed] [Google Scholar]
- Cobb M. H., Goldsmith E. J. How MAP kinases are regulated. J Biol Chem. 1995 Jun 23;270(25):14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Martin G. S. Identification of a 42-kilodalton phosphotyrosyl protein as a serine(threonine) protein kinase by renaturation. Mol Cell Biol. 1990 Jun;10(6):3020–3026. doi: 10.1128/mcb.10.6.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbers D. L. Guanylyl cyclase receptors and their endocrine, paracrine, and autocrine ligands. Cell. 1992 Oct 2;71(1):1–4. doi: 10.1016/0092-8674(92)90258-e. [DOI] [PubMed] [Google Scholar]
- Garbers D. L., Koesling D., Schultz G. Guanylyl cyclase receptors. Mol Biol Cell. 1994 Jan;5(1):1–5. doi: 10.1091/mbc.5.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough U. W. Green yeast. Cell. 1992 Aug 21;70(4):533–538. doi: 10.1016/0092-8674(92)90424-b. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991 Feb 1;192(2):262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Herrero P., Fernández R., Moreno F. The hexokinase isoenzyme PII of Saccharomyces cerevisiae ia a protein kinase. J Gen Microbiol. 1989 May;135(5):1209–1216. doi: 10.1099/00221287-135-5-1209. [DOI] [PubMed] [Google Scholar]
- Kalab P., Visconti P., Leclerc P., Kopf G. S. p95, the major phosphotyrosine-containing protein in mouse spermatozoa, is a hexokinase with unique properties. J Biol Chem. 1994 Feb 4;269(5):3810–3817. [PubMed] [Google Scholar]
- Kamps M. P. Determination of phosphoamino acid composition by acid hydrolysis of protein blotted to Immobilon. Methods Enzymol. 1991;201:21–27. doi: 10.1016/0076-6879(91)01005-m. [DOI] [PubMed] [Google Scholar]
- Klages S., Adam D., Class K., Fargnoli J., Bolen J. B., Penhallow R. C. Ctk: a protein-tyrosine kinase related to Csk that defines an enzyme family. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2597–2601. doi: 10.1073/pnas.91.7.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurjan J. Pheromone response in yeast. Annu Rev Biochem. 1992;61:1097–1129. doi: 10.1146/annurev.bi.61.070192.005313. [DOI] [PubMed] [Google Scholar]
- Maurer R. A. Isolation of a yeast protein kinase gene by screening with a mammalian protein kinase cDNA. DNA. 1988 Sep;7(7):469–474. doi: 10.1089/dna.1.1988.7.469. [DOI] [PubMed] [Google Scholar]
- Moore K. L., Kinsey W. H. Identification of an abl-related protein tyrosine kinase in the cortex of the sea urchin egg: possible role at fertilization. Dev Biol. 1994 Aug;164(2):444–455. doi: 10.1006/dbio.1994.1214. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Pearson R. B., Kemp B. E. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarmby L. M., Hartzell H. C. Dissection of eukaryotic transmembrane signalling using Chlamydomonas. Trends Pharmacol Sci. 1994 Sep;15(9):343–349. doi: 10.1016/0165-6147(94)90029-9. [DOI] [PubMed] [Google Scholar]
- SAGER R., GRANICK S. Nutritional control of sexuality in Chlamydomonas reinhardi. J Gen Physiol. 1954 Jul 20;37(6):729–742. doi: 10.1085/jgp.37.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Small L., Goodenough U. W. Activation of adenylyl cyclase in Chlamydomonas reinhardtii by adhesion and by heat. J Cell Biol. 1993 Jul;122(1):137–147. doi: 10.1083/jcb.122.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell W. J. Mating in Chlamydomonas: a system for the study of specific cell adhesion. I. Ultrastructural and electrophoretic analyses of flagellar surface components involved in adhesion. J Cell Biol. 1976 Jan;68(1):48–69. doi: 10.1083/jcb.68.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z., Shoelson S. E., Chaudhuri M., Gish G., Pawson T., Haser W. G., King F., Roberts T., Ratnofsky S., Lechleider R. J. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993 Mar 12;72(5):767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- Stein J. C., Howlett B., Boyes D. C., Nasrallah M. E., Nasrallah J. B. Molecular cloning of a putative receptor protein kinase gene encoded at the self-incompatibility locus of Brassica oleracea. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8816–8820. doi: 10.1073/pnas.88.19.8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias C. M., Howlett B., Nasrallah J. B. An Arabidopsis thaliana Gene with Sequence Similarity to the S-Locus Receptor Kinase of Brassica oleracea: Sequence and Expression. Plant Physiol. 1992 May;99(1):284–290. doi: 10.1104/pp.99.1.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. C., Zhang R. Relationship of a putative receptor protein kinase from maize to the S-locus glycoproteins of Brassica. Nature. 1990 Jun 21;345(6277):743–746. doi: 10.1038/345743a0. [DOI] [PubMed] [Google Scholar]
- Witman G. B., Carlson K., Berliner J., Rosenbaum J. L. Chlamydomonas flagella. I. Isolation and electrophoretic analysis of microtubules, matrix, membranes, and mastigonemes. J Cell Biol. 1972 Sep;54(3):507–539. doi: 10.1083/jcb.54.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Zhang F., Ebert D., Cobb M. H., Goldsmith E. J. Activity of the MAP kinase ERK2 is controlled by a flexible surface loop. Structure. 1995 Mar 15;3(3):299–307. doi: 10.1016/s0969-2126(01)00160-5. [DOI] [PubMed] [Google Scholar]
- Zhang Y. H., Ross E. M., Snell W. J. ATP-dependent regulation of flagellar adenylylcyclase in gametes of Chlamydomonas reinhardtii. J Biol Chem. 1991 Dec 5;266(34):22954–22959. [PubMed] [Google Scholar]
- Zhang Y., Snell W. J. Differential regulation of adenylylcyclases in vegetative and gametic flagella of Chlamydomonas. J Biol Chem. 1993 Jan 25;268(3):1786–1791. [PubMed] [Google Scholar]
- Zhang Y., Snell W. J. Flagellar adhesion-dependent regulation of Chlamydomonas adenylyl cyclase in vitro: a possible role for protein kinases in sexual signaling. J Cell Biol. 1994 May;125(3):617–624. doi: 10.1083/jcb.125.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Geer P., Hunter T., Lindberg R. A. Receptor protein-tyrosine kinases and their signal transduction pathways. Annu Rev Cell Biol. 1994;10:251–337. doi: 10.1146/annurev.cb.10.110194.001343. [DOI] [PubMed] [Google Scholar]