Abstract
Atypical presentation is the most common form of celiac disease (CD). Although the terminologies like latent, silent and potential have expressed different aspects of clinical and pathological behaviour of CD, they also have contributed in some extent to confusion between clinicians and patients due to the multiple definitions and uncertainty around them. In the light of new advances and the discovery of entities such as non-celiac gluten sensitivity, using subclinical instead of silent and atypical instead of potential/latent may simplify the understanding behind the clinical behaviour of atypical CD. The evidence behind a lower threshold for starting a gluten free diet (GFD) in non-celiac gluten sensitive patients would strongly support applying a GFD treatment strategy in any forms of CD.
Keywords: Subclinical, Celiac disease, Atypical, Microscopic enteritis, Gluten sensitivity
Introduction
Using multiple terminology in defining atypical celiac disease (CD) has confused many of the clinicians to recognise atypical forms of this common disorder. CD is not considered an uncommon disorder any longer and is not a disease of essentially European origin (1). Nevertheless, recognising the existence of atypical forms known under the old terminologies like latent, silent and potential CD has introduced a new insight on clinical behaviour of this condition. That way the age of presentation of the disease has changed dramatically (1, 2) and the factors responsible for this change are mainly attributed to advances in diagnostic tools in recognising atypical and subclinical forms of the disease. Adult presentation is increasingly common, and subclinical CD can occur at any age. Population screening with serological tests, have shown a CD screening prevalence of the order of 1% in the western hemisphere (3). European and Asian studies involving healthy blood donors found a prevalence rate of 1 in 166-330 (4, 5) subclinical CD. According to the previous studies, screening based on antibodies only would underestimate the prevalence of CD due to false-negative results caused by the low sensitivity of tests (6–10). However, some studies suggest that the overestimation of CD frequency could also result from antibody based screening programmes due to a high rate of false positives (11).
Sub-clinical Celiac Disease
Terminologies like latent, silent and potential celiac disease can be confusing for clinicians and patients. Silent CD is not absolutely silent after all; patients show signs of CD with no significant symptoms. Potential and latent are defined differently in different studies. T-cell-mediated autoimmune processes are initiated by gluten exposure, leading to both intestinal and atypical extraintestinal manifestations. More and more diseases are proven to be associated with CD. In these conditions, screening is strongly recommended. However, a typical CD patient today has merely mild abdominal symptoms. Malabsorption can be silent like a mild anaemia (better defined under subclinical), or there is usually only moderate malabsorption, if any at all. Diagnostic difficulties may further emerge when minor mucosal changes are found (12). Should the presence of CD be ascertained in every symptomatic patient with atypical presentation? Since gluten sensitivity is no longer limited to overt villous atrophy, and given the results of many studies (13–15), we believe the answer is yes.
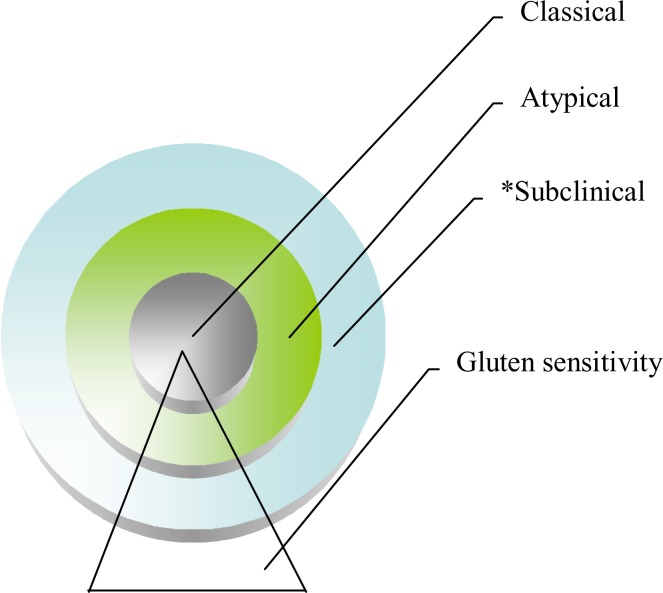
Subclinical or so called silent CD cases are being detected in increasing numbers because of raised awareness of the disease. Presentations with atypical symptoms are the dominant form of disease manifestation and these comprise the sole and main part of the celiac iceberg (16). Whether they have positive serology with negative biopsy or increased γδ T Cells receptors with symptoms compatible with CD they could be classified as atypical CD. We are moving towards a lower threshold in implementing a gluten free diet (GFD). Villous atrophy is not mandatory any longer to qualify a patient for GFD. In fact a large number of patients present with non-celiac gluten sensitivity with completely normal biopsy and negative serology. They also seem to benefit from a GFD. In such circumstances there is a need to re-define the terminologies according to the modified treatment strategy in gluten related disorders. The main strategy for treatment should target symptomatic typical or atypical patients, and not asymptomatic cases. We propose a simplified classification by dividing and replacing previous terminology to typical, atypical and subclinical instead of silent/latent and potential. (See Figure 1).
Figure 1.
Celiac and non-celiac gluten sensitivity
* Suclinical: previously known as silent and atypical as known under latent and potential CD
Immunogenetics involvement in Celiac Disease
The spectrum of gluten related disorders seems to be beyond HLA DQ2-8. Our knowledge of CD pathogenesis has made significant progress in the last decade. The disorder is now considered the result of a complex interaction between genetic and environmental factors such as gluten that classified from subclinical to severe malabsorption. In contrast to gluten sensitivity celiac disease development has a strong genetic component with a sibling relative risk (lambda (s)) of 30. Recent studies using the human genome screening technique in families with multiple siblings suffering from CD have suggested the presence of at least 4 different chromosomes in the predisposition to suffer from CD (17). One susceptibility locus is the MHC (major histocompatibility complex) region, with a particular association with the HLA-DQ alleles DQA1*0501 and DQB1*0201. However, shared-haplotype studies suggest that genes within the MHC complex contribute no more than 40% to the sibling familial risk of disease. Early studies showed that gliadin elicits an inflammatory T-cell reaction when added to intestinal biopsy specimens of celiac patients in vitro and a link to the genetic predisposition was provided by the isolation of gliadin-specific HLA-DQ2-restricted T-cell clones from CD mucosa (18, 19). However, the prevalence of HLA-DQ2 is high in the normal population (25-45%), suggesting the involvement of additional, and probably non-HLA-linked genes in CD pathogenesis.
Microscopic enteritis
The pathologic spectrum of the mucosal abnormalities seen on small intestinal biopsies, range from microscopic enteritis (Marsh 0-II) to macroscopic forms (Marsh IIIa-c) (20). Not every gluten-sensitised individual inevitably develops CD and not every celiac patient develops the destructive lesions such as Marsh III. Celiac disease is not exclusively due to antibody production either. A large proportion of the patients present with microscopic enteritis (Marsh I-II) whose diagnoses may actually be missed (20–23). Five major histopathological features that define CD have been recognized in the previous study. These 6 distinct and sequential phases of the CD are microscopic enteritis (ME) Marsh (0-II). Marsh 0 with normal small bowel mucosa where intraepithelial lymphocytes are below 25/100 entrecotes. Some patients could still have subtle abnormalities at this stage like increased γδ T cell receptors or alteration of enterocytes and microvillis. i) Recruitment of T-lymphocytes > 25/100 enterocytes (intestinal-intraepithelial lymphocyte or IEL; Marsh I), ii) lymphocyte infiltration and crypt hyperplasia (Marsh II), iii) macroscopic enteritis (Marsh IIIa-c) partial villous atrophy (Marsh IIIa), iv) subtotal villous atrophy (Marsh IIIb) and v) total atrophy (Marsh IIIb) and total villous atrophy (Marsh IIIc) (6, 8). This sequential cascade suggests that a T-cell response to gliadin precedes, and very likely produces, the complete pattern of CD. The statistical comparison between antibody-positive and antibody-negative cases shows that the appearance of antibodies was seen predominantly in cases with serious mucosal damage in which IELs was highly increased. However, it is hard to rule out the contribution of antibodies in genesis of an autoimmune condition like CD.
The screening value of autoantibodies has been too optimistically overestimated, especially those on tissue transglutaminase antibodies (tTGA) (24, 25). However, comparing the tTGA to EMA and AGA, the sensitivity of tTGA does not offer any advantages over EMA for screening of the populations at high risk of CD (26–28). It is time to re-evaluate our perception of intestinal pathology (29) in such terms, rather than by continued use of subjective degrees of villous atrophy (VA), since absence of VA is not evidence of absence of CD. Such terminology obscures the recognition of fundamental changes occurring within small bowel mucosa. In simple words, increased density of IEL's and crypt hyperplasia form an essential phase in the disease pathogenesis sequence of progression. As CD with milder enteropathy is the most common form, histology cannot be considered as the gold standard any longer. Therefore, treatment should target the symptoms and not the immunohistology (29–32).
Gluten sensitivity and Celiac disease
Gluten sensitivity (GS) is characterised by negative antibodies and normal histology; it is defined as a non-allergic and non-autoimmune condition in which the consumption of gluten can lead to symptoms similar to those seen in CD. Until recently the terms GS and CD were used synonymously in literature (33) and it is not clear yet whether patients affected by GS will have some subtle intestinal and mucosal changes consistent with microscopic enteritis. Yet we know very little about the pathogenic mechanism behind gluten sensitivity. Some GS patients would tolerate even more than 5g gluten/day and still remain symptom free with negative serology (34, 35).
GS patients are gluten intolerant and gluten consumption does not lead to small intestinal damage, so it is not accompanied by the concurrence of tTG autoantibodies or autoimmune disease. In the study by Kaukinen et al. out of 94 adults with GI symptoms, 63% were affected by gluten foods and neither classified as CD nor as allergic (36).
On the other hand around 50% of the GS patients were DQ2/DQ8 positive, which is similar to that of the general population, while celiac patients carry more than 95% in most regions of the word. However, while the prevalence of CD is roughly 1% within the general population, GS is thought to affect 6 to 10% of the general population (37). In some cases GS can present with normal or milder enteropathy seen as increased intestinal permeability, IBS, abdominal discomfort, pancreatic disorders, pain or diarrhoea; or it may present with a variety of extraintestinal symptoms including lymphoma, attention deficit disorder and neuropathy, autism and schizophrenia, infertility, IBD, muscular disturbances as well as osteopenia and osteoprosis (38–42). According to current literature, a GFD is recommended to gluten sensitive cases with/without enteropathy. This policy includes a range of symptomatic gluten sensitive cases with atypical presentation including those with small bowel microscopic changes (Marsh 0-II) who are antibody negative or show characteristic features of other conditions.
Conclusion
The spectrum of gluten related disorders is widening. This is because these common systemic disorders have multifactorial etiology with a multitude of symptoms and complications inside and outside the small bowel. We still don't know how seriously subclinical CD will be affected by the long term complications if they are not treated with GFD.
A marked increase in the prevalence of CD, especially the subclinical CD forms and non-celiac gluten sensitivity, seem to become a major health problem (43–46). The clinician may often face the variability of histological and clinical aspects of CD (46) with uncertainty, as they might not quite fit into the diagnostic models in the current guidelines. Accumulated evidence supports that decreasing the treatment threshold for cases with atypical CD and those with gluten sensitivity, as the life quality of these cases will improve with GFD and the long-term health benefit of this strategy, would perhaps be also cost effective.
(Please cite as: Rostami Nejad M, Hogg-Kollars S, Ishaq S, Rostami K. Subclinical celiac disease and gluten sensitivity. Gastroenterol Hepatol Bed Bench 2011;4(3):102-108).
References
- 1.Vilppula A, Collin P, Maki M, Valve R, Luostarinen M, Krekela I, et al. Undetected coeliac disease in the elderly: a biopsy-proven population based study. Dig Liver Dis. 2008;40:809–13. doi: 10.1016/j.dld.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Baudon JJ, Dabadie A, Cardona J, Digeon B, Giniés JL, Larchet M, et al. Incidence of symptomatic celiac disease in French children. Presse Med. 2001;30:107–10. [PubMed] [Google Scholar]
- 3.Rostami Nejad, Rostami K, Emami MH, Zali MR, Malekzadeh R. Epidemiology of Celiac disease in Iran; A Review. Middle East Journal of Digestive Diseases. 2011;3:74–77. [PMC free article] [PubMed] [Google Scholar]
- 4.Rostami K, Mulder CJ, Werre JM, van Beukelen FR, Kerchhaert J, Crusius JB, Pena AS, Willekens FL, Meijer JW. High prevalence of celiac disease in apparently healthy blood donors suggests a high prevalence of undiagnosed celiac disease in the Dutch population. Scand J Gastroenterol. 1999;34:276–79. doi: 10.1080/00365529950173681. [DOI] [PubMed] [Google Scholar]
- 5.Shahbazkhani B, Malekzadeh R, Sotoudeh M, Fayaz Moghadam K, Farhadi M, Ansari R, et al. High prevalence of celiac disease in apparently healthy Iranian blood donors. Eur J Gastroenterol Hepatol. 2003;15:475–78. doi: 10.1097/01.meg.0000059118.41030.96. [DOI] [PubMed] [Google Scholar]
- 6.Rostami K, Kerckhaert J, Tiemessen R, von Blomberg BM, Meijer JW, Mulder CJ. Anti-endomysium and Antigliadin antibodies in untreated celiacs: disappointing in clinical practice. Am J Gastroenterol. 1999;94:888–94. doi: 10.1111/j.1572-0241.1999.983_f.x. [DOI] [PubMed] [Google Scholar]
- 7.Bottaro G, Rotolo N, Spina M, Sciuto C, Castiglione S, Sanfilippo G, Musumeci S. Evaluation of sensitivity and specificity of antigliadin antibodies for the diagnosis of celiac disease in childhood. Minerva Pediatr. 1995;47:505–10. [PubMed] [Google Scholar]
- 8.Dickey W, McMillan SA, Hughes DF. Sensitivity of serum tissue transglutaminase antibodies for endomysial antibody positive and negative coeliac disease. Scand J Gastroenterol. 2001;36:511–14. doi: 10.1080/003655201750153359. [DOI] [PubMed] [Google Scholar]
- 9.Prasad S, Thomas P, Nicholas DS, Sharer NM, Snook JA. Adult endomysial antibody-negative coeliac disease and cigarette smoking. Eur J Gastroenterol Hepatol. 2001;13:667–71. doi: 10.1097/00042737-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Tursi A, Brandimarte G, Giorgetti G, Gigliobianco A, Lombardi D, Gasbarrini G. Low prevalence of antigliadin and anti-endomysium antibodies in subclinical/silent celiac disease. Am J Gastroenterol. 2001;96:1507–10. doi: 10.1111/j.1572-0241.2001.03744.x. [DOI] [PubMed] [Google Scholar]
- 11.Emami MH, Karimi S, Kouhestani S, Hashemi M, Taheri H. Diagnostic accuracy of IgA anti-tissue transglutaminase in patients suspected of having coeliac disease in Iran. J Gastrointestin Liver Dis. 2008;17:141–6. [PubMed] [Google Scholar]
- 12.Kaukinen K, Maki M, Partanen J, Sievänen H, Collin P. Celiac disease without villous atrophy: revision of criteria called for. Dig Dis Sci. 2001;46:879–87. doi: 10.1023/a:1010729207320. [DOI] [PubMed] [Google Scholar]
- 13.Fine KD, Ogunji F, Saloum Y, Beharry S, Crippin J, Weinstein J. Celiac sprue: another autoimmune syndrome associated with hepatitis C. Am J Gastroenterol. 2001;96:138–45. doi: 10.1111/j.1572-0241.2001.03464.x. [DOI] [PubMed] [Google Scholar]
- 14.Khoshbaten M, Rostami Nejad M, Farzady L, Sharifi N, Hashemi SH, Rostami K. Fertility disorder associated with celiac disease in males and females: fact or fiction? J Obstet Gynaecol Res. 2011 May 11; doi: 10.1111/j.1447-0756.2010.01518.x. [DOI] [PubMed] [Google Scholar]
- 15.Matteoni CA, Goldblum JR, Wang N, Brzezinski A, Achkar E, Soffer EE. Celiac disease is highly prevalent in lymphocytic colitis. J Clin Gastroenterol. 2001;32:225–7. doi: 10.1097/00004836-200103000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Rostami Nejad M, Rostami K, Pourhoseingholi MA, et al. Atypical Presentation is Dominant and Typical for Coeliac Disease. J Gastrointestin Liver Dis. 2009;18:285–91. [PubMed] [Google Scholar]
- 17.Peña AS, Garrote JA, Crusius JB. Advances in the immunogenetics of coeliac disease. Clues for understanding the pathogenesis and disease heterogeneity. Scand J Gastroenterol Suppl. 1998;225:56–8. [PubMed] [Google Scholar]
- 18.King AL, Ciclitira PJ. Celiac disease: strongly heritable, oligogenic, but genetically complex (Abstract) Mol Genet Metab. 2000;71:70–5. doi: 10.1006/mgme.2000.3067. [DOI] [PubMed] [Google Scholar]
- 19.Rostami Nejad M, Romanos J, Rostami K, Ganji G, Mohebbi S, Bakhshipour A, et al. HLA-DQ2 and -DQ8 genotypes in celiac disease and healthy Iranian population using Tag Single Nucleotide Polymorphisms. Govaresh. 2010;15:28. [Google Scholar]
- 20.Dickey W, Hughes DF, McMillan SA. Disappearance of endomysial antibodies in treated celiac disease does not indicate histological recovery. Am J Gastroenterol. 2000;95:712–14. doi: 10.1111/j.1572-0241.2000.01838.x. [DOI] [PubMed] [Google Scholar]
- 21.Rostami K, Villanacci V. Microscopic enteritis: novel prospect in coeliac disease clinical and immuno-histogenesis. Evolution in diagnostic and treatment strategies. Dig Liver Dis. 2009;41:245–52. doi: 10.1016/j.dld.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Mulder CJ, Rostami K, Marsh MN. When is a coeliac a coeliac? Gut. 1998;42:594. doi: 10.1136/gut.42.4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rostami K, Al Dulaimi D, Rostami Nejad M, Danciu M. Microscopic enteritis and patho-mechanism of malabsorption. Autoimmun Highlights. 2010;1:37–38. doi: 10.1007/s13317-010-0006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.SulkanenS, Halttunen T, Laurila K, Kolho KL, Korponay-Szabó IR, Sarnesto A. Tissue transglutaminase autoantibody enzyme-linked immunosorbent assay in detecting celiac disease. Gastroenterology. 1998;115:1322–28. doi: 10.1016/s0016-5085(98)70008-3. [DOI] [PubMed] [Google Scholar]
- 25.Sblattero D, Berti I, Trevisiol C, Marzari R, Tommasini A, Bradbury A, et al. Human recombinant tissue transglutaminase ELISA: an innovative diagnostic assay for celiac disease. Am J Gastroenterol. 2000;98:1253–57. doi: 10.1111/j.1572-0241.2000.02018.x. [DOI] [PubMed] [Google Scholar]
- 26.Nachmana F, Sugaia E, Mauriñoa E, Bai JC. Serological tests for celiac disease as indicators of long-term compliance with the gluten-free diet. Eur J Gastroenterol Hepatol. 2011;23:473–480. doi: 10.1097/MEG.0b013e328346e0f1. [DOI] [PubMed] [Google Scholar]
- 27.Koop I, Ilchmann R, Izzi L, Adragna A, Koop H, Barthelmes H. Detection of autoantibodies against tissue transglutaminase in patients with celiac disease and dermatitis herpetiformis. Am J Gastroenterol. 2000;95:2014. doi: 10.1111/j.1572-0241.2000.02086.x. [DOI] [PubMed] [Google Scholar]
- 28.Biagi F, Ellis HJ, Yiannakou JY, Brusco G, Swift GL, Smith PM, et al. Tissue transglutaminase antibodies in celiac disease. Am J Gastroenterol. 2000;94:2187–92. doi: 10.1111/j.1572-0241.1999.01292.x. [DOI] [PubMed] [Google Scholar]
- 29.Mulder CJ. When is a coeliac a coeliac? Report of working group of the United Gastroenterology Week in Amsterdam 2001. Eur J Gastroenterol Hepatol. 2001;13:1–6. doi: 10.1097/00042737-200109000-00023. [DOI] [PubMed] [Google Scholar]
- 30.Volta U, Villanacci V. Celiac disease: diagnostic criteria in progress. Cell Mol Immunol. 2011;8:96–102. doi: 10.1038/cmi.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans KE, Aziz I, Cross SS, Sahota GR, Hopper AD, Hadjivassiliou M. A Prospective Study of Duodenal Bulb Biopsy in Newly Diagnosed and Established Adult Celiac Disease. Am J Gastroenterol. 2011 May 24; doi: 10.1038/ajg.2011.171. [DOI] [PubMed] [Google Scholar]
- 32.Hopper AD, Sanders DS. The duodenal bulb biopsy “myth”: is there now enough evidence to change clinical practice? J Clin Gastroenterol. 2009;43:692–93. doi: 10.1097/MCG.0b013e31819cccf1. [DOI] [PubMed] [Google Scholar]
- 33.Hadjivassiliou M, Williamson CA, Woodroofe N. The Immunology Of Gluten Sensitivity: Beyond The Gut. Trends Immunol. 2004;25:578–82. doi: 10.1016/j.it.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Hopman EG, von Blomberg ME, Batstra MR, Morreau H, Dekker FW, Koning F, et al. Gluten tolerance in adult patients with celiac disease 20 years after diagnosis? Eur J Gastroenterol Hepatol. 2008;20:423–29. doi: 10.1097/MEG.0b013e3282f4de6e. [DOI] [PubMed] [Google Scholar]
- 35.Matysiak-Budnik T, Malamut G, de Serre NP, Grosdidier E, Seguier S, Brousse N, et al. Long-term follow-up of 61 coeliac patients diagnosed in childhood: evolution toward latency is possible on a normal diet. Gut. 2007;56:1379–86. doi: 10.1136/gut.2006.100511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaukinen K, Turjanmaa K, Mäki M, Partanen J, Venäläinen R, Reunala T, et al. Intolerance to cereals is not specific for celiac disease. Scandinavian Journal of Gastroenterology. 2000;35:942–946. doi: 10.1080/003655200750022995. [DOI] [PubMed] [Google Scholar]
- 37.Anderson LA, McMillan SA, Watson RG, Monaghan P, Gavin AT, Fox C, et al. Maligancy and mortality in a population-based cohort of patients with celiac disease or ‘gluten sensitivity’. World J Gastroenterol. 2007;13:146–51. doi: 10.3748/wjg.v13.i1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sapone A, Lammers KM, Mazzarella G, Mikhailenko I, Cartenì M, Casolaro V, et al. Differential mucosal IL-17 expression in two gliadin-induced disorders: gluten sensitivity and the autoimmune enteropathy celiac disease. Int Arch Allergy Immunol. 2010;152:75–80. doi: 10.1159/000260087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ford RP. The gluten syndrome: a neurological disease. Med hypotheses. 2009;73:438–40. doi: 10.1016/j.mehy.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 40.Elder JH, Shankar M, Shuster J, Theriaque D, Burns S, Sherrill L. The gluten-free, casein-free diet in autism: results of a preliminary double blind clinical trial. J Autism Dev Disord. 2006;36:413–20. doi: 10.1007/s10803-006-0079-0. [DOI] [PubMed] [Google Scholar]
- 41.Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Leister F, et al. Markers of gluten sensitivity and celiac disease in recent-onset psychosis and multi-episode schizophrenia. Biol Psychiatry. 2010;68:100–104. doi: 10.1016/j.biopsych.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 42.Hadjivassiliou M, Chattopadhyay AK, Grünewald RA, Jarratt JA, Kandler RH, Rao DG, et al. Myopathy associated with gluten sensitivity. Muscle Nerve. 2007;35:443–50. doi: 10.1002/mus.20709. [DOI] [PubMed] [Google Scholar]
- 43.Kurppa K, Collin P, Viljamaa M, Haimila K, Saavalainen P, Partanen J, et al. Diagnosing mild enteropathy celiac disease: a randomized, controlled clinical study. Gastroenterology. 2009;136:816–23. doi: 10.1053/j.gastro.2008.11.040. [DOI] [PubMed] [Google Scholar]
- 44.Nachman F, del Campo MP, González A, Corzo L, Vázquez H, Sfoggia C, et al. Long-term deterioration of quality of life in adult patients with celiac disease is associated with treatment noncompliance. Dig Liver Dis. 2010;42:685–91. doi: 10.1016/j.dld.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 45.Bold J, Rostami K. Gluten tolerance; potential challenges in treatment strategies. Gastroenterol Hepatol Bed Bench. 2011;4:53–57. [PMC free article] [PubMed] [Google Scholar]
- 46.Fasano A, Catassi C. Current approaches to diagnosis and treatment of celiac disease: an evolving spectrum. Gastroenterology. 2001;120:636–51. doi: 10.1053/gast.2001.22123. [DOI] [PubMed] [Google Scholar]