Abstract
Colorectal cancer is one of the most common malignancy in the world and the second cancer-related death, many molecular and genetic aspects of this disease have been cleared as chromosomal instability and the role of some key proteins as WNT/β catenin, trypsin and others. Also recently the role of folate turnover and some neurotransmitters as serotonin were also considered. The scope of this review is to describe some details about new molecular pathways suggested for occurrence or progress of this disease.
Keywords: Colorectal cancer, Molecular pathology, WNT protein, Trypsin, Serotonin
Introduction
Colorectal cancer disease (CRC) is the fourth most common malignancy and the second cause of cancer-related deaths. For example only in 2008, 148,810 cases of colorectal cancer have been diagnosed and 49,960 were died from this malignancy (1). Considerable progress in curing this disease has been achieved during 12 the past years, and some new therapeutic agents have been introduced by which the survival rate of patients has been improved (1). Pathologic stage represents the most important prognostic factor for patients with colorectal cancer. According to tumor-node-metastasis (TNM) system, as defined by the American Joint Committee on Cancer, considering regional lymph node involvement, and presence of distant sites of disease (2, 3), the stage of disease is divided from T1 (invasion of the submucosa) to T4 (invasion into the serosa or adjacent structures) for tumors and N0 to N3 for nodes involvement and M0 to M1 for metastasis (Table 1). In patients with resectable colorectal cancer, many other pathologic and clinical aspects have been clarified that are associated with an increased risk for tumor recurrence. These include poorly differentiated histology, lymphovascular invasion, perineural invasion, T4 tumor penetration, bowel perforation, clinical bowel obstruction and an increased preoperative plasma level of carcinoembryonic antigen (4, 5). During recent years, some advances have been happening in the treatment of CRC (colorectal cancer) and control of its' metastatic stages. The first line for the treatment of this malignancy is surgery. Patients with obstruction, perforation and/ or expression of some biomarkers such as hetrozigocity in chromosome q 18 or mutation in TGF-β gene need chemotherapy after surgery. The metastatic patients have some limitations in treatment and their treatments with current methods are difficult and they are recommended for surgery and are given some palliative chemotherapy (6).
Table 1.
Classification of stages of CRC according to tumor-node -metastasis (TNM) system
| T0 | Not present any lesion |
| Tis | Invasion limited to mucosa |
| T1 | Invasion to submucosa |
| T2 | Invasion to muscularis properia |
| T3 | Invasion into subserosa or fats around colon |
| T4 | Invasion into serosa and adjacent structure |
| N0 | Not any invasion into the nodes |
| N1 | 1 to 3 nodes around the colon are positives |
| N2 | 4 nodes or more around the colon are positives |
| N3 | Any node as long as a blood vessel is positive |
| M0 | Not present any metastases |
| M1 | Presence of any distal metastases |
Microsatellite instability and loss of heterozygosity
Microsatellite instability and loss of heterozygosity at chromosome q18 are the 2 best-defined molecular prognostic markers (7) (Table 2). Microsatellite instability results from mutations or promoter hypermethylation of DNA mismatch repair genes leading to errors in DNA replication and changes in short and repeated sequences of DNA. It is present in the most majority of tumors from patients with hereditary nonpolyposis colon cancer, but also is found in 15 to 20% of patients with sporadic colon cancer (7, 8). Patients with tumors possessing a high degree of microsatellite instability have a more favorable prognosis than those patients whose tumors are microsatellite stable (8, 9). Loss of heterozygosity at chromosome q18 has been reported in approximately 50% of colon cancers and has been associated with a worse prognosis (9, 10).
Table 2.
Prognostic markers for CRC
| Microsatellite instability | >Mutations or promoter hypermethylation of DNA mismatch repair genes | >Errors in DNA replication and changes in short, repeated sequences of DNA |
| loss of heterozygosity | >Loss of heterozygosity at chromosome q18 | >has been reported in approximately 50% of colon cancers>worse prognosis |
Metastases
The progression of tumor beyond the colorectal and regional lymph nodes defines the M stage of the American Joint Committee on Cancer classification system, with M1 indicating the presence of tumor metastases to distant sites. Approximately 20% of patients with metastatic disease and 30% to 40% of patients with localized disease ultimately develop metastases. The liver reflects the most common initial site of disease spread, but metastases to other organs during the course of the disease are common, including lungs, peritoneum and intra-abdominal lymph nodes. Patients with a small number of isolated, organ-confined metastases may be cured of their disease by surgical resection; many patients with metastatic disease are candidates for systemic chemotherapy to palliate symptoms and prolong life (11). As the stage of disease increases from stage I to stage IV, the year overall survival rates declines dramatically: stage I,greater than 90%; stage II, 70%–85%; stage III, 25%–80%;and stage IV, less than 10% (12, 13).
Molecular pathology from stem cells to cancer
Stem cells of intestine are localized towards the bottom of the crypt in the large intestine, and superior to the Paneth cells in the small intestine (14). Their differentiating progeny migrate upwards through the transit amplifying zone in the lower-to-middle region of the crypt, before becoming terminally differentiated, and are eventually led into the lumen (15). The Paneth cell is one of five cell types into which the small intestinal epithelial stem cell can differentiate; the other cell types are the absorptive enterocyte, hormone secreting endocrine cells, mucus producing goblet cells and ‘M’ cells. M cells are involved in the transport of antigens from the gut lumen into Peyer’s patche (16).
Regulation of intestinal stem cells functions by WNT pathways
WNT/ β-catenin pathway is important for regulating gut epithelial stem cell function. WNT genes encode a large family of secreted molecules that have important roles in the regulation of proliferation and apoptosis of epithelial cells. In humans, there are at least 19 members of the WNT family and at least 10 members of its receptor family, FZ (frizzled) (17). The Wnt receptor complex is combined with FZ plus LRP5 and LRP6 (LDL receptor related proteins), which are homologs of the LDL receptor (17) binding of WNT to FZ activates one of two pathways (Table 3): the canonical pathway, which involves β-catenin (Figure 1) and controls cellular proliferation; and the planar pathway, which involves Ca2+ and is important in cellular movement and polarity (18).
Table 3.
WNT signaling pathway in pathophysiology of colon cancer
| Canonical | Involves β-Catenin | Controls cell proliferation |
| Planar | Involves Ca++ | Control cellular movement and polarity |
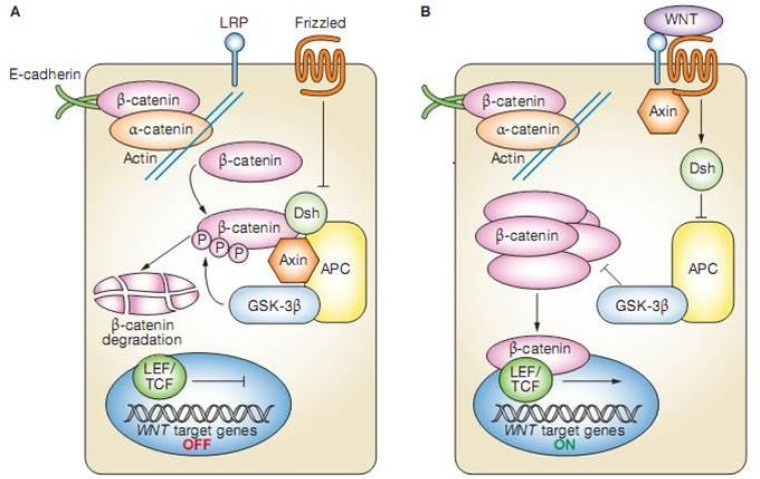
Figure 1.
The Wnt signaling pathway. (A) In the absence of WNT binding to frizzled,dishevelled remains unactivated and β-catenin is targeted for degradtion via the adenomatous polyposis coli/axin/glycogen-synthase-kinase 3β pathway. (B) When Wnt binds to frizzled(right), dishevelled is activated and uncouples β-catenin from the adenomatous polyposis coli; β-catenin can then associate with transcription factors and mediate transcription of the target gene. PC, adenomateous polyposis coli; Dsh, dishevelled; GSK-3β, glycogen synthase kinase 3β; LEF, lymphoid enhancer-binding factor, LRP, LDL-receptor-related protein; P, phosphorylation; TCF, T-cell factor (51).
Briefly, in the canonical pathway, in the absence of WNT, cytosolic β-catenin, which is normally bound to membranous E-cadherin, interacts with the ‘destruction complex’, which is composed of the tumor-suppressor protein APC (adenomatous polyposis coli), glycogen synthase kinase 3β and axin (19). This results in serine phosphorylation of β-catenin, its recognition by an E3 ubiquitin ligase, and its degradation (19). But if WNT is present, it couples with FZ receptor then the kinase activity of the destruction complex is blocked, and β-catenin remains unphosphorylated, resulting in accumulation of β-catenin in the nucleus (19). This enables β-catenin to bind to the transcription factor TCF4 that can activate downstream target genes such as the protooncogene MYC that promotes entry of the cell into the S-phase of the cell cycle (20). Data indicate that the TCF4/β-catenin pathway also has a role in the maintenance of intestinal progenitor cells in adult mammalian crypts. According to Van de Wetering and colleagues' reports of a DNA microarray study in human colon adenocarcinoma cell lines that had inducible dominant-negative TCF4 mutations: by inhibiting TCF4–β-catenin complex formation, growth was halted in the G1 phase of the cell cycle. It is possible that the TCF4–β-catenin complex acts as a master switch that controls the balance of proliferation and differentiation in healthy and malignant intestinal epithelial cells (19).
Cancer and stem cells
Cancer is the disease of stem cells, because only stem cells have the ability to self-renew and neoplasia is essentially dysregulated of self renewal (21). In addition, stem cells are one of the few cell types that are sufficiently long-lived to acquire the necessary number of sequential mutations to convert a cell from the normal to malignant state (21). Tumors themselves probably contain stem cells—so-called cancer stem cells (CSCs) (22).
In a heterogeneous population of cells, it is only CSCs that have the ability to self-renew and therefore, sustain and increase the tumor cell population. It was not until more recently that CSCs were described in the NOD/SCID (nonobese diabetic/severe combined immunodeficient) mouse model by Dick and colleagues (23). As yet, CSCs have not been isolated from colorectal cancers but have been successfully isolated from some breast and brain tumors (24–26).
Trypsin in colorectal cancer
Recently, it has been shown that Trypsin (a digestive enzyme) has considerable role in developing of neoplasia, and invasion and metastasis in several cancers (27, 28). Trypsin has the potential as a prognosticator (29, 30) and patients with trypsin-positive colorectal cancers (CRCs) have shorter overall and disease-free survival than patients with trypsin-negative CRC (28). However, the biological mechanisms by which trypsin contributes to the poor outcome in cancer in general and in CRC in particular, are not well cleared.
Trypsin is one of characterized serine proteinases. Proteinases play essential roles not only in many physiological processes (eg food digestion, blood coagulation, fibrinolysis, and control of blood pressure) but also in a wide range of important pathological processes (eg atherosclerosis, inflammation and cancer) (31, 32). Trypsin exhibits selective proteolytic activity against the peptide bonds in protein molecules that have carboxyl groups donated by the amino acids arginine and lysine. For physiological protection against premature activity, as known from pancreatic physiology, trypsin is secreted as an inactive zymogen (trypsinogen) in the pancreatic juice, and is activated by conversion to trypsin by an enteropeptidase in the alkaline milieu of the duodenal lumen. Secondly, trypsinogen may be activated into active trypsin by an enteropeptidase found in duodenal enterocytes (33, 34). Interestingly,adenocarcinoma cells of the duodenum (32), as well as other tissues expressing trypsin, have a trypsin-activating enteropeptidase (29, 30). Lastly, the antiproteinase mediator pancreatic secretory trypsin inhibitor (PSTI) protects from premature activity. An imbalance in this ‘proteinase–antiproteinase-system’ plays a pathophysiological role in the development of pancreatitis and seems to present an increased risk for developing pancreatic adenocarcinoma (29–31) PSTI is excreted by the mucosa of the normal gastrointestinal tract, where it serves to protect the cells from proteolytic breakdown. The same peptide is secreted by tumour cells, and is often referred to as ‘tumour associated trypsin inhibitor’ (TATI), which is identical to PSTI. Trypsin expression is increased in human cancer cells of the ovary, prostate, lung, stomach, colon, and others. Trypsin in cancer is often referred to as ‘tumour-associated trypsin’, and generally represents trypsin-1 and trypsin-2 (35). The contributing role of the tumor environment and its constituents (ie stroma cells, signal molecules, matrix enzymes) in the invasive and metastatic process is emerging (Figure2, 3) (36). Recently evidence declare that the adverse effects of trypsin are mediated through interplay with other proteinases systems (Figure 3), such as the MMPs (37) and the recently explored PAR-2 (37–40). The current a mechanism by which trypsin induces invasion and metastasis are manifold. Firstly, as a proteolytic enzyme, trypsin may directly degrade extracellular proteins by itself, by attacking type 1 collagen of the basal membrane (39). Secondly, its effect is likely to be mediated indirectly through the activation and effect of other latent proteolytic cascades, the most important being the MMPs. Lastly, recent evidence points to the activation of signal molecules, such as PAR-2.
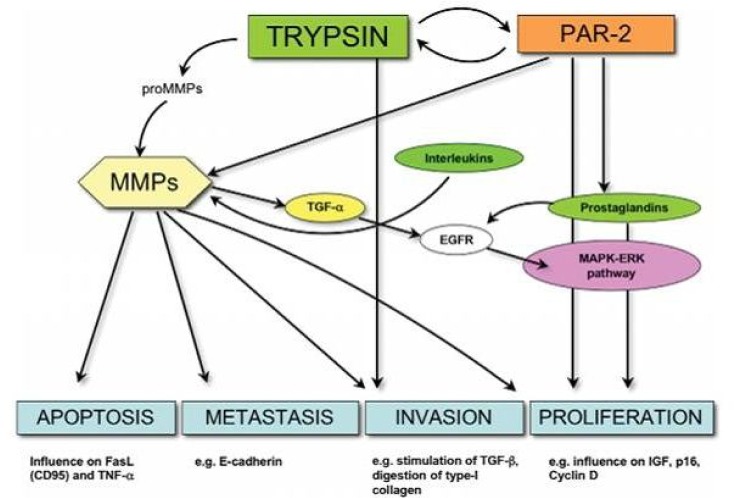
Figure 3.
Simplified model showing current knowledge of trypsin interaction with proteinase-activated receptor 2 (PAR-2) and the matrix metalloproteinase (MMPs). The arrows indicate activation (52).
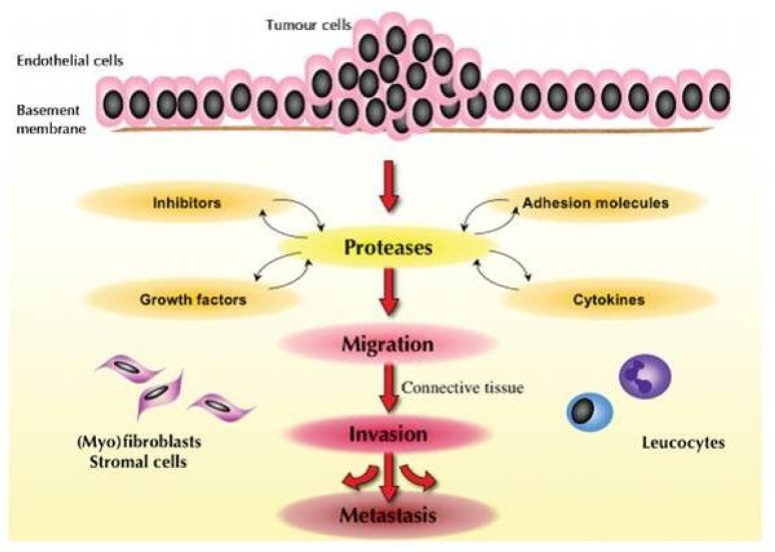
Figure 2.
Ggeneral model illustrating the stepwise influence of proteinase systems and related co-factors from tumour cells, extracellular matrix and immune system on cancer invasion and metastasis. Proteinases, such as trypsin, may play a major role in this process by direct and indirect activation of other proteinase cascades and related factors (52).
Overexpression of trypsin associated with poor prognosis in CRC patients has been reported with several evidences. Yamamoto et al. found that trypsin Immunorectivity was more intense at the invasive front than in the superficial part of the tumor (28). Trypsin positivity (defined as> 30% positive cells at the invasive front of CRCs) correlated significantly with the depth of invasion, lymphatic and venous invasion, lymph node and distant metastasis, advanced pathological tumor-node-metastasis stage, as well as recurrence. Trypsin activates matrilysin (MMP-7), which plays an important role in CRC progression, and may explain the adverse effect of trypsin on CRC prognosis.
Trypsin as a target for the therapy of cancer cells show as an analogy to the natural protective mechanisms of trypsin by expressing TATI (Tumor associated trypsin inhibitor) at increased levels in CRCs compared with adjacent normal mucosa (41). This suggests that an innate protective mechanism occurs even in tumors (42). Although TATI is highly co-expressed in CRC, experimental inhibition of trypsin with TATI is only partially successful (43), and TATI is not a useful tumor marker compared with carcinoembryonic antigen. Inhibition of trypsin-mediated invasion is feasible with derivatives of tetracycline (43). However, trypsin interacts with other cascades, making single targeted inhibition difficult and probably ineffective (Table 4).
Table 4.
Role of trypsin in colorectal cancer
| Trypsin, a digestive enzyme has considerable role in developing of neoplasia, invasion and metastasis | > Has potential as a prognosticator | > trypsin-positive(CRCs) : shorter overall and disease-free survival |
| PSTI excreted by the mucosa of the normal gastrointestinal tract, serves to protect the cells from proteolytic breakdown | > The same peptide secreted by tumour cells, and is often referred to as ‘tumour associated trypsin inhibitor’ (TATI), which is identical to PSTI | |
| Trypsin expression in human cancer cells of the ovary, prostate, lung, stomach, colon, and others | >tumor-associated trypsin’, generally represents trypsin-1 and trypsin-2 | |
| Recently evidences: adverse effects of trypsin are mediated through MMPs and the recently explored PAR-2 | > as a proteolytic enzyme, directly degrade extracellular proteins by itself, by attacking type 1 collagen of the basal membrane. | > indirectly through the activation other latent proteolytic cascades, the most important, the MMPs. Lastly, activation of signal molecules, such as PAR-2 |
| Trypsin activates Matrilysin(MMP-7) | > plays an important role in CRC progression, may explain the adverse effect of trypsin on CRC prognosis | |
| Trypsin as a target for therapy of cancer cells | >Natural protective mechanisms of trypsin by expressing TATI (Tumor associated trypsin inhibitor) > increased levels in CRCs compared with adjacent normal mucosa (Innate protective mechanism occurs even in tumors). | > Although TATI is highly coexpressed in CRC, experimental inhibition of trypsin with TATI is only partially successful. |
Polymorphisms in the one carbonated metabolic pathway
In recent years it has been shown that one-carbon (e.g., folate) metabolism has a crucial role in the etiology of colorectal cancer (CRC). Cytosolic serine hydroxymethyltransferase (cSHMT), methylenetetrahydrofolate dehydrogenase (MTHFD1) and glutamate carboxypeptidase (IIGCPII) are key genes involved in this pathway (44). Low intake or low levels of circulating folate, a key component of one-carbon metabolism, has been consistently associated with increased risk of colorectal cancer (CRC) (45, 46). Functional polymorphisms in folate-metabolizing genes are capable of modifying the risk of CRC as shown by (Jia Chen et al) (46) studies on 2 such genes, the methylenetetrahydrofolate reductase (MTHFR) and the thymidylate synthase (TS). Specifically, it was reported that the 677TT genotype of the MTHFR 677C3T polymorphism (47, 48) and the 2R/2R genotype of the tandem repeated polymorphism in the TS promoter (48) were associated with reduced risk of CRC. The TS 2R/2R genotype, associated with low TS expression, was also associated with better survival after CRC diagnosis (48). Additionally, both polymorphisms modified plasma folate and total homocysteine (tHcy) levels. Serine hydroxymethyltransferase (SHMT), methylenetetrahydrofolate dehydrogenase (MTHFD1) and glutamate carboxypeptidase II (GCPII) are 3 of the constellation of genes involved in folate-dependent one-carbon metabolism. Several polymorphisms in these genes have recently been clarified and their functionality has also been implicated.
Role of serotonin and serotonin receptors in CRC
Serotonin (5-hydroxytryptamine, 5-HT) is a neurotransmitter that mediates a wide variety of physiological effects including peripheral and central action through the binding of multiple receptor subtypes. Mitogenic effects of serotonin receptors in prostate, bladder and breast cancer, especially 5-HT1A-D and 5HT2A receptors had been shown before. Recently we have shown that also 5HT can have mitogenic role in CRC and role of 5HT1B receptors is with much more importance (49–51). It was suggested that mitogenic role of serotonin receptors in cancer is via the activation of (Phospholipase C-β/protein Kinase C) and also the activation of MAPKinase and extracellular Kinase controlled with signal, which can induce some intracellular phosphorylation events leading to the activation of some transcription factors as TCF4 (49, 50).
Conclusion
According to a recent review about pathophysilology of colorectal cancer we can consider this malignancy as a multifactorial disease in which many biochemical and genetic factors are involved and the role of stem cells and their regulatory proteins as WNT/β catenin are with importance and some chromosomal and genetic mutations as microsatellite instability and loss of heterozygosity at chromosome 18q are associated with poor prognosis. Also we can consider a correlation between trypsin overexpression and matrilysin protein activation with CRC progression. Recently the role of folate turnover and serotonin receptors and their signaling pathways are also considered. Our knowledge about accurate pathophysiology of this disease is incomplete and needs more investigations and more molecular studies especially on intracellular and extracellular pathways and intermediate proteins. This disease is correlated closely with our diets and with our living habbits; as with much more exercises and better diet regimes (low fat and free radicals foods and high bran, folate and antioxidants diets) we can prevent much better this malignancy evenly in old ages.
Acknowledgements
We appreciate very much the efforts of Effat Hayati and Sara Ghobadi for their collaboration in collecting and arranging informations and the editing of article.
(Please cite as: Ziapour P, Ataee R, Shadifar R, Vaillancourt C, Ahmadi A, Jafari -Sabet M, et al. New intracellular and molecular aspects in pathophysiology of colorectal cancer. Gastroenterology and Hepatology From Bed to Bench 2011;4(2):43-52).
Future Perspectives
As role of folate and antioxidants have been shown effective in the prevention of colorectal cancer, it can be suggested to use these agent especially curcumin and melatonin as potent antioxidants in diets regimes for high risk people. Also research developments on trypsin inhibitors and monoclonal antibodies against carcinoemberyonic antigen and anti serotonergic drugs can be novel approaches in chemotherapy of CRC. Further researches on stem cells for exploring other genetical and molecular signaling pathways are important for better understanding of molecular pathophysiology of this malignancy.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Greene F, Page DL, Fleming ID, Fritz A, Haller DJ, Morrow M, et al., editors. AJCC cancer staging handbook. 6th ed. New York: Springer; 2002. [Google Scholar]
- 3.Greene FL, Stewart AK, Norton HJ. A new TNM staging strategy for node-positive (stage III) colon cancer: an analysis of 50,042 patients. Ann Surg. 2002;236:416–21. doi: 10.1097/00000658-200210000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Compton C, Fenoglio-Preiser CM, Pettigrew N, Fielding LP. American Joint Committee on Cancer Prognostic Factors Consensus Conference: Colorectal Working Group. Cancer. 2000;88:1739–57. doi: 10.1002/(sici)1097-0142(20000401)88:7<1739::aid-cncr30>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 5.Wanebo HJ, Rao B, Pinsky CM, Hoffman RG, Stearns M, Schwartz MK, et al. Preoperative carcinoembryonic antigen level as prognostic indicator in colorectal cancer. N Engl J Med. 1978;299:448–451. doi: 10.1056/NEJM197808312990904. [DOI] [PubMed] [Google Scholar]
- 6.Lurje G, Zhang W, Lenz HJ. Molecular prognostic markers in locally advanced colon cancer. Clin Colorectal Cancer. 2007;6:683–90. doi: 10.3816/CCC.2007.n.037. [DOI] [PubMed] [Google Scholar]
- 7.Gryfe R, Kim H, Hsieh ET, Aronson MD, Holowaty EJ, Bull SB, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med. 2000;342:69–77. doi: 10.1056/NEJM200001133420201. [DOI] [PubMed] [Google Scholar]
- 8.Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N Engl J Med. 2005;352:1851–60. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe T, Wu TT, Catalano PJ, Ueki T, Satriano R, Haller DG, et al. Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N Engl J Med. 2001;344:1196–206. doi: 10.1056/NEJM200104193441603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313–27. doi: 10.1200/JCO.2006.08.2644. [DOI] [PubMed] [Google Scholar]
- 11.Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–46. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 12.O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96:1420–25. doi: 10.1093/jnci/djh275. [DOI] [PubMed] [Google Scholar]
- 13.Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005;352:476–87. doi: 10.1056/NEJMra040958. [DOI] [PubMed] [Google Scholar]
- 14.Mayhall EA, Paffett-Lugassy N, Zon LI. The clinical potential of stem cells. Curr Opin Cell Biol. 2004;16:713–20. doi: 10.1016/j.ceb.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Lanza RP. Handbook of stem cells: adult and fetal stem cells. Burlington: Elsevier Academic Press; 2004. [Google Scholar]
- 16.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. III. Entero-endocrine cells. Am J Anat. 1974;141:503–19. doi: 10.1002/aja.1001410405. [DOI] [PubMed] [Google Scholar]
- 17.Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- 18.Leedham SJ, Brittan M, McDonald SA, Wright NA. Intestinal stem cells. J Cell Mol Med. 2005;9:11–24. doi: 10.1111/j.1582-4934.2005.tb00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, et al. The β-catenin/ TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–50. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 20.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 21.Radtke F, Clevers H. Self-renewal and cancer of the gut: two sides of a colon. Science. 2005;307:1904–1909. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- 21.Pinto D, Clevers H. WNT, stem cells and cancer in the intestine. Biol Cell. 2005;97:185–96. doi: 10.1042/BC20040094. [DOI] [PubMed] [Google Scholar]
- 22.Dick JE, O’Brien CA, Pollett A, Gallinger S. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–10. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 23.Bhatia M, Wang JC, Kapp U, Bonnet D, Dick JE. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc Natl Acad Sci USA. 1997;94:5320–25. doi: 10.1073/pnas.94.10.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clarke RB. Isolation and characterization of human mammary stem cells. Cell Prolif. 2005;38:375–86. doi: 10.1111/j.1365-2184.2005.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galderisi U, Cipollaro M, Giordano A. Stem cells and brain cance. Cell Death Differ. 2006;13:5–11. doi: 10.1038/sj.cdd.4401757. [DOI] [PubMed] [Google Scholar]
- 26.Paju A, Vartiainen J, Haglund C, Itkonen O, von Boguslawski K, Leminen A, et al. Expression of trypsinogen-1, trypsinogen- and tumor-associated trypsin inhibitor in ovarian cancer:prognostic study on tissue and serum. Clin Cancer Res. 2004;10:4761–68. doi: 10.1158/1078-0432.CCR-0204-03. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto H, Iku S, Adachi Y, Imsumran A, Taniguchi H, Nosho K, et al. Association of trypsin expression with tumour progression and matrilysin expression in human colorectal cancer. J Pathol. 2003;199:176–84. doi: 10.1002/path.1277. [DOI] [PubMed] [Google Scholar]
- 28.Solakidi S, Tiniakos DG, Petraki K, Stathopoulos GP, Markaki I, Androulakis G, et al. Co-expression of trypsin and tumour-associated trypsin inhibitor (TATI) in colorectal adenocarcinomas. Histol Histopathol. 2003;18:1181–88. doi: 10.14670/HH-18.1181. [DOI] [PubMed] [Google Scholar]
- 29.Williams SJ, Gotley DC, Antalis TM. Human trypsinogen incolorectal cancer. Int J Cancer. 2001;93:67–73. doi: 10.1002/ijc.1304. [DOI] [PubMed] [Google Scholar]
- 30.Nyberg P, Moilanen M, Paju A, Sarin A, Stenman UH, Sorsa T, et al. MMP-9 activation by tumor trypsin-2 enhances in vivo invasion of human tongue carcinoma cells. J Dent Res. 2002;81:831–35. doi: 10.1177/154405910208101207. [DOI] [PubMed] [Google Scholar]
- 31.Bjartell A, Paju A, Zhang WM, Gadaleanu V, Hansson J, Landberg G, et al. Expression of tumor-associated trypsinogens (TAT-1 and TAT-2) in prostate cancer. Prostate. 2005;64:29–39. doi: 10.1002/pros.20236. [DOI] [PubMed] [Google Scholar]
- 32.Aron ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 33.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–70. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 34.Moilanen M, Sorsa T, Stenman M, Nyberg P, Lindy O, Vesterinen J, et al. Tumor-associated trypsinogen-2 (trypsinogen-2)activates procollagenases (MMP-1, -8, -13) and stromelysin-1(MMP-) and degrades type I collagen. Biochemistry. 2003;42:5414–20. doi: 10.1021/bi020582s. [DOI] [PubMed] [Google Scholar]
- 35.Mueller MM, Fusenig NE. Friends or foes bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–49. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 36.Turpeinen U, Koivunen E, Stenman UH. Reaction of a tumour-associated trypsin inhibitor with serine proteinases asso-iated with coagulation and tumour invasion. Biochem J. 1988;254:911–14. doi: 10.1042/bj2540911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishibori M, Mori S, Takahashi HK. Physiology and pathophysiology of proteinase-activated receptors (PARs): PAR-2-mediated proliferation of colon cancer cell. J Pharmacol Sci. 2005;97:25–30. doi: 10.1254/jphs.fmj04005x5. [DOI] [PubMed] [Google Scholar]
- 38.Yada K, Shibata K, Matsumoto T, Ohta M, Yokoyama S, Kitano S. Protease-activated receptor-2 regulates cell proliferation and enhances cyclooxygenase-2 mRNA expression in human pancreatic cancer cells. J Surg Oncol. 2005;89:79–85. doi: 10.1002/jso.20197. [DOI] [PubMed] [Google Scholar]
- 39.Darmoul D, Gratio V, Devaud H, Laburthe M. Protease-activated receptor 2 in colon cancer: trypsin-induced MAPK phosphorylation and cell proliferation are mediated by epidermal growth factor receptor transactivation. J Biol Chem. 2004;279:20927–34. doi: 10.1074/jbc.M401430200. [DOI] [PubMed] [Google Scholar]
- 40.Tomita N, Doi S, Higashiyama M, Morimoto H, Murotani M, Kawasaki Y, et al. Expression of pancreatic secretory trypsin inhibitor gene in human colorectal tumor. Cancer. 1990;66:2144–49. doi: 10.1002/1097-0142(19901115)66:10<2144::aid-cncr2820661017>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 41.Higashiyama M, Monden T, Tomita N, Murotani M, Kawasaki Y, Morimoto H, et al. Expression of pancreatic secretory trypsin inhibitor (PSTI) in colorectal cancer. Br J Cancer. 1990;62:954–58. doi: 10.1038/bjc.1990.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Solakidi S, Dessypris A, Stathopoulos GP, Androulakis G, Sekeris CE. Tumour-associated trypsin inhibitor, carcinoembryonic antigen and acute-phase reactant proteins CRP and alpha1-antitrypsin in patients with gastrointestinal malignancies. Clin Biochem. 2004;37:56–60. doi: 10.1016/j.clinbiochem.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Giovannucci E, Rimm E, Ascherio A, Stampfer M, Colditz G, Willett W. Alcohol, low-methionine: low-folate diets, risk of colon cancer in men. J Natl Cancer Inst. 1995;87:265–73. doi: 10.1093/jnci/87.4.265. [DOI] [PubMed] [Google Scholar]
- 44.Kato I, Dnistrian A, Schwartz M, Toniolo P, Koenig K, Shore R, et al. Serum folate, homocysteine and colorectal cancer risk in women: a nested case - control study. Br J Cancer. 1999;79:1917–22. doi: 10.1038/sj.bjc.6690305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen J, Giovannucci E, Kelsey K, Rimm E, Stampfer M, Colditz G, et al. A methylenetetrahydrofolate reductase polymorphism and the risk of colorectal cancer. Cancer Res. 1996;56:4862–64. [PubMed] [Google Scholar]
- 46.Ma J, Stampfer M, Giovannucci E, Artigas C, Hunter D, Fuchs C, et al. Methylenetetrahydro-folate reductase polymorphism, dietary interactions, risk of colorectal cancer. Cancer Res. 1997;57:1098–102. [PubMed] [Google Scholar]
- 47.Chen J, Hunter D, Stampfer M, Kyte C, Chan W, Wetmur J, et al. A prospective study on relations of a polymorphism in the Thymidylate Synthase Promoter Enhancer Region and the risk and survival of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:953–57. [PubMed] [Google Scholar]
- 48.Ataee R, Ajdary S, Zarrindast M, Rezayat M, Hayatbakhsh MR. Anti-mitogenic and apoptotic effects of 5-HT1B receptor antagonist on HT29 colorectal cancer cell line. J Cancer Res Clin Oncol. 2010;136:1461–69. doi: 10.1007/s00432-010-0801-3. [DOI] [PubMed] [Google Scholar]
- 49.Ataee R, Zarrindast M, Ajdary S, Rezayat M. Study of 5HT3 and 5HT4 receptors expression in HT29 cell line and human colon adenocarcinoma tissues. Arch Iran Med. 2010;13:120–25. [PubMed] [Google Scholar]
- 50.Ataee R, Zarrindast M, Ajdary S, Rezayat M, Ataee A. Y25130 Hydrochloride,a selective 5HT3 receptor antagonist has potent antimitogenic and antiapoptotic effect on HT29 colorectal cancer cell line. Eur J Cancer Prev. 2010;19:138–43. doi: 10.1097/CEJ.0b013e3283354901. [DOI] [PubMed] [Google Scholar]
- 51.McDonald SA, Preston SL, Lovell MJ, Wright NA, Jankowski JA. Mechanisms of disease: from stem cells to colorectal cancer. Nat Clin Pract Gastroenterol Hepatol. 2006;3:267–74. doi: 10.1038/ncpgasthep0473. [DOI] [PubMed] [Google Scholar]
- 52.Soreide K, Janssen EA, Körner H, Baak JP. Trypsin in colorectal cancer: molecular biological mechanisms of proliferation, invasion, and metastasis. J Pathol. 2006;209:147–56. doi: 10.1002/path.1999. [DOI] [PubMed] [Google Scholar]