Abstract
Aim
The purpose of this study is to explore whether the types and quality of breakfast could influence energy levels (blood glucose levels) and propose ideal breakfast models.
Background
It is widely considered that a regular breakfast provides a number of health benefits; however, there is no general scientific agreement regarding what kind of food should be consumed. Evidence supports the importance of balancing blood glucose levels by low glycaemic index/load (L-GI/L) and increased protein diets, in particular in metabolic disorders, which non-alcoholic fatty liver disease (NAFLD) has a close relation to.
Patients and methods
This study was conducted by using a valid and standard questionnaire at the University of Worcester to evaluate the breakfast and dietary habits and energy levels. The Kruskal-Wallis test was used for statistical analysis.
Results
No significant differences were found either between breakfast consumption, energy levels, types of snack and amount of caffeine intake in the morning or between types of breakfast, energy levels, types of snack, and amount of caffeine intake in the morning. However, potential differences in energy levels were found across the groups of breakfast types: glycaemia (GL) (p=.057) and protein intake (p=.056).
Conclusion
The types and quality of breakfast would be key as regular breakfast consumption alone did not show adequate health benefits. Lower GL foods and higher protein intake at breakfast were found to be associated with higher energy levels. It is therefore recommended that breakfast foods should be low in GL and high in protein. These changes may lead to better health status and prevention of disease, especially metabolic and liver disorders, in the long term.
Keywords: Food habits, Blood glucose, Glycaemic index, Diet therapy
Introduction
According to an old phrase, breakfast is considered the most important meal of the day, although it is the meal which is most often missed and the most underestimated (1, 2). This saying has recently acquired scientific support (3). The reported health benefits from regular breakfast consumption include a better nutritional profile (4), reduced body mass index (5), better cognitive functions (6), reduced incidence of chronic degenerating diseases including type 2 diabetes and cardiovascular disease (7), a healthier lifestyle (8), healthier food choices (9), and regular eating and exercise patterns (1).
Despite a large number of studies supporting the importance of breakfast consumption, there is no general scientific agreement as to what kind of food should be consumed for breakfast (10), and few studies have investigated how the types and quality of breakfast influence the health benefits (11). When considering factors leading to the health benefits of breakfast consumption, it could be argued that the influence of types of food on blood glucose levels may be the most important point, since low and slow glucose release is believed to keep the energy levels balanced, preventing ‘energy dips’ as well as providing long satiety between meals (12). The beneficial effects of low glycaemic index/load (LGI/L) foods include improved glucose and lipid metabolism (13, 14), low GI/L foods can increase long-term satiety, reduce hunger and lower subsequent voluntary food intake (15, 16). Nilsson et al. (2008) (17) argue that LGI foods are capable of keeping blood glucose levels lower and stable during the course of a whole day, and thus this could be expected to further add to the beneficial effects of breakfast, providing an ideal ‘nutritional start’ in the morning.
Furthermore, a large amount of research supports the fact that imbalanced blood glucose levels are associated with chronic metabolic disorders. LGI/L diets were shown to reduce fasting and post-prandial insulin, glucose, triacylglycerol, total cholesterol, and non-esterified fatty acid concentrations, and thus this type of diet is considered to be associated with a wide range of benefits with respect to established metabolic risk factors (18–20). Fatty liver disease is now considered to be strongly associated with insulin resistance (21); it has been found to be highly correlated with all the components of metabolic syndrome (22). A concern for non-alcoholic liver disease (NAFLD) is growing in clinical hepatology (23); for example one in four or five American adults are considered to have NAFLD (24). Resent research discovered that high glycaemic index (HGI) foods were related to increased hepatic fat (25–27), and low glycaemic index (LGI)/L diet, emphasising on complex carbohydrates with fibres and moderately high protein intake (15-20%), has shown to be significantly effective in the treatment of the patients with non-alcoholic steatohepatitis (NASH) (28).
However, caution should be exercised in food choices which are solely based on the GI/L, as the foods may be energy dense and contain substantial amount of sugars (sucrose), or undesirable fatty acids which contribute to the reduction of glycaemic response (29, 30). Furthermore, unlike the GL, the GI cannot be solemnly relied on, as the GI and amount of a food eaten are all used to determine the postprandial glycaemic response (30). Therefore, it would appear that the GL concept, which is based on how much carbohydrate there is in a serving (31), may be more straightforward when applied to the public.
Increased protein intake has also been discovered to be associated with improved glycaemic response, resulting in balanced energy levels (32, 33), and protein content in a meal is considered to be key for satiety and appetite regulation (34). Protein source has also been considered to be a determinant of satiating efficacy (35, 36). For example, several human studies found that whey protein increases satiety more than other types of protein, such as casein, soy, and egg albumin (35, 37, 38). This is considered to be due to its quick digestion and absorption which can result in rapid and larger increase in plasma amino acids (39), although this property was found to be associated with a negative effect, a faster release of insulin (40). Since hyperglycaemia and hyperinsulinaemia are both factors of insulin resistance, the insulinotropic component of milk products could be a cause of concern for health (41, 42).
Given the impact of glycaemic level in metabolic and digestive health, the overall aim of this study is to explore whether breakfast consumption and the types and quality of food eaten influence blood glucose levels (energy levels) later in the morning, and formulate recommendations on ideal breakfast models developing from the findings of this empirical work as well as the literature review.
Patients and Methods
The site of this study is the University of Worcester in United Kingdom, and from where a sample population of staff and students was selected. The sample size of this study consisted of a mixture of 93 males and females: 24 males and 69 females; of these 83 were students and the remaining were staff. Two people were withdrawn from the sample due to insufficient data collected. A self-completion structured questionnaire was chosen to extract the data, and this consisted of three parts: part one: demographic questions, part two: questions about breakfast habits and part three: questions about snacking and caffeine intake habits. An overall summary of the participants can be found in Table 1.
Table 1.
An overall summary of the participants
| Categories | Summaries |
|---|---|
| Demographic |
|
| Breakfast |
|
| Weight |
|
| Energy levels Snacking |
|
| Caffeine intake |
|
The GL of breakfast was grouped into low-GL (such as oat porridge, ‘All Bran’, bran flakes, fruits (except bananas) and vegetables, wholemeal pita bread), medium-GL (MGL) (such as ‘Special K’, muesli, wholemeal bread, pastries, ‘Weetabix’, shredded wheat, ‘Cheerios’, bananas) and high-GL (such as white bread, cornflakes, ‘Coco Pops’, ‘Nesquick’), and types of snack into LGL and HGL, according to the GL information (12, 43). The amount of caffeine consumption for each participant was calculated based on the published caffeine content information of each beverage (44, 45).
The Kruskal-Wallis test was used to determine whether there are significant differences among variables. Breakfast consumption habits (regular breakfast eaters, non-regular breakfast eaters, and complete breakfast skippers), the GL of breakfast (low, medium and high), and protein intake (none, one portion, two portions, and three portions) at breakfast were used as categorical independent variables, while energy levels (a 5-point scale), types of snacks (no intake, LGL, and HGL), and the amount of caffeine intake (mg) were used as dependent variables. In this study, statistical analyses were performed by the Statistical Package for the Social Science (SPSS) statistical software package version 14.0 for Windows (46).
Results
The associations between breakfast consumption and variables (energy levels, the GL of snacks consumed and the amount of caffeine intake)
The Kruskal-Willis test did not find statistically significant differences between breakfast consumption and all these variables at the 5% level (energy levels= p=.55, the GL of snacks= p=.56, and the amount of caffeine intake= p=.50).
The association between types (GL) of breakfast and variables
Energy levels
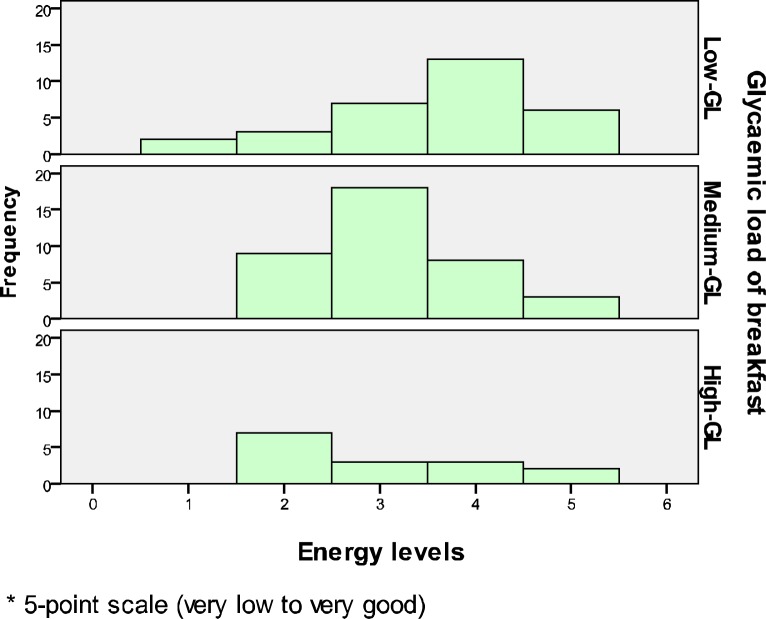
The Kruskal-Willis test found p=.057 (Gp1, n=31: LGL; Gp2, n=38: MGL; Gp3, n=15: HGL), X2 (2, n=84)=5.72, p=.057). This figure is very close to the significant level of p<.05, and thus this suggests that there is a potential difference in energy levels across the three GL groups, although it is not statistically significant enough (Fig. 1).
Figure 1.
The association between GL of breakfast and energy levels
Types (GL) of snack
Although the Kruskal-Willis test found p=.33 (Gp1, n=31: LGL; Gp2, n=38: MGL; Gp3, n=15: HGL), X2 (2, n=84)=2.23, p=.33), the mean ranks (median) show that both an LGL- and MGL-breakfast are the lowest overall ranking, which corresponds to the lowest score of snack groups.
Caffeine intake
The Kruskal-Willis test did not find a statistically significant difference in the amount of caffeine intake across the three levels of GL breakfast (Gp1, n=31: LGL; Gp2, n=38: MGL; Gp3, n=15: HGL), X2 (2, n=84)=3.78, p=.15. However, the mean ranks show that low-GL has the lowest overall ranking which corresponds to the lowest amount of caffeine intake.
The association between protein intake at breakfast
Energy levels
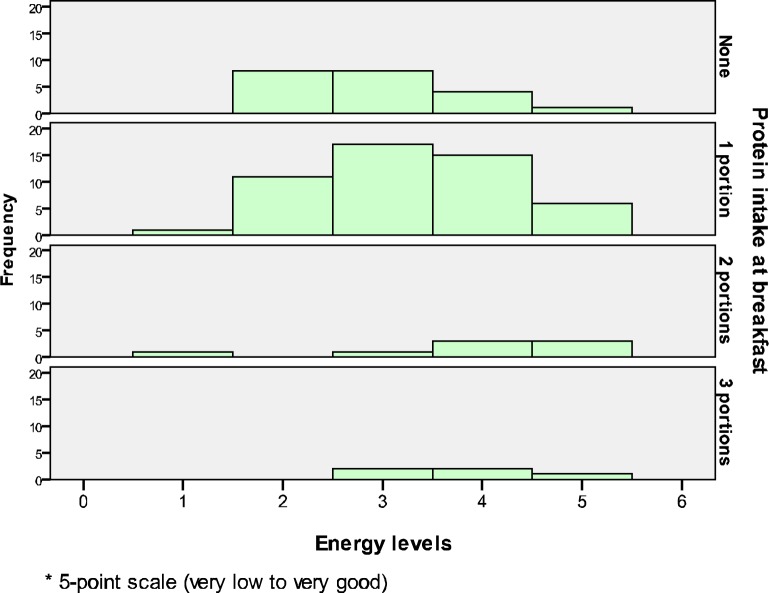
The Kruskal-Willis test found p=.056 (Gp1, n=21: none; Gp2, n=50: 1 portion; Gp3, n=8: 2 portions; Gp4, n=5: 3 portions), X2 (3, n=84) =7.56, p=.056). This figure is very close to the significant level (p<.05), and thus this suggests that there is a potential difference in energy levels across the four groups of protein intake (Fig. 2). The mean ranks (median) show that both two and three portions of protein intake have the highest overall ranking (4.0) which corresponds to the highest score on energy levels.
Figure 2.
The association between protein intake at breakfast and energy levels
Types (GL) of snack
The Kruskal-Willis test did not find a statistically significant difference in GL levels of snacks across the four groups of protein intake (Gp1, n=21: none; Gp2, n=50: 1 portion; Gp3, n=8: 2 portions; Gp4, n=5: 3 portions), X2 (3, n=84) =1.95, p=.58). However, the mean ranks show that protein intake at breakfast, including all three different portions, has the lower overall ranking, which corresponds to a lower GL of snacks than the non-protein intake group.
Amount of caffeine intake
The Kruskal-Willis test did not find a statistically significant difference in the amount of caffeine intake across the four groups of protein intake (Gp1, n=21: none; Gp2, n=50: 1 portion; Gp3, n=8: 2 portions; Gp4, n=5: 3 portions), X2 (3, n=84) =.88, p=.83). However, the mean ranks show that three portions of protein intake at breakfast have the lowest overall rank, which corresponds to the lowest amount of caffeine intake.
To conclude, all the results were not significant at the 5%, however, potential differences in energy levels were found across the groups of breakfast GL (p=.057) and protein intake at breakfast (p=.056). Moreover, trends were also observed in all other sets of associations.
Discussion
The empirical work in this study demonstrated that lower levels of glycaemic load and higher portions of protein intake at breakfast were associated with higher levels of energy, possibly by controlling blood glucose levels. The findings both from this present study and the literature review suggest that the concept of the LGL and increased protein intake are one of the most essential factors to be applied to breakfast, as well as any other meals of the day.
Marsh & Brand-Miller (2008) (47) are of the strong belief that using the GI is fairly easy for most people, as it simply means substituting one HGI food for one LGI food in the same food group, rather than making major dietary changes. The examples can be found in Table 2.
Table 2.
| HGL foods should be switched to | LGL foods |
|---|---|
| Bread – both white (HGL) and wholemeal (MGL) |
|
| Processed breakfast cereals |
|
| Plain biscuits or crackers Cakes and muffins Potato |
|
| Rice – white short grain rice, such as jasmine rice |
|
It could be summarised that, in order to adapt the benefits of the LGI/L, individuals should be advised to increase their consumption of fruit, vegetables and legumes, choose wholegrain products which have been minimally and traditionally processed, such as stone-ground, sourdough, or pumpernickel bread and old-fashioned oatmeal, and limit the intake of potatoes and sugar (48). These recommendations would tend to promote diets high in fibre, micronutrients and antioxidants and low in energy density (12, 47).
As for ideal amount of protein intake, a number of studies suggest that the dietary reference values (DRVs) (15% or 0.75g of protein per kg body weight per day (0.75g/kg/d)) are not adequate, particularly for older adults since a moderately higher protein intake of 1.0-1.3g/kg/d would be required to maintain nitrogen balance, as well as offset decreased protein synthetic efficiency and insulin action (49, 50). Diets with a moderately higher protein intake (20-35% of total energy) have not appeared to be associated with negative health outcomes (51, 52).
Furthermore, de Castro (2004) (53) argues that adults require a minimum of 15g of essential amino acids (AAs) or at least 30g of total protein at each meal to fully stimulate skeletal muscle protein synthesis, and Layman (2009) (54) supports this view. 30g of protein at breakfast appears to be appropriate and thus could be a target amount, as the recommendations above can result in about 90g of protein intake: 19% of daily intake results in 90g of intake if the person is a female aged between 19 and 50 years-old (1900kcalx19%÷4kcal=90.25g).
Moreover, 1.3g/kg/d results in 91g of intake if a person with 70kg of body weight is considered (70×1.3=91g).
The literature review indicates that the choice of good protein foods would be difficult, due to the potential health concerns of cow’s milk. Melnik (2009) (55) also consider its containing active insulin-like growth factor (IGF) 1 and IGF-2 as another health concern because of an enormous impact on the human GH/insulin/IGF-1 axis, disturbing most sensitive hormonal regulatory signalling networks, which has an impact on most chronic diseases in Western societies. These include acne (56), atherosclerosis (57), diabetes (48), obesity (58), cancer (59) and neurodegenerative diseases (60). Furthermore, IGF1 has also been found to be associated with fibrosis and steatosis of non-alcoholic liver disease (NALD) (61).
The problem is that milk and dairy products may be the most commonly consumed protein sources at breakfast due to easy access and the governments’ recommendations, for example in the UK and the USA, in particular for its calcium content (55, 62). However, calcium can be obtained from other foods and more highly absorbed from beans and most greens (40-64%) than milk (32%), and calcium from the fortified products, such as cereals, juice and soy milk, can be absorbed nearly as well as dairy calcium (63).
Therefore, it could be argued that the recommended protein sources include eggs, legumes, nuts/seeds, fish and poultry. The addition of non-dairy protein powder, such as soy, pea and hemp protein, to foods could be useful to boost protein intake (64). The rationales of these choices can be found in Table 3. Table 4 suggests the ideal portion sizes of protein foods, targeting 30g (1.3g/kg/d) of protein, and suitable combinations with carbohydrate foods for breakfast.
Table 3.
Good protein sources
| Sources | Rationales (references) |
|---|---|
| Eggs |
|
| Beans and pulses |
|
| Nuts and seeds |
|
| Fish | |
| Poultry |
Table 4.
| Food groups | Suggestions |
|---|---|
| Eggs |
|
| Legumes |
|
| Nuts and seeds |
|
| Fish |
|
| Poultry |
|
P = protein, TTL = total, GTTL = ground total
It could be concluded that choosing the right kinds of cereal (LGL) and milk, as well as increasing protein intake, would be key for energy (blood glucose) balancing, ideal breakfast, as these seem to constitute the breakfast menu consumed by the majority of people. In the long term, these changes may lead to better health status and prevention of disease, especially metabolic disorders, which may be linked to liver health. Finally, we strongly hope that healthier ready-to-eat cereals which are enriched with protein and extra fibre, as well as being made from wholegrain (preferably oats due to its LGL and nutrient-rich properties (65)) and containing less sugar, will be available on the market in the near future. This is believed to promote improved health to the public.
Acknowledgements
I am grateful for all the support I have received whilst researching and writing up this dissertation. I especially thank the Language Centre and International Office at the University of Worcester for their help with data collection.
(Please cite as: Kamada I, Truman L, Bold J, Mortimore D. The impact of breakfast in metabolic and digestive health. Gastroenterology and Hepatology From Bed to Bench 2011;4(2):76-85).
References
- 1.Affenito SG. Breakfast: a missed opportunity. J Am Diet Assoc. 2007;107:565–69. doi: 10.1016/j.jada.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Marangoni F, Poli A, Agostoni C, Di Pietro P, Cricelli C, Brignoli O, et al. A consensus document on the role of breakfast in the attainment and maintenance of health and wellness. Acta Biomed. 2009;80:166–71. [PubMed] [Google Scholar]
- 3.Leidy HJ, Bossingham MJ, Mattes RD, Campbell WW. Increased dietary protein consumed at breakfast leads to an initial and sustained feeling of fullness during energy restriction compared to other meal times. Br J Nutr. 2008;101:798–803. doi: 10.1017/s0007114508051532. [DOI] [PubMed] [Google Scholar]
- 4.Rampersaud GC, Pereira MA, Girard BL, Adams J, Metzl JD. Breakfast habits, nutritional status, body weight, and academic performance in children and adolescents. J Am Diet Assoc. 2005;105:743–60. doi: 10.1016/j.jada.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Rea-Jeng Y, Edward W, Yeu-Sheng H, Mei-Yen C. Irregular breakfast eating and health status among adolescents in Taiwan. BMC Public Health. 2006;6:295–302. doi: 10.1186/1471-2458-6-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Widenhorn-Muller K, Hille K, Klenk J, Weiland U. Influence of having breakfast on cognitive performance and mood in 13-to 20-year-old high school students: results of a crossover trial. Pediatrics. 2008;122:279–84. doi: 10.1542/peds.2007-0944. [DOI] [PubMed] [Google Scholar]
- 7.Timlin MT, Pereira MA. Breakfast frequency and quality in the etiology of adult obesity and chronic diseases. Nutr Rev. 2007;65:268–81. doi: 10.1301/nr.2007.jun.268-281. [DOI] [PubMed] [Google Scholar]
- 8.Keski-Rahkonen A, Kaprio J, Rissanen A, Virkkunen M, Rose RJ. Breakfast skipping and health-compromising behaviors in adolescents and adults. Eur J Clin Nutr. 2003;57:842–53. doi: 10.1038/sj.ejcn.1601618. [DOI] [PubMed] [Google Scholar]
- 9.Matthys C, De Henauw S, Bellemans M, De Maeyer M, De Backer G. Breakfast habits affect overall nutrient profiles in adolescents. Public Health Nutr. 2007;10:413–21. doi: 10.1017/S1368980007248049. [DOI] [PubMed] [Google Scholar]
- 10.Preziosi P, Galan P, Deheeger M, Yacoub N, Drewnowski A, Hercberg S. Breakfast Type, Daily Nutrient Intakes and Vitamin and Mineral Status of French Children, Adolescents and Adults. J Am Coll Nutr. 1999;18:171–78. doi: 10.1080/07315724.1999.10718846. [DOI] [PubMed] [Google Scholar]
- 11.Rampersaud GC. Benefits of breakfast for children and adolescents: update and recommendations for practitioners. Am J Lifestyle Med. 2009;3:86–103. [Google Scholar]
- 12.The University of Sydney. The Glycemic Index. 2010. [Online] Available from: http://www.glycemicindex.com. Accessed at: 9th April 2010.
- 13.Livesey G, Taylor R, Hulshof T, Howlett J. Glycemic response and health a systematic review and meta-analysis: relations between dietary glycemic properties and health outcomes. Am J Clin Nutr. 2008;87:258S–68. doi: 10.1093/ajcn/87.1.258S. [DOI] [PubMed] [Google Scholar]
- 14.Brand-Miller JC. Glycemic Load and Chronic Disease. Nutr Rev. 2003;61:1. doi: 10.1301/nr.2003.may.S49-S55. [DOI] [PubMed] [Google Scholar]
- 15.Anderson GH, Woodend D. Effect of glycemic carbohydrates on short-term satiety and food intake. Nutr Rev. 2003;61:17–26. doi: 10.1301/nr.2003.may.S17-S26. [DOI] [PubMed] [Google Scholar]
- 16.Warren JM, Henry CJK, Simonite V. Low glycemic index breakfasts and reduced food intake in preadolescent children. Pediatrics. 2003;112:414–19. doi: 10.1542/peds.112.5.e414. [DOI] [PubMed] [Google Scholar]
- 17.Nilsson AC, Ostman EM, Granfeldt Y, Bjorck IME. Effect of cereal test breakfasts differing in glycemic index and content of indigestible carbohydrates on daylong glucose tolerance in healthy subjects. Am J Clin Nutr. 2008;87:645–54. doi: 10.1093/ajcn/87.3.645. [DOI] [PubMed] [Google Scholar]
- 18.Beulens JWJ, de Bruijne LM, Stolk RP, Peeters PH, Bots ML, et al. High Dietary Glycemic Load and Glycemic Index Increase Risk of Cardiovascular Disease Among Middle-Aged Women A Population-Based Follow-Up Study. J Am Coll Cardiol. 2007;50:14–21. doi: 10.1016/j.jacc.2007.02.068. [DOI] [PubMed] [Google Scholar]
- 19.Rizkalla SW, Bellisle F, Slama G. Health benefits of low glycaemic index foods, such as pulses, in diabetic patients and healthy individuals. Br J Nutr. 2002;88:255–62. doi: 10.1079/BJN2002715. [DOI] [PubMed] [Google Scholar]
- 20.Schulze MB, Liu S, Rimm EB, Manson JAE, Willett WC, Hu FB. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am J Clin Nutr. 2004;80:348–56. doi: 10.1093/ajcn/80.2.348. [DOI] [PubMed] [Google Scholar]
- 21.Rector RS, Thyfault JP, Wei Y, Ibdah JA. Non-alcoholic fatty liver disease and the metabolic syndrome: an update. World Journal of Gastroenterology: WJG. 2008;14:185–92. doi: 10.3748/wjg.14.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kotronen A, Yki-Jarvinen H. Fatty Liver: A Novel Component of the Metabolic Syndrome. Arterioscler Thromb Vasc Biol. 2008;28:27–38. doi: 10.1161/ATVBAHA.107.147538. [DOI] [PubMed] [Google Scholar]
- 23.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–23. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 24.Falck-Ytter Y, Younossi ZM, Marchesini G, McCullough AJ. Clinical features and natural history of nonalcoholic steatosis syndromes. Semin Liver Dis. 2001;21:15–22. doi: 10.1055/s-2001-12926. [DOI] [PubMed] [Google Scholar]
- 25.Toshimitsu K, Matsuura B, Ohkubo I, Niiya T, Furukawa S, Hiasa Y, et al. Dietary habits and nutrient intake in non-alcoholic steatohepatitis. Nutrition. 2007;23:46–52. doi: 10.1016/j.nut.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Papandreou D, Rousso I, Malindretos P, Makedou A, Moudiou T, Pidonia I, et al. Are saturated fatty acids and insulin resistance associated with fatty liver in obese children? Clin Nutr. 2008;27:233–40. doi: 10.1016/j.clnu.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Valtueña S, Pellegrini N, Ardigò D, Del Rio D, Numeroso F, Scazzina F, et al. Dietary glycemic index and liver steatosis. Am J Clin Nutr. 2006;84:136–42. doi: 10.1093/ajcn/84.1.136. [DOI] [PubMed] [Google Scholar]
- 28.Huang MA, Greenson JK, Chao C, Anderson L, Peterman D, Jacobson J, et al. One-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis: a pilot study. Am J Gastroenterol. 2005;100:1072–81. doi: 10.1111/j.1572-0241.2005.41334.x. [DOI] [PubMed] [Google Scholar]
- 29.Freeman J. The glycemic index debate: does the type of carbohydrate really matter? Diabetes Forecast. 2005;58:11. [PubMed] [Google Scholar]
- 30.Venn BJ, Green TJ. Glycemic index and glycemic load: measurement issues and their effect on diet–disease relationships. Eur J Clin Nutr. 2007;61:S122–31. doi: 10.1038/sj.ejcn.1602942. [DOI] [PubMed] [Google Scholar]
- 31.Murry M, Pizzorno J, Pizzorno L. The Encyclopedia of Healing Foods. New York: Atria Books; 2005. [Google Scholar]
- 32.Gannon MC, Nuttall FQ, Saeed A, Jordan K, Hoover H. An increase in dietary protein improves the blood glucose response in persons with type 2 diabetes. Am J Clin Nutr. 2003;78:734–41. doi: 10.1093/ajcn/78.4.734. [DOI] [PubMed] [Google Scholar]
- 33.Karamanlis A, Chaikomin R, Doran S, Bellon M, Bartholomeusz FD, Wishart JM, et al. Effects of protein on glycemic and incretin responses and gastric emptying after oral glucose in healthy subjects. Am J Clin Nutr. 2007;86:1364–68. doi: 10.1093/ajcn/86.5.1364. [DOI] [PubMed] [Google Scholar]
- 34.Paddon-Jones D, Westman E, Mattes RD, Wolfe RR, Astrup A, Westerterp-Plantenga M. Protein, weight management, and satiety. Am J Clin Nutr. 2008;87:1558S–61S. doi: 10.1093/ajcn/87.5.1558S. [DOI] [PubMed] [Google Scholar]
- 35.Anderson GH, Tecimer SN, Shah D, Zafar TA. Protein source, quantity, and time of consumption determine the effect of proteins on short-term food intake in young men. J Nutr. 2004;134:3011–15. doi: 10.1093/jn/134.11.3011. [DOI] [PubMed] [Google Scholar]
- 36.Veldhorst MAB, Nieuwenhuizen AG, Hochstenbach-Waelen A, Westerterp KR, Engelen MP, Brummer RJ, et al. A breakfast with alpha-lactalbumin, gelatin, or gelatin TRP lowers energy intake at lunch compared with a breakfast with casein, soy, whey, or whey-GMP. Clin Nutr. 2009;28:147–55. doi: 10.1016/j.clnu.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Bowen J, Noakes M, Clifton PM. Appetite regulatory hormone responses to various dietary proteins differ by body mass index status despite similar reductions in ad libitum energy intake. J Clin Endocrinol Metab. 2006;91:2913–19. doi: 10.1210/jc.2006-0609. [DOI] [PubMed] [Google Scholar]
- 38.Veldhorst MAB, Nieuwenhuizen AG, Hochstenbach-Waelen A, van Vught AJ, Westerterp KR, Engelen MP, et al. Dose-dependent satiating effect of whey relative to casein or soy. Physiol Behav. 2009;96:675–82. doi: 10.1016/j.physbeh.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Hall WL, Millward DJ, Long SJ, Morgan LM. Casein and whey exert different effects on plasma amino acid profiles, gastrointestinal hormone secretion and appetite. Br J Nutr. 2003;89:239–48. doi: 10.1079/BJN2002760. [DOI] [PubMed] [Google Scholar]
- 40.Calbet JAL, MacLean DA. Plasma Glucagon and Insulin Responses Depend on the Rate of Appearance of Amino Acids after Ingestion of Different Protein Solutions in Humans. J Nutr. 2002;132:2174–82. doi: 10.1093/jn/132.8.2174. [DOI] [PubMed] [Google Scholar]
- 41.Nilsson M, Stenberg M, Frid AH, Holst JJ, Bjorck IME. Glycemia and insulinemia in healthy subjects after lactose-equivalent meals of milk and other food proteins: the role of plasma amino acids and incretins. Am J Clin Nutr. 2004;80:1246–53. doi: 10.1093/ajcn/80.5.1246. [DOI] [PubMed] [Google Scholar]
- 42.von Post-Skagegård M, Vessby B, Karlström B. Glucose and insulin responses in healthy women after intake of composite meals containing cod-, milk-, and soy protein. Eur J Clin Nutr. 2006;60:949–54. doi: 10.1038/sj.ejcn.1602404. [DOI] [PubMed] [Google Scholar]
- 43.Condé Nast Digital. Nutrition Data. 2009. [Online] Available from: http://www.nutritiondata.com/ [Accessed 28th April 2010]
- 44.Energyfiend.com. Caffeine Content of Drinks. 2010. [Online] Available from: http://www.energyfiend.com/the-caffeine-database. [Accessed 28th April 2010]
- 45.The Centre for Science for the Public Interest. Caffeine Content of Food & Drugs. 2010. [Online] Available from: http://carbc.ca/portals/0/resources/FS%20Caffeine.pdf. [Accessed 23rd March 2010]
- 46.SPSS Inc. The Statistical Package for the Social Science. 2006. p. 14.0.
- 47.Marsh K, Brand-Miller J. State of the Art Reviews: Glycemic Index, Obesity, and Chronic Disease. Am J Lifestyle Med. 2008;2:142–50. [Google Scholar]
- 48.Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287:2414–23. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- 49.Campbell WW, Trappe TA, Wolfe RR, Evans WJ. The recommended dietary allowance for protein may not be adequate for older people to maintain skeletal muscle. Journals of Gerontology Series A: Biological and Medical Sciences. 2001;56:373–80. doi: 10.1093/gerona/56.6.m373. [DOI] [PubMed] [Google Scholar]
- 50.Symons TB, Schutzler SE, Cocke TL, Chinkes DL, Wolfe RR, Paddon-Jones D. Aging does not impair the anabolic response to a protein-rich meal. Am J Clin Nutr. 2007;86:451–56. doi: 10.1093/ajcn/86.2.451. [DOI] [PubMed] [Google Scholar]
- 51.Hayashi Y. Application of the concepts of risk assessment to the study of amino acid supplements. J Nutr. 2003;133:2021S–24S. doi: 10.1093/jn/133.6.2021S. [DOI] [PubMed] [Google Scholar]
- 52.Cordain L, Miller JB, Eaton SB, Mann N, Holt SHA, Speth JD. Plant-animal subsistence ratios and macronutrient energy estimations in worldwide hunter-gatherer diets. Am J Clin Nutr. 2000;71:682–92. doi: 10.1093/ajcn/71.3.682. [DOI] [PubMed] [Google Scholar]
- 53.de Castro JM. The time of day of food intake influences overall intake in humans. J Nutr. 2004;134:104–11. doi: 10.1093/jn/134.1.104. [DOI] [PubMed] [Google Scholar]
- 54.Layman DK. Dietary Guidelines should reflect new understandings about adult protein needs. Nutr Metab (Lond) 2009;6:1–6. doi: 10.1186/1743-7075-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melnik BC. Milk-the promoter of chronic Western diseases. Med Hypotheses. 2009;72:631–39. doi: 10.1016/j.mehy.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 56.Vora S, Ovhal A, Jerajani H, Nair N, Chakrabortty A. Correlation of facial sebum to serum insulin-like growth factor-1 in patients with acne. Br J Dermatol. 2008;159:990–91. doi: 10.1111/j.1365-2133.2008.08764.x. [DOI] [PubMed] [Google Scholar]
- 57.Zhu B, Zhao G, Witte DP, Hui DY, Fagin JA. Targeted overexpression of IGF-I in smooth muscle cells of transgenic mice enhances neointimal formation through increased proliferation and cell migration after intraarterial injury. Endocrinology. 2001;142:3598–606. doi: 10.1210/endo.142.8.8331. [DOI] [PubMed] [Google Scholar]
- 58.Falorni A, Bini V, Cabiati G, Papi F, Arzano S, Celi F, Sanasi M. Serum levels of type I procollagen C-terminal propeptide, insulin-like growth factor-I (IGF-I), and IGF binding protein-3 in obese children and adolescents: Relationship to gender, pubertal development, growth, insulin, and nutritional status. Metab Clin Exp. 1997;46:862–71. doi: 10.1016/s0026-0495(97)90071-8. [DOI] [PubMed] [Google Scholar]
- 59.Fürstenberger G, Senn HJ. Insulin-like growth factors and cancer. The lancet oncology. 2002;3:298–302. doi: 10.1016/s1470-2045(02)00731-3. [DOI] [PubMed] [Google Scholar]
- 60.Taguchi A, White MF. Insulin-like signaling, nutrient homeostasis, and life span. Annu Rev Physiol. 2008;70:191–212. doi: 10.1146/annurev.physiol.70.113006.100533. [DOI] [PubMed] [Google Scholar]
- 61.Ichikawa T, Nakao K, Hamasaki K, Furukawa R, Tsuruta S, Ueda Y, et al. Role of growth hormone, insulin-like growth factor 1 and insulin-like growth factor-binding protein 3 in development of non-alcoholic fatty liver disease. Hepatol Int. 2007;1:287–94. doi: 10.1007/s12072-007-9007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.The Food Standards Agency. Food Standards Agency. 2010. [Online] Available from: http://www.eatwell.gov.uk/healthydiet/ [Accessed 13th May 2010]
- 63.Keller JL, Lanou AJ, Barnard ND. The consumer cost of calcium from food and supplements. J Am Diet Assoc. 2002;102:1669–71. doi: 10.1016/s0002-8223(02)90355-x. [DOI] [PubMed] [Google Scholar]
- 64.Kevin A. Increase in Demand for Non-dairy Protein Products and Alternative Protein Supplements. 2009. [Online] Available from http://www.articlesnatch.com/Article/Increase-In-Demand-For-Non-dairy-Protein-Products-And-Alternative-Protein-Supplements/934392. [Accessed 8th June 2010]
- 65.Butt MS, Tahir-Nadeem M, Khan MKI, Shabir R, Butt MS. Oat: unique among the cereals. Eur J Nutr. 2008;47:68–79. doi: 10.1007/s00394-008-0698-7. [DOI] [PubMed] [Google Scholar]
- 66.Gray J, Griffin B. Eggs and dietary cholesterol-dispelling the myth. Nutr Bull. 2009;34:66–70. [Google Scholar]
- 67.Riedel WJ, Klaassen T, Schmitt JAJ. Tryptophan, mood, and cognitive function. Brain Behav Immun. 2002;16:581–89. doi: 10.1016/s0889-1591(02)00013-2. [DOI] [PubMed] [Google Scholar]
- 68.Winham A, Webb D, Barr A. Beans and Good Health. Nutr Today. 2008;43:201–9. [Google Scholar]
- 69.Venter CS. Health benefits of soy beans and soy products: a review. Journal of Family Ecology and Consumer Sciences/Tydskrif vir Gesinsekologie en Verbruikerswetenskappe. 1999;27:2–11. [Google Scholar]
- 70.Xiao CW. Health Effects of Soy Protein and Isoflavones in Humans. J Nutr. 2008;138:1244S–9S. doi: 10.1093/jn/138.6.1244S. [DOI] [PubMed] [Google Scholar]
- 71.Coates AM, Howe PRC. Edible nuts and metabolic health. Curr Opin Lipidol. 2007;18:25–30. doi: 10.1097/MOL.0b013e3280123a47. [DOI] [PubMed] [Google Scholar]
- 72.Kris-Etherton PM, Hu FB, Ros E, Sabate J. The Role of Tree Nuts and Peanuts in the Prevention of Coronary Heart Disease: Multiple Potential Mechanisms. J Nutr. 2008;138:1746S–51. doi: 10.1093/jn/138.9.1746S. [DOI] [PubMed] [Google Scholar]
- 73.Ruxton CHS, Reed SC, Simpson MJA, Millington KJ. The health benefits of omega-3 polyunsaturated fatty acids: a review of the evidence. J Hum Nutr Diet. 2004;17:449–59. doi: 10.1111/j.1365-277X.2004.00552.x. [DOI] [PubMed] [Google Scholar]
- 74.Calder PC. n-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83:S1505–19. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 75.The Agricultural Research Service. Nutrient Data Laboratory. 2010. [Online] Available from: http://www.nal.usda.gov/fnic/foodcomp/search/ Accessed: 15th May 2010]