Abstract
Colorectal cancer (CRC) is the second leading cause of cancer death. Progress has been made in the development of chemotherapy for advanced CRC. Targeted therapies against VEGF or EGFR are now commonly used. However, many cases show that tolerance develops to such treatments; therefore, new strategies are required to replace or complement current therapies. Nuclear factor-κB (NF-κB) transcription factors play a key role in many physiological processes such as innate and adaptive immune responses, cell proliferation, cell death, and inflammation. It has become clear that aberrant regulation of NF-κB and the signaling pathways that control its activity are involved in cancer development and progression, as well as in resistance to chemo- and radio- therapies. Hence, anti-NF-κB therapy may rescue many cases of CRC and should be considered as a therapeutic target.
Keywords: Colorectal cancer, NF-κB
Introduction
Cancer is a leading cause of death in industrialized countries. Although mortality rates have declined in recent years due to earlier detection and more options in treatment, most cancers remain incurable. Colorectal cancer (CRC) is the third most commonly diagnosed cancer in the world but more than half of all deaths from the disease occur in the more developed regions of the world (1, 2). The symptoms of CRC depend on the location of tumor in the bowel and whether it has spread elsewhere in the body. Symptoms and signs are divided into local, constitutional, and metastatic. Certain factors increase a person's risk of developing the disease including age, polyps of the colon, history of cancer, heredity, smoking, diet, physical inactivity, viruses, low levels of selenium, inflammatory bowel disease, environmental factors, exogenous hormones, and alcohol (3–5).
In recent years, great progress has been made in the development of chemotherapy for advanced CRC and new treatment options are now available. For example, 5-fluorouracil (5-FU) was reformulated (6) and two new drugs, oxaliplatin and irinotecan, were investigated as adjunctive therapies (7). Targeted therapies against vascular endothelial growth factor (VEGF), bevacizumab, or epidermal growth factor receptor (EGFR), cetuximab, are now commonly used as treatments for metastatic CRC (8–10). Meanwhile, many cases show that tolerance develops to such treatments (11). Therefore, treatment of advanced CRC requires new strategies to replace or complement current therapies. In this sense, targeting transcription factors has attracted growing attention. Nuclear factor-κB (NF-κB) is a transcription factor that participates in the induction of several genes for cytokines and enzymes that play important functional roles in various cell types (12). Since the identification of the NF-kB transcription factors and the cloning of the NF-kB and IkB-coding genes, a large number of experimental evidence has been accumulated demonstrating that these factors play a major role in the development and progression of various human cancers (13–15). The NF-kB signaling pathway is implicated in a variety of physiological and pathological processes. Moreover, there is growing evidence indicating the relationship between cancer development and NF-kB (16–18).
NF-κB transcription factors and their signaling pathways
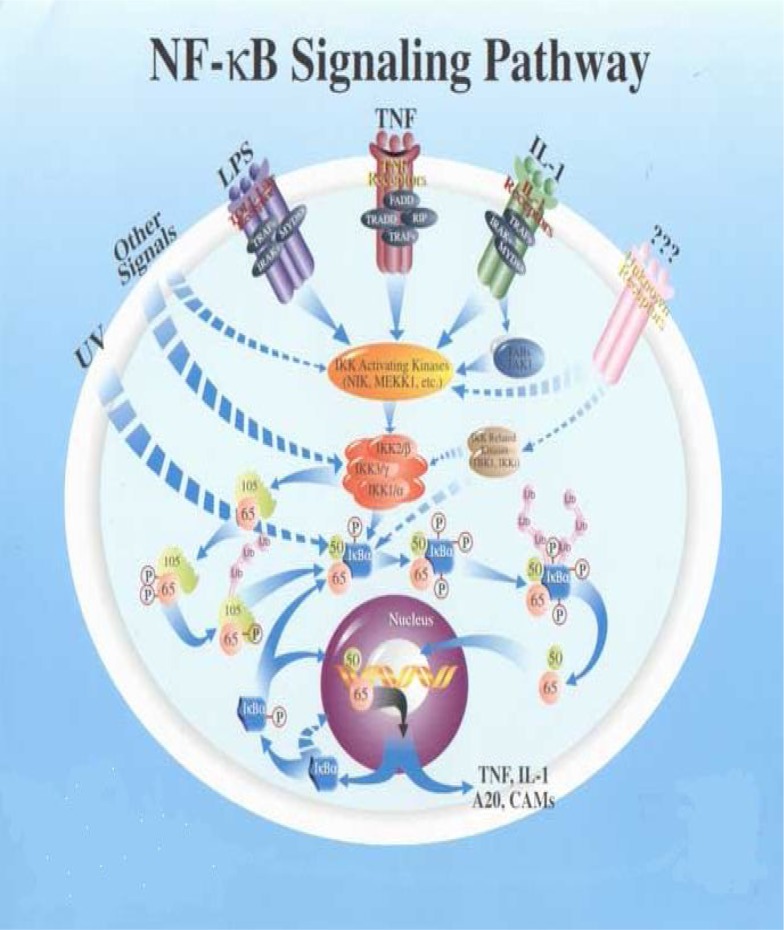
In mammals, the NF-κB family is composed of five members, RelA (p65), RelB, cRel (Rel), NF-κB1 (p50 and its precursor p105) and NF-κB2 (p52 and its precursor p100) (Figure 1).
Figure 1.
NF-κB signaling pathways
These proteins form homo- and heterodimeric complexes, the activity of which is regulated by two major pathways. The first one, known as the classical NF-κB activation pathway, mainly applies to RelA: p50 dimers which under non-stimulated conditions are sequestered in the cytoplasm through interactions with inhibitory proteins of the IκB family. Following stimulation with a broad range of stimuli such as TNF-α (tumor necrosis factor- alpha) or IL-1 (interleukin- 1), viruses, genotoxic agents and ionizing radiation, the IκB molecules are phosphorylated by the IκB kinase complex (IKK) at specific serine residues leading to their ubiquitination and degradation by the proteasome pathway. RelA:p50 dimers are subsequently released and free to translocate to the nucleus where they activate transcription of various target genes (19). This pathway plays a major role in the control of innate immunity and inflammation (20, 21). The second pathway, the so-called alternative NF-κB signaling pathway, is stimulated by a more restricted set of cytokines that all belong to the TNF superfamily (e.g. BAFF, CD40L, LTβ). This pathway involves the upstream kinase NF-κB-inducing kinase (NIK) which activates IKKα, thereby leading to the phosphorylation and proteasome-dependent processing of p100, the main RelB inhibitor, thus resulting in RelB:p52 and RelB:p50 nuclear translocation and DNA binding (22–25).
Most importantly, all studies point out to a crucial role for the alternative pathway in controlling the development, organization and function of secondary lymphoid organs and B-cell maturation and survival (26, 27). Activation of NF-κB pathways relies on the inducible phosphorylation of IκB inhibitory proteins (IκBα for the classical pathway and p100 for the alternative pathway) by the IKK complex and its subunits. IKK is composed of two catalytic subunits, IKKα and IKKβ, and a regulatory subunit, NEMO/IKKγ. Disruption of genes encoding individual subunits has demonstrated that IKKβ and NEMO/IKKγ are required for activation of the classical NF-κB pathway by inflammatory signals, a pathway in which IKKα does not play an essential role. In contrast, RelB:p50 and RelB:p52 activation is absolutely dependent on IKKα, but not on IKKβ or NEMO/IKKγ (28).
Constitutive activation of NF-kB in CRC
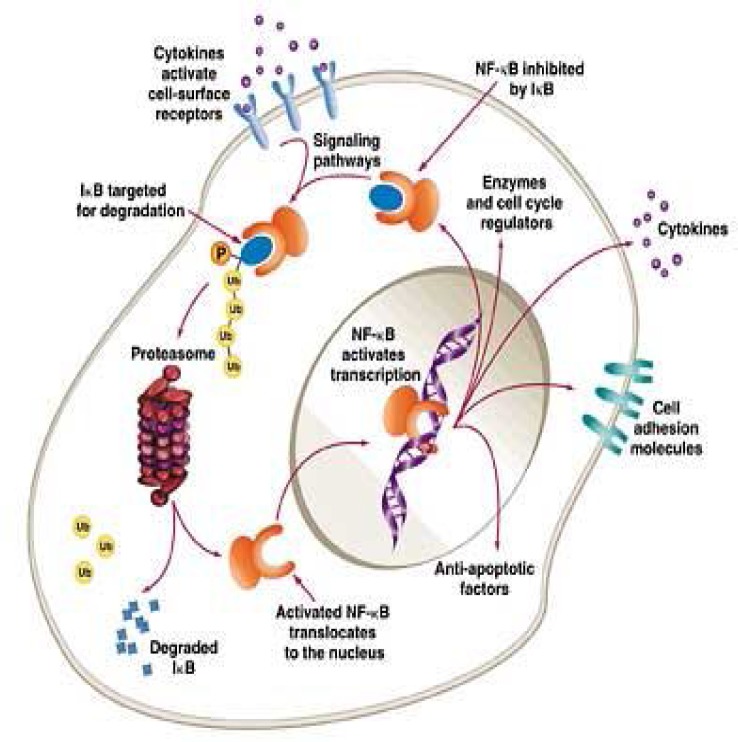
It has been reported that induction of NF-kB activation leads to the resistance to chemotherapy. Constitutively activated NF-kB can often be seen in various cancer cells, cell lines, xenograft animal models or clinical sites. As for CRC, constitutively activated NF-kB was observed in 66% of CRC cell lines and 40% of human CRCs (29, 30). Other reports found constitutively activated NF-kB in almost 60 to 80% of CRCs (31) (Figure 2).
Figure 2.
Constitutive NF-kB activation
Constitutively activated NF-kB promotes the proliferation of cancer cells and rescues the cancer cells from cell death. When the constitutive activation of NF-kB was inhibited by knocking down IKKg with siRNA (KD cells), greater apoptosis was induced in KD cells than in control cells by stimulation with TNF-a or 5-FU. In a xenograft model of nude mice, the tumor volume of KD cells was significantly smaller than the tumors of control cells. In the tumor of the KD cells, more cell death was observed than in the tumors of control cells. Since the constitutive activation of NF-kB is present in a remarkable number of patients with CRC, many patients who showed resistance to chemotherapy could account for the constitutive activation of NF-kB (30, 31). Therefore, anti-NF-kB therapy may rescue many cases of CRC.
Novel anti-NF-kB agents
1. Proteasome inhibitors and bortezomib
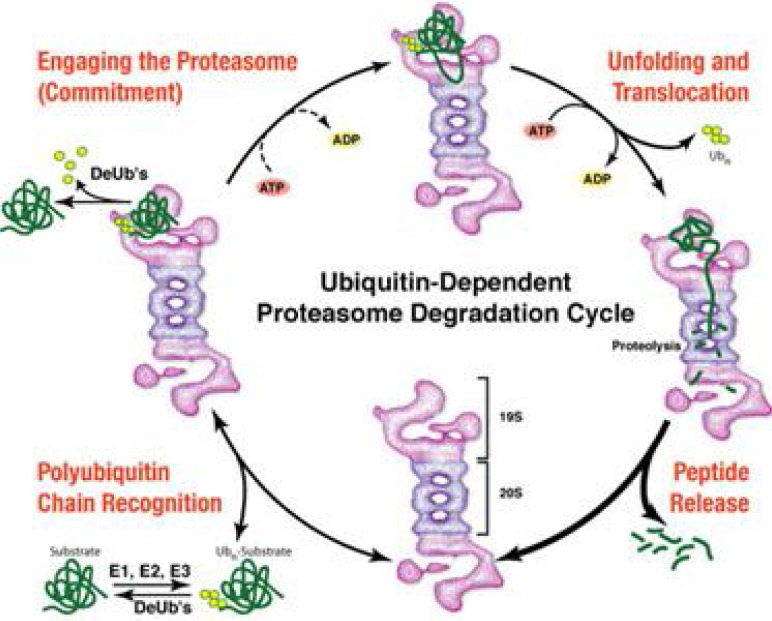
The proteasome is a 26S multiprotein complex that is mainly located in the cytoplasm and nucleus. It consists of a 19S regulatory subunit and a 20S catalytic subunit that contains six unique ATP-dependent serine protease sites, two of each with chymotrypsin, trypsin, and caspase-like activities (32, 33) (Figure 3).
Figure 3.
Proteasome degradation cycle
Ubiquitinated proteins are recognized by the 19S subunit which results in both the liberation of ubiquitin chains that are recycled, and the formation of a denatured protein that is transferred to the outer ring or the 20S core unit. This outer ring only allows such denatured proteins to enter the catabolic site of the proteasome where they are hydrolyzed into small polypeptides (34). The proteasome inhibitor bortezomib is an IV drug that disappears from the plasma compartment almost completely within 15 min of injection but has a long elimination half life (>40 h) (35). Clinical dose monitoring is thus inferred from the measure of residual proteasome activity in blood cells. In general, dose of 1.3 mg/m2 inhibits 65% of proteasome activity with a peak inhibition at 1 h and a return to baseline activity within 48–72 h. Primate experiments have also shown that bortezomib is distributed evenly in most organs with the notable exception of the CNS in areas protected by the blood–brain barrier (36).
2. IKK inhibitors
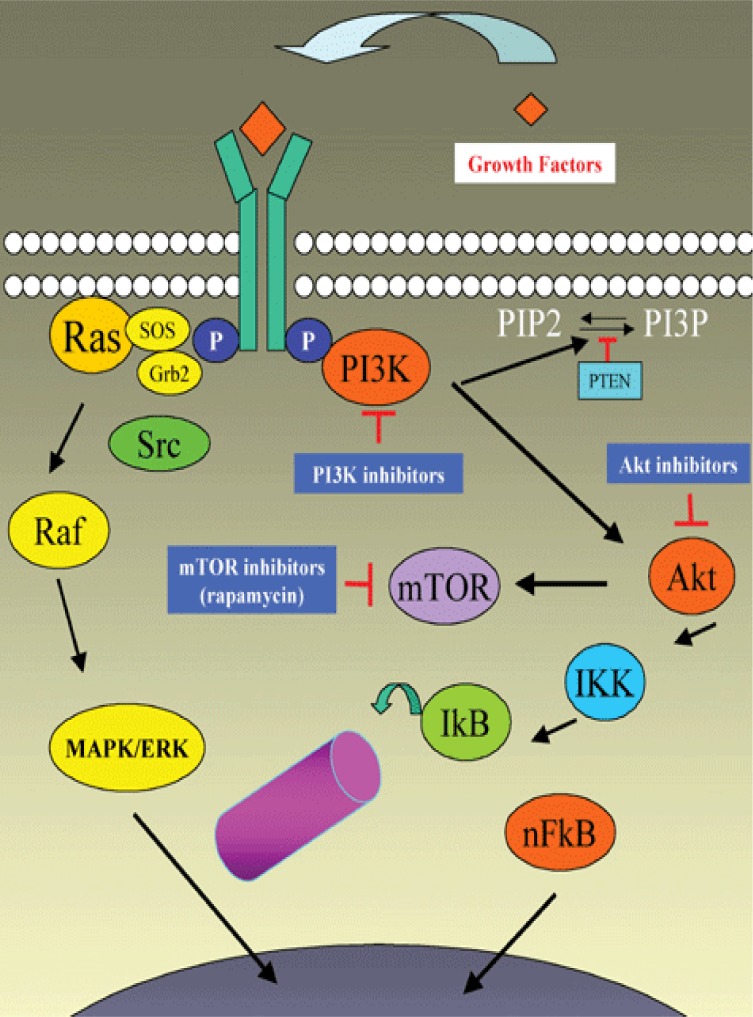
Since the activation and nuclear translocation of the most commonly expressed NF-kB dimers require an IKKb-dependent phosphorylation of their cytoplasmic IkB partners, IKK constitutes a key target for the development of small NF-kB inhibitors. IKK consists of two catalytic (IKKa and IKKb) and a regulatory (IKKg or NEMO) subunits. The activity of IKKa or IKKb depends on their phosphorylation at serine residues located in their respective kinase domains that also contains a critical cysteine residue (Figure 4).
Figure 4.
Activation of IKK pathway
A variety of old drugs such as aspirin, salicylates, sulindac, sulfasalazine and thalidomide inhibit the IKKs catalytic activity. Thiol-reactive drugs such as arsenic trioxide (As2O3) and the gold compound auranofin alter IKKb Cys-179 and also inhibit IKK-dependent NF-kB activation (37, 38). As2O3 has recently been used in clinical trials against promyelocytic leukaemia and solid tumors (39–41) with the nuance that several signalling pathways may be involved in its anti-tumor effect (42). Auranofin is also known to induce apoptosis in promyelocytic leukaemia cells (43).
3. The target molecule candidates
The NF-kB signal starts from ligands such as cytokines or LPS (lipopolysaccharide), goes through receptors, cytosolic proteins, intranuclear proteins and DNA, and arrives at newly produced proteins. Targeting the ligands or receptors such as IL-1 and its receptors may be useful to some extent. With the view of preventing carcinogenesis, an IL-1 receptor antagonist was reported to be upregulated in the remission of ulcerative colitis (44). In advanced CRC, a study reported that a nucleotide polymorphism of the IL-1 receptor antagonist gene was correlated with the prognosis of CRC. A clinical study on the IL-1 receptor antagonist against CRC has not been conducted, but it is used in rheumatoid arthritis (45). IKKs or IkBs are now the main targets of NF-kB inhibitors. Many researchers have made various inhibitors attenuating IKKa, IKKb, IKKg or IkBa that some of them were effective in animal models. According to Hayakawa et al., NEMO binding domain peptide (NBD), one of the inhibitors of IKKb, exerts chemopreventive effects in CRC mice model (46).
Conclusion
In recent years, surgical resections as well as chemotherapy have been the only effective therapies in treating CRC, however, not very successful. As target therapy will prolong the prognosis of the patients with CRC, therefore, the exploration of more useful targets will be of great importance. NF-kB signaling is currently a well studied area that recently has become an obvious target of cancers. It appears to be a promising focus and deserves further investigations for use as a therapeutic target. Although NF-kB inhibitors have been used clinically, they seem to be far from ideal drugs because of their harmful side effects. As aforementioned, NF-kB is a key element of various physiological activation in the whole body, therefore, some toxic side effects of inhibiting NF-kB may be unavoidable.
(Please cite as: Hassanzadeh P. Colorectal cancer and NF-κB signaling pathway. Gastroenterol Hepatol Bed Bench 2011;4(3):127-132).
References
- 1.Levin KE, Dozois RR. Epidemiology of large bowel cancer. World J Surg. 1991;15:562–67. doi: 10.1007/BF01789199. [DOI] [PubMed] [Google Scholar]
- 2.Strate LL, Syngal S. Hereditary colorectal cancer syndromes. Cancer Causes Control. 2005;16:201–13. doi: 10.1007/s10552-004-3488-4. [DOI] [PubMed] [Google Scholar]
- 3.DeCosse JJ, Tsioulias GJ, Jacobson JS. Colorectal cancer: detection, treatment, and rehabilitation. 1994;44:27–42. doi: 10.3322/canjclin.44.1.27. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton SR. Colorectal carcinoma in patients with Crohn's disease. Gastroenterology. 1985;89:398–407. doi: 10.1016/0016-5085(85)90343-9. [DOI] [PubMed] [Google Scholar]
- 5.Longnecker MP. Alcohol consumption in relation to risk of cancers of the breast and large bowel. Alcohol Health Res World. 1992;16:223–29. [Google Scholar]
- 6.Chu QS, Hammond LA, Schwartz G, Ochoa L, Rha SY, Denis L, et al. Phase I and pharmacokinetic study of the oral fluoropyrimidine S-1 on a once-daily-for-28-day schedule in patients with advanced malignancies. Clin Cancer Res. 2004;10:4913–21. doi: 10.1158/1078-0432.CCR-04-0469. [DOI] [PubMed] [Google Scholar]
- 7.Tournigand C, Andre T, Achille E, Liedo G, Flesh M, Mery-Mignard D. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–37. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 8.Arsene D, Galais MP, Bouhier-Leporrier K, Reimund JM. Recent developments in colorectal cancer treatment by monoclonal antibodies. Expert Opin Biol Ther. 2006;6:1175–92. doi: 10.1517/14712598.6.11.1175. [DOI] [PubMed] [Google Scholar]
- 9.Fakih M. Anti-EGFR monoclonal antibodies in metastatic colorectal cancer: time for an individualized approach? Expert Rev Anticancer Ther. 2008;8:1471–80. doi: 10.1586/14737140.8.9.1471. [DOI] [PubMed] [Google Scholar]
- 10.Prat A, Casado E, Cortes J. New approaches in angiogenic targeting for colorectal cancer. World J Gastroenterol. 2007;13:5857–66. doi: 10.3748/wjg.v13.i44.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez J, Zarate R, Bandres E. Combining chemotherapy and targeted therapies in metastatic colorectal cancer. World J Gastroenterol. 2007;13:5867–76. doi: 10.3748/wjg.v13.i44.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agrawal A, Cha-Molstad H, Samols D, Kushner I. Overexpressed nuclear factor-κB can participate in endogenous C-reactive protein induction, and enhances the effects of C/EBP( and signal transducer and activator of transcription-3. Immunology. 2003;108:539–47. doi: 10.1046/j.1365-2567.2003.01608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rayet B, Gelinas C. Aberrant rel/nfkb genes and activity in human cancer. Oncogene. 1999;18:6938–47. doi: 10.1038/sj.onc.1203221. [DOI] [PubMed] [Google Scholar]
- 14.Pikarsky E, Ben-Neriah Y. NF-kappaB inhibition: a double-edged sword in cancer? Eur J Cancer. 2006;42:779–84. doi: 10.1016/j.ejca.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Kim HJ, Hawke N, Baldwin AS. NF-kappaB and IKK as therapeutic targets in cancer. Cell Death Differ. 2006;13:738–47. doi: 10.1038/sj.cdd.4401877. [DOI] [PubMed] [Google Scholar]
- 16.Kamata H, Honda S, Maeda S. Reactive oxygen species promote TNF alpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–61. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 17.Maeda S, Omata M. Inflammation and cancer: role of nuclear factor-kappaB activation. Cancer Sci. 2008;99:836–42. doi: 10.1111/j.1349-7006.2008.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clevers H. At the crossroads of inflammation and cancer. Cell. 2004;118:671–74. doi: 10.1016/j.cell.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109:S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 20.Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–77. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- 21.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–88. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Derudder E, dejardin E, Pritchard LL, Green DR, Korner M, Baud V. RelB/p50 dimers are differentially regulated by tumor necrosis factor-alpha and lymphotoxin-beta receptor activation: critical roles for p100. J Biol Chem. 2003;278:23278–84. doi: 10.1074/jbc.M300106200. [DOI] [PubMed] [Google Scholar]
- 23.Dejardin E, Droin NM, delhase M, Haas E, Cao Y, Markis C, et al. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity. 2002;17:525–35. doi: 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- 24.Xiao G, Harhaj EW, Sun S. NF-kappaB-Inducing Kinase Regulates the Processing of NF-kappaB2 p100. Mol Cell. 2001;7:401–09. doi: 10.1016/s1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]
- 25.Claudio E, Brown K, Park S, Wang H, Siebenlist U. BAFF-induced NEMO-independent processing of NF-kappa B2 in maturing B cells. Nat Immunol. 2002;3:958–65. doi: 10.1038/ni842. [DOI] [PubMed] [Google Scholar]
- 26.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–88. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Dejardin E. The alternative NF-kappaB pathway from biochemistry to biology: pitfalls and promises for future drug development. Biochem Pharmacol. 2006;72:1161–79. doi: 10.1016/j.bcp.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Pomerantz JL, Baltimore D. Two pathways to NF-kappaB. Mol Cell. 2002;10:693–95. doi: 10.1016/s1097-2765(02)00697-4. [DOI] [PubMed] [Google Scholar]
- 29.Sakamoto K, Maeda S, Hikiba Y. Constitutive NF-kB activation in colorectal carcinoma plays a key role in angiogenesis, promoting tumor growth. Clin Cancer Res. 2009;15:2248–58. doi: 10.1158/1078-0432.CCR-08-1383. [DOI] [PubMed] [Google Scholar]
- 30.Voboril R, Weberova-Voborilova J. Constitutive NF-kappaB activity in colorectal cancer cells: impact on radiation-induced NF-kappaB activity, radiosensitivity, and apoptosis. Neoplasma. 2006;53:518–23. [PubMed] [Google Scholar]
- 31.Lind DS, Hochwald SN, Malaty J. Nuclear factor-kB is upregulated in colorectal cancer. Surgery. 2001;130:363–69. doi: 10.1067/msy.2001.116672. [DOI] [PubMed] [Google Scholar]
- 32.Adams J. Proteasome inhibition: a novel approach to cancer therapy. Trends Mol Med. 2002;8:S49–54. doi: 10.1016/s1471-4914(02)02315-8. [DOI] [PubMed] [Google Scholar]
- 33.Kisselev AF, Garcia-Calvo M, Overkleeft HS, Peterson E, Pennington MW, Ploegh HL. The caspase-like sites of proteasomes, their substrate specificity, new inhibitors and substrates, and allosteric interactions with the trypsin-like sites. J Biol Chem. 2003;278:35869–77. doi: 10.1074/jbc.M303725200. [DOI] [PubMed] [Google Scholar]
- 34.Dick TP, Nussbaum AK, Deeg M, Heinemeyer W, Groll M, Schirle M. Contribution of proteasomal betasubunits to the cleavage of peptide substrates analyzed with yeast mutants. J Biol Chem. 1998;273:25637–46. doi: 10.1074/jbc.273.40.25637. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz R, Davidson T. Pharmacology, pharmacokinetics, and practical applications ofbortezomib. Oncology. 2004;18:14–21. [PubMed] [Google Scholar]
- 36.Lightcap ES, McCormack TA, Pien CS, Chau V, Adams J, Elliott PJ. Proteasome inhibition measurements: clinicalapplication. Clin Chem. 2000;46:673–83. [PubMed] [Google Scholar]
- 37.Jeon KI, Byun MS, Jue DM. Gold compound auranofin inhibits IkappaB kinase (IKK) by modifying Cys-179 of IKKbeta subunit. Exp Mol Med. 2003;35:61–6. doi: 10.1038/emm.2003.9. [DOI] [PubMed] [Google Scholar]
- 38.Mathas S, Lietz A, Janz M, Hinz M, Jundt F, Scheidereit C. Inhibition of NF-kappaB essentially contributes to arsenic-induced apoptosisM. Blood. 2003;102:1028–34. doi: 10.1182/blood-2002-04-1154. [DOI] [PubMed] [Google Scholar]
- 39.Kim KB, Bedikian AY, Camacho LH, Papadopoulos NE, McCullough C. A phase II trial of arsenic trioxide in patients with metastatic melanoma. Cancer. 2005;104:1687–92. doi: 10.1002/cncr.21386. [DOI] [PubMed] [Google Scholar]
- 40.Mathews V, George B, Lakshmi KM, Viswabandya A, Bajel A, Balasubramanian P. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: durable remissions with minimal toxicity. Blood. 2006;107:2627–32. doi: 10.1182/blood-2005-08-3532. [DOI] [PubMed] [Google Scholar]
- 41.Murgo AJ. Clinical trials of arsenic trioxide in hematologic and solid tumors: overview of the National Cancer Institute Cooperative Research and Development Studies. Oncologist. 2001;6:22–8. doi: 10.1634/theoncologist.6-suppl_2-22. [DOI] [PubMed] [Google Scholar]
- 42.Woo SY, Lee MY, Jung YJ, Yoo ES, Seoh JY, Shin HY. Arsenic trioxide inhibits cell growth in SH-SY5Y and SKN SKN- AS neuroblastoma cell lines by a different mechanism. Pediatr Hematol Oncol. 2006;23:231–43. doi: 10.1080/08880010500506818. [DOI] [PubMed] [Google Scholar]
- 43.Kim IS, Jin JY, Lee IH, Park SJ. Auranofin induces apoptosis and when combined with retinoic acid enhances differentiation of acute promyelocytic leukaemia cells in vitro. Br J Pharmacol. 2004;142:749–55. doi: 10.1038/sj.bjp.0705708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakimura K, Omori T, Iwashita E. Clinical response is associated with elevated plasma interleukin-1 receptor antagonist during selective granulocyte and monocyte apheresis in patients with ulcerative colitis. Dig Dis Sci. 2006;51:1525–31. doi: 10.1007/s10620-005-9012-1. [DOI] [PubMed] [Google Scholar]
- 45.Graziano F, Ruzzo A, Canestrari E. Variations in the interleukin-1 receptor antagonist gene impact on survival of patients with advanced colorectal cancer. Pharmacogenomics J. 2009;9:78–84. doi: 10.1038/tpj.2008.16. [DOI] [PubMed] [Google Scholar]
- 46.Hayakawa Y, Maeda S, Nakagawa H, Hikiba Y, Shibata W, Sakamoto K, et al. Effectiveness of IkB kinase inhibitors in murine colitis-associated tumorigenesis. J Gastroenterol. 2009;44:935–43. doi: 10.1007/s00535-009-0098-7. [DOI] [PubMed] [Google Scholar]