Abstract
Aim
In this study the anticancer activity of Lavender aqueous extract against MKN45 cell line was evaluated.
Background
Plant-based drugs are regarded as promising therapies. Lavender is a plant that has been cultivated from ancient times. An aqueous extract of Lavender has shown therapeutic effects on the nervous system in the high doses based on in-vivo studies. Gastric cancer is one of the frequent cancers in Iranian population. We therefore assessed the effect of Lavender upon a gastric cancer cell line.
Patients and methods
The MKN45 cancer cell line was selected for treatment with aqueous extract of Lavender. Survival of MKN45 cell line was studied in the presence of various concentrations of Lavender extract by MTT assay method. Morphological studies were performed via microscopic analyses. Flow cytometry and proteomics techniques were applied to determining pharmaceutical mechanism of lavender cytotoxic effects.
Results
The survival and morphological studies revealed anticancer characteristics of extract. Flow cytometry findings indicate that Lavender extract had a cytotoxic effect upon the cell line. Proteomics analysis identified a significant alternation in gastric cellular proteome expression after treating with the extract. Among 1000 spots, more than 700 spots showed changes in protein expression levels by informatics analysis. Of these proteins, expression of three cancer biomarkers, Annexin1, Anolase1 and HSP70 were suppressed by extract.
Conclusion
This study suggests that Lavender extract is cytotoxic and alter protein expression in a gastric cancer cell line.
Keywords: MKN45 Cell Line, Lavender, Proteomics, Flow cytometry
Introduction
Gastric cancer is the fourth most common cancer worldwide (1). There is a high incidence of gastric cancer in developing countries, and based on 2008 reports, more than 990000 cases come about worldwide per year (2, 3). Also, it is one of the major health problems in Iran. The north and northwest regions of Iran are the high risk areas for this type of cancer (4). Both race and gender play an important role in incident of disease and subsequent mortality rate. Therefore, this cancer is more frequent in older population particularly in seventh and eight decades of their life (3). Adenocarcinoma is the most prevalent malignant tumors of the stomach, accounting for almost 90% of gastric tumors (5). Pathologically, there are two types of gastric adenocarcinoma based upon location: cardia, or proximal, and distal, noncardia adenocarcinomas. Histologically, gastric cancer is divided into two main types: well-differentiated, intestinal type, and undifferentiated, diffuse type (6). There are lots of risk factors linked to adenocarcinoma. Many environmental factors including smoking, high salt intake, and a diet with an insufficient level of antioxidants are involved in the pathogenesis of gastric cancer endogenous and host factors, including those related to male gender (7). In addition to this, there are a variety of genes that increase the risk of stomach cancer. These specific genes such as MCC, APC, and p53 tumor suppressor genes have been identified in a large percentage of gastric cancers (8–10).
The lavenders (Lavandula) are a genus of 39 species of angiosperms plants and belong to mint family, Lamiaceae. An Old World genus, distributed from Micronesia across Africa, the Mediterranean, South-West Asia, Arabia, Western Iran and South-East India. Lavender is a most known genus that is used in therapeutic means. Lavandula Angustifolia is an essential oil and has known anticancer, antibacterial, antifungal, analgesic, antioxidant and sedative effect due to its linalool and linalyl acetate component (11–21). Also aqueous extracts of Lavandula stoechas reduced mitotic index, but induced chromosomal aberrations and mitotic aberrations (22). Recently it is reported that aqueous extract of Lavandula angustifolia has a considerably effects on alzheimer disease (23).
Proteomic analysis is the study of the proteome by the two dimensional gel electrophoresis has generated numerous datasets of potential diagnostic prognostic, and therapeutic significance by comparing the proteomic profiles between a healthy or control sample and a diseased or drug-treated sample, proteins altered in their expression levels and patterns can be identified and characterized in a cancer study (24). The aim of this study was to assess possible anticancer properties of a Lavender aqueous extract.
Methods
Cell culture
The cell culture medium (RPMI), fetal bovine serum (FBS), penicillin and streptomycin were provided by Gibco BRL (Life Technologies, Paisley, Scotland). Cell lines were obtained from cell bank (Pastuer Institute, Tehran, Iran). 3-(4, 5-dimethyl-thiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), Annexin V-FLOUS staining kit (Cat. No. 11 988 549 001) was provided from Roche Diagnostics GmbH (Germany).
The adenocarcinoma gastric cell line (MKN45) was cultured in the RPMI medium, that had been treated with FBS (10%, v/v), streptomycin (100 µg/mL), and penicillin (100 U/mL). Cultures were maintained at 37°C in 5% CO2 and 95% air, and the medium changed two times per week. The cells were seeded, in the 96 well plates and incubated at 37 °C under 5% CO2 atmosphere for 48 h. Then, the various filtered concentrations of extract (1,5,10,20,30,50,70,100,120,and 150) and 0 as control pattern were induced into cells for 48h for MTT assay proposes.
The inhibition of cellular proliferation was measured by the modified MTT 3-(4, 5 dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide]) assay, based on the ability of live cells to converting thiazolyl blue to dark blue formazan (25). Approximately 2.5 - 103 cells were seeded into 96-well culture plates and treated with or without Lavender extract at 48 hrs. After treatment, 20 ml MTT (5 g/l) was added into the wells and incubation continued at 37°C for 4 hrs, and 100 ml DMSO was pipette to solubilize the formazan product for 30 min at room temperature. The absorbency at 570 nm was measured using ELISA reader:
Percent of Cytotoxicity = (1 - mean absorbance of toxicant - treated cells) × 100 Mean absorbance of negative control Percent Viability = 100 – percent of Cytotoxicity
Microscopic study
In order to compare the cell morphology and pattern of cell distribution in the absence (without extract) and presence of the extract an inverted microscope (Ceti) was used.
Flow Cytometry analysis
For Flow Cytometry analysis, MKN45 cells were cultured into 6-well plates at a density of 1 × 106 cells in the presence and absence of the cytotoxic agents for 48 hrs. All floated and adherent cells were harvested and centrifuged at 200 ×g for 10 min. Cell pellet was washed with 1X phosphate buffer saline solution and centrifuged at 200 ×g for 10 min. The cell pellet was then re-suspended in 100 µL of Annexin V/FLUOS labeling solution (predilute 20 µLAnnexin V/FLUOS labeling reagent in 1 mL incubation buffer and add 20 µL propidium iodide solution), and incubated at 15-25 °C for 10-15 min. It was then employed to analyze the cell population analyzed by Flow Cytometer (Bio-Rad, USA). In this experiment, the cells were aspirated by PBS, and then 1×106, MKN45 cells were used. The samples were read in a FACS Flow Cytometer (USA) using 488 nm excitation and a 515 nm bandpass filter for fluorescein detection and a filter > 600 nm for propidium iodide detection. Analyses were performed by Cell Quest software supplied in the instrument.
The sample preparation for 2DE
The harvested cells were washed three times using washing buffer (250 mM D-Sorbitol and 10 mMTris, pH 7.0), subsequently lysed with lysis buffer containing 8 M urea, 4% CHAPS (3-(3-cholamidopropyl) dimethylammonio- 1-propanesulfonate), 40 mM dithiothreitol (DTT), 2% pharmalyte (pH 3–10NL), 1 mM phenylmethylsulfonyl fluoride (PMSF) and 1 mM ethylene diamine tetra-acetic acid (EDTA). The lysed cells were sonicated with a probe sonicator for 5 min followed by a centrifuge at 40 000 g for 30 min. After quantification of proteins by the Bradford method, the supernatants were stored at -20C until use for electrophoresis.
2D electrophoresis and image analysis
Isoelectric focusing electrophoresis was carried out with 17 cm (pH 3–10NL) IPG strips at -20°C according to the manufacturer's instructions. Approximately1 mg protein was loaded onto each gel and triplicate gels for each sample were run to achieve reproducible 2DE results. Briefly, the strips were rehydrated without voltage for 4 h and with 50 V for 8 h. The iso-electric focusing was programmed at a gradient mode, which was first focused for 3 hrs at the different voltages, 500, 1000 and 8000 V, respectively, then continued at 8000 V until a total of 50 KVh. The focused strips were equilibrated in buffer with 6 M urea, 50 mM Tris–HCl, 30% glycerol, 2% SDS and trace bromophenol blue, and were subsequently treated by the reduction of DTT and alkylation of iodoacetamide. The treated strips were transferred onto 12% uniform SDS polyacrylamide gels running in 2.5 W each gel for 30 min and 15 W each gel until the bromophenol blue dye reached the bottom of the gel. The gels were visualized by Coomassie brilliant blue staining. The gels were scanned by Bio Rad Image Scanner and the image analysis was conducted with Image Master 2D Platinum licensed by Bio Rad Biosciences. The threshold as the significant change in 2DE spots was detected.
Results
MTT assay is a common method for studying cell survival (26). Viability of MKN45 cell line was studied in the presence of various concentrations of Lavender extract at incubation time of 48 hrs.
Figures 1 represents viability of MKN45 cell line in the presence of filtered extract of Lavender. The extract has inhibited cell proliferation. MTT assay revealed that extract has a significant effect on cell survival. For more investigating the cell morphology in the presence and absence of extract is studied via microscopic assessments (Figure 2). The morphological study indicated that extract induced cell death accompanied by gross morphological change alteration. To identify the mechanisms of extract effect against the cell line, flow cytometry was applied; the results are presented in Figure 3. Flow cytometric findings indicated that the considerable numbers of cells (about %60) were dead via necrosis (see Figure 3, upper left) and that the rate of apoptosis was negligible (Figure 3).
Figure 1.
Viability percentage of MKN45 Cell line in the presence of 0, 1, 5, 10, 20, 30, 50, 70, 100, 120 and 150 g/mL concentrations of Lavender filtered extract at 48 hours incubation time. Results are presented as mean ± SD. Significant levels are *p < 0.05, *p < 0.01 and ***p < 0.001
Figure 2.
MKN45 cell morphology in the a) absence, b) presence of 150 g/mL, and c) presence of 30 g/mL concentration of extract
Figure 3.
A flow cytometry scheme in evaluation of: (left) Control group (the cells in the absence of extract) (right) Sample cells (the cells in the presence of 30concentration of filtered extraction) after incubation for 48 h. The cells were harvested, stained with Annexin V/FLUOS (FL-I) and propidium iodide (PI, FL-2) and analyzed by flow cytometry. Four populations are resolved. Living cells or Annexin- V/FLUOS (-) /PI (-) (LL) are seen in the lower left quadrant. Cells that are Annexin V/FLUOS (+)/PI (-) (LR) are apoptotic (lower right). The cell population with Annexin V/FLUOS (+)/PI (+) (UR) has been described as necrotic or advanced apoptotic (upper right) and Annexin V/FLUOS (-)/PI (+) (UL) may be bare nucle cells in late necrosis, or cellular debris (upper left)
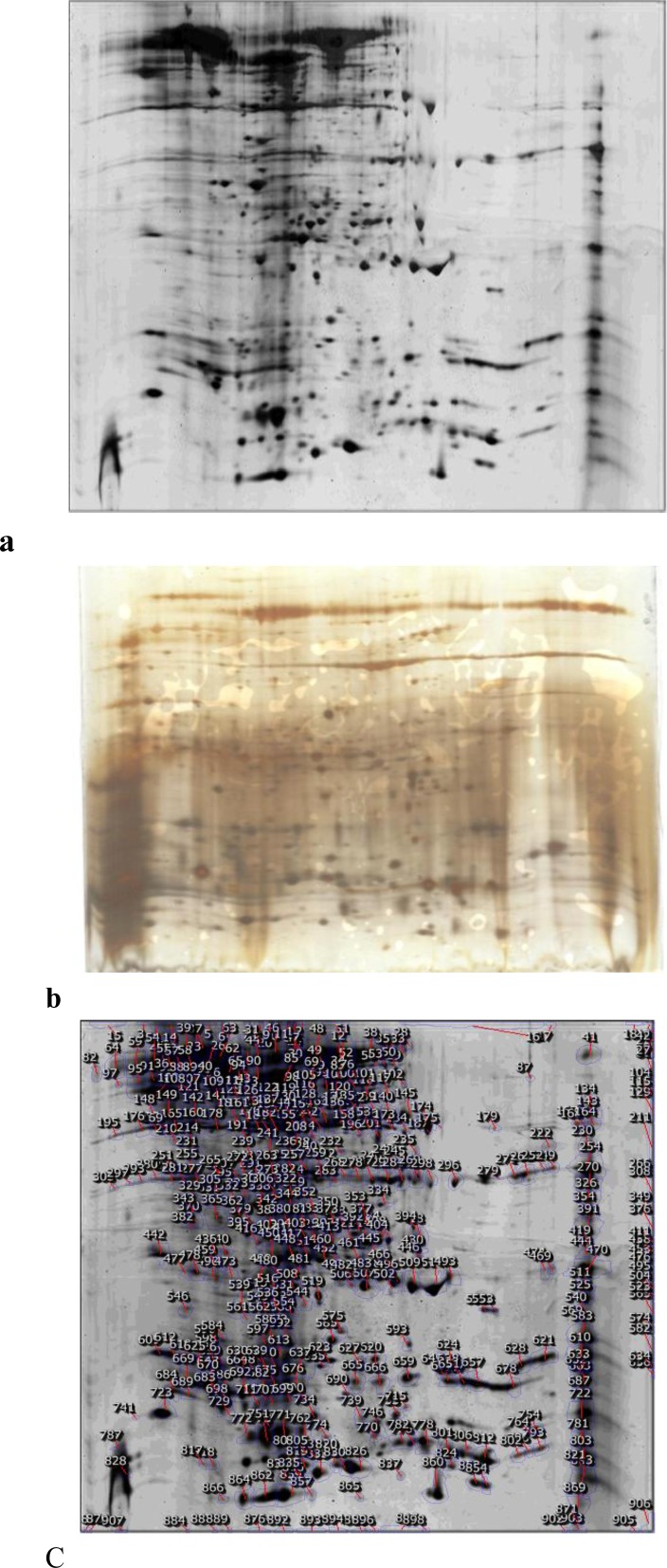
As flow cytometry has revealed that necrosis is the main mechanism of the cell death, Proteomics was used for determining effect of the Lavender extract upon gene expression. It was expected that, in correspond to necrosis, rate of alternation in gene expression will be noticeable. A large number of proteins are candidates as biomarkers of malignant transformation expression. Proteomes of the gastric cancer cell line in the presence and absence of extract were provided and compared (See Figure 4). Surprisingly, there was a considerable difference in the proteomes of the cell line treated with Lavender extract and the control cell line. As shown in Figure 4, there are about 1000 spots that are comparable between two gels. Clustering as a logical method for proteins classification and also showing schematically representation of their relation is a useful method for better understanding of protein expression alteration (27).
Figure 4.
Comparison of 2DE gels presented as a control MKN45 (a) and treated cells with 30 g/mL concentration of Lavender extract (b). The gel of untreated cells is selected as reference gel and the spots are marked on this gel (c). Due to expanding dimension of alternations, protein clustering has been done (the result are not shown).
Clustering of proteins that their expressions show considerable alteration is done by same spot software (see Figure 5). As it is depicted in Figure 5, the proteins that their expression is changed, the rates of alteration is significant and are grouped in four clusters.
Figure 5.
Clustering analysis of proteins on the gels of untreated (condition 1) and treated (condition 2) cell line with extract. There are four distinct clusters and the proteins that their expression are remarkably changed, are located in one cluster.
Discussion
Since there is no significant evidence of anticancer properties of Lavender aqueous extract, various concentrations of extract were provided and treated with MKN45 cell line for survival examination. As it is shown in Figure 1, 5 µg/mL concentration of extract will reduces cell survival up to 80%. Therefore, microscopic study of cell morphology was performed. The results (see Figure 2) are consisted with MTT assay findings. Understanding Lavender aqueous extract effects on cell proliferation mechanisms is important so flow cytometry as a one of the best choices (26) was applied for this assessment. As it is shown in Figure 3, necrosis is the main mechanism that the extract induces in MKN45 cells viability. Occurrence of about 60% necrosis versus about 4% apoptosis indicates that extract induces non programmed death. Previously Soheili Kashani et al. reported that this extract had pharmacological properties and no poisonous effect on rats at concentration of 200 µg/mL (28). It can be concluded that in-vivo and in-vitro effects of lavender and also its effect of cellular and organism levels are significantly different. This is confirmed by several reported data about other kinds of herbs (26). Proteomics is a powerful technique to study protein expression process (29, 33). Gel analysis by same spot software has shown that expression of the huge groups of the genes is altered by the Lavender extract. The proteins with altered expression are located in one cluster. Since clustering is a method based on cellular compartment, molecular function and biological process aspects of proteins, it can be concluded that the proteins may be localized to a single cellular compartment or related to a single cellular function
The findings of this study indicate that, Lavender aqueous extract has a significant effect on cell proliferation and inhibits their growth via necrosis. This effect is accompanied by considerable changes in the gastric tumor cells protein expression. Finally lavender extract may be a suitable candidate for more investigations as an anti cancer drug.
(Please cite as: Zamanian- Azodi M, Rezaie-Tavirani M, Heydari-Kashal S, kalantari S, Dailian S, Zali H. Proteomics analysis of MKN45 cell line before and after treatment with Lavender aqueous extract. Gastroenterol Hepatol Bed Bench 2012;5(1):35-42).
References
- 1.Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol. 2009;472:467–77. doi: 10.1007/978-1-60327-492-0_23. [DOI] [PubMed] [Google Scholar]
- 2.De Vries AC, Kuipers EJ. Epidemiology of premalignant gastric lesions: implications for the development of screening and surveillance strategies. Helicobacter. 2007;12:22–31. doi: 10.1111/j.1523-5378.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- 3.Katanoda K, Yako-Suketomo H. Comparison of time trends in stomach cancer incidence (1973–2002) in Asia, from cancer incidence in five continents, vols IV–IX. Jpn J Clin Oncol. 2009;39:71–72. doi: 10.1093/jjco/hyn150. [DOI] [PubMed] [Google Scholar]
- 4.Malekzadeh R, Derakhshan MH, Malekzadeh Z. Gastric Cancer in Iran: Epidemiology and Risk Factor. Arch Iranian Med. 2009;12:576–83. [PubMed] [Google Scholar]
- 5.Fuchs CS, Mayer RJ. Gastric carcinoma. N Engl J Med. 1995;333:32–41. doi: 10.1056/NEJM199507063330107. [DOI] [PubMed] [Google Scholar]
- 6.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinaltype carcinoma. An attempt at a histo-clinical classification. ActaPatholMicrobiolScand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 7.Derakhshan MH, Liptrot S, Paul J, Brown IL, Morrison D, McColl KE. Oesophageal and gastric intestinal-type adenocarcinomas show the same male predominance due to a 17 year delayed development in females. Gut. 2009;58:16–23. doi: 10.1136/gut.2008.161331. [DOI] [PubMed] [Google Scholar]
- 8.Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P, et al. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392:402–5. doi: 10.1038/32918. [DOI] [PubMed] [Google Scholar]
- 9.Oliveira C, Seruca R, Carneiro F. Hereditary gastric cancer. Best Pract Res Clin Gastroenterol. 2009;23:147–57. doi: 10.1016/j.bpg.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs CS, Mayer RJ. Gastric carcinoma. N Engl J Med. 1995;333:32–41. doi: 10.1056/NEJM199507063330107. [DOI] [PubMed] [Google Scholar]
- 11.Sugawara Y, Hino Y, Kawasaki M, Hara C, Tamura K, Sugimoto N, et al. Alteration of perceived fragrance of essential oils in relation to type of work: a simple screening test for efficacy of aroma. Chem Senses. 1999;24(4):415–21. doi: 10.1093/chemse/24.4.415. [DOI] [PubMed] [Google Scholar]
- 12.Satoh T, Sugawara Y. Effects on humans elicited by inhaling the fragrance of essential oils: sensory test, multi-channel thermometric study and forehead surface potential wave measurement on basil and peppermint. Anal Sci. 2003;19(1):139–46. doi: 10.2116/analsci.19.139. [DOI] [PubMed] [Google Scholar]
- 13.Sugawara Y, Hino Y, Kawasaki M, Hara C, Tamura K, Sugimoto N, et al. Sedative effect on humans of inhalation of essential oil of linalool: sensory evaluation and physiological measurements using optically active linalools. Analytica Chimica Acta. 1998;365:293–99. [Google Scholar]
- 14.Buchbauer G, Jirovetz L, Jager W, Dietrich H, Plank C. Aromatherapy: evidence for sedative effects of the essential oil of lavender after inhalation. Z Naturforsch (C) 1991;46:1067–72. doi: 10.1515/znc-1991-11-1223. [DOI] [PubMed] [Google Scholar]
- 15.Ghelardini C, Galeotti N, Salvatore G, Mazzanti G. Local anaesthetic activity of the essential oil of Lavan dulaan gustifolia. Planta Med. 1999;65:700–3. doi: 10.1055/s-1999-14045. [DOI] [PubMed] [Google Scholar]
- 16.Cosentino S, Tuberoso CI, Pisano B, et al. In -vitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Lett Appl Microbiol. 1999;29:130–35. doi: 10.1046/j.1472-765x.1999.00605.x. [DOI] [PubMed] [Google Scholar]
- 17.Subramanyam Pattnaik S. Antibacterial and antifungal activity of aromatic constituents of essential oils. Microbios. 1997;89:39–46. [PubMed] [Google Scholar]
- 18.Adam K, Sivropoulou A, Kokkini S, Lanaras T, Arsenakis M. Antifungal activities of Origanum vulgare subsp. hirtum, Mentha spicata, Lavandula angustifolia, and Salvia fruticosa essential oils against human pathogenic fungi. J Agricul Food Chem. 1998;46:1739–45. [Google Scholar]
- 19.Yarnell E. Essential oils against lice. Quarterly Review of Natural Medicine. 1998;3:177–84. [Google Scholar]
- 20.Hink W, Fee J. Toxicity of D-limonene, the major component of citrus peel oil, to all stages of the cat flea, Ctenocephalides felis . J Med Entomol. 1986;23:400–4. doi: 10.1093/jmedent/23.4.400. [DOI] [PubMed] [Google Scholar]
- 21.Hink WF, Liberati TA, Collart MG. Toxicity of linalool to life stages of the cat flea, Ctenocephalides felta (Siphonaptera pulicidae) and its eficacy in capres and on animals. J Med Entomol. 1988;25:1–4. doi: 10.1093/jmedent/25.1.1. [DOI] [PubMed] [Google Scholar]
- 22.Perrucci S, Cioni PL, Flamini G, Morelli I, Macchioni G. Acaricidal agents of natural origin against Psoroptescuniculi. Parassitologia. 1994;36:269–71. [PubMed] [Google Scholar]
- 23.O'Brien DJ. Treatment of psoroptic mange with reference to epidemiology and history. Vet Parasitol. 1999;83:177–85. doi: 10.1016/s0304-4017(99)00056-4. [DOI] [PubMed] [Google Scholar]
- 24.Jager W, Buchbauer G, Jirovetz L, Fritzer M. Percutaneous absorption of lavender oil from massage oil. Journal of the Society of Cosmetic Chemists. 1992;43:49–54. [Google Scholar]
- 25.Celik TA, Aslantürk OS. Investigation of cytotoxic and genotoxic effects of Ecballium elaterium juice based on Allium test. Methods Find Exp Clin Pharmacol. 2009;31:591–96. doi: 10.1358/mf.2009.31.9.1434629. [DOI] [PubMed] [Google Scholar]
- 26.Soheili Kashani M, Salami M, Talaei Zavareh A, Hashemi M, Motaghi M, Rezaei Tavirani M. Attenuating effects of aqueous extract of Lavandulaangusti folia on Alzheimeric rat's spatial learning. Medical Science Journal of Islamic Azad University, Tehran Medical Branch; 2011. pp. 221–27. [Google Scholar]
- 27.Reymond MA, Schlegel W. Proteomics in Cancer. Adv Clin Chem. 2007;44:103–42. doi: 10.1016/s0065-2423(07)44004-5. [DOI] [PubMed] [Google Scholar]
- 28.Mosmann T. Tim Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;16(65):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 29.Ardeshiry-lajimi AB, Rezaie-Tavirani M, Mortazavi SA, Barzegar M, Moghadamnia SH, Rezaee MB. Study of Anti Cancer Property of Scrophularia striata extract on the Human Astrocytoma Cell Line (1321) Iranian Journal of Pharmaceutical Research. 2010;9:403–10. [PMC free article] [PubMed] [Google Scholar]
- 30.Pucci-Minafra I, Cancemi P, Albanese NN, Di Cara G, Marabeti MR, Marrazzo A, et al. New Protein Clustering of Breast Cancer Tissue Proteomics Using Actin Content as a Cellularity Indicator. J Proteome Res. 2008;7:1412–18. doi: 10.1021/pr700748m. [DOI] [PubMed] [Google Scholar]
- 31.Soheili Kashani M, Rezaei Tavirani M, Talaei A, Salami M. Aqueous extract of lavender (Lavandula angustifolia) improves the spatial performance of a rat model of Alzheimer's disease. Neurosci Bull. 2011;27:99–106. doi: 10.1007/s12264-011-1149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hennig L. Patterns of beauty – omics meets plant development. Trends Plant Sci. 2007;12:287–93. doi: 10.1016/j.tplants.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Bachi A, Bonaldi T. Quantitative proteomics as a new piece of the systems biology puzzle. J Proteomics. 2007;71:357–67. doi: 10.1016/j.jprot.2008.07.001. [DOI] [PubMed] [Google Scholar]