Abstract
Aim
Since data about prevalence of JC virus in Iranian population is scarce, this study was designed to evaluate the prevalence of JC virus in healthy individuals who had attended Fajr hospital and Farjam clinical laboratory in Tehran, Iran.
Background
JC virus is the causative agent of progressive multifocal encephalopathy (PML) in individuals with suppressed immune system. There are some evidences that this virus is responsible for some forms of cancers for example colorectal and gastric cancers in humans.
Patients and methods
Urine samples from 133 healthy individuals older than 18 years old were collected and after extraction of viral DNA, PCR was performed to determine the presence of virus. Results of the test and demographic data of subjects were entered into SPSS program and were analyzed by it.
Results
71 subjects were male and 62 individuals were female. Mean age of the population was 42.23 ± 13.47. From the total number of 133 subjects, 51 (38.3%) individuals were positive for the presence of JC virus. Gender had statistically significant relationship with JC virus presence (p= 0.042). Age was not significantly related to JC virus presence status (p= 0.3).
Conclusion
Obtained rate of JC prevalence in this study is similar to the results of studies in India and Philippine. Because of this virus's role in AIDS and the role of this virus in gastrointestinal cancers have been revealed in recent years, the more extended studies on the prevalence of this virus in different populations in Iran is necessary.
Keywords: JC virus, Prevalence, Iran
Introduction
JC virus is a double stranded circular DNA virus which belongs to the Polyomaviridae family. Members of this family are small non-enveloped viruses, which infect a wide range of hosts (1). Other members of this family including BK virus and Simian Vacuolating virus 40 (SV40 virus) are well known for their role in kidney allograft infection and tumorogenetic properties, respectively (2, 3). JC is the initials of a patient's name with progressive multifocal leukoencephalopathy (PML); this virus was extracted from its brain in 1971 (4). Transmission routes of this virus are not completely understood, but since this virus has been detected in untreated sewage, the fecal-oral route is probably the main route of transmission (5).
After primary infection in tonsils, this virus establishes a latent infection- which persists lifetime- in epithelium cells of kidney (6). In case of immune suppression, for example in AIDS patients or those who undergo organ transplantation, this virus reactivates and causes PML, which in 20 to 50 percent of patients is lethal in the first three months of diagnosis (7). Whether the virus in PML patients came from kidney or virus is dormant in CNS is still unclear.
There are some evidences that this virus has roles in some forms of cancer in human. It is speculated that it is responsible for some forms of brain cancer (8), colorectal cancer (9, 10) and gastric cancer (11). This virus encodes a protein transforming antigen (T-Ag), which virus tumorogenicity is ascribed to this protein. It has been shown in Enam study that JCV T-antigen interacts with beta-Catenin which deregulates the Wnt signaling pathway in the tumor cells. This in turn, enhances the transcription from c-myc promoter. This paves the way for tumorogenesis of this virus (10).
In recent years there have been many studies, which investigated the relationship between the oncogenic viruses like JC virus and gastrointestinal tumors in humans (12, 13). These studies suggest that JC virus has a role in esophageal cancer (14). A recent study by Shin and colleagues demonstrated the expression of the oncogenic JC virus protein T-Ag in a subset of gastric cancer cases (15). These findings were confirmed by Murai and his colleagues (16). As stated before this virus upregulate the beta-Catenin protein in cancerous cells in colorectal patients (10). This has been reported by other researchers as well (17, 18).
The infection with this virus occurs before adulthood and high percentage of the population are positive by the age of 18 (19). Antibody against this virus has been found in different parts of the world from Europe to south Pacific Islands and from North America to remote parts of Brazil (20). The prevalence rate of this virus is diverse in different countries and populations. The rate varies from 44 to 77 percent in USA and England to 85 to 97 percent in Brazil and Japan (21). In Iran, a study was performed in AIDS patients by Behbahani and colleagues to evaluate the JC virus. They reported that excretion of JC virus in urine is not related to HIV infection (22).
There is scarce information about prevalence of this virus in general population in Iran. The aim of this study was to determine the infection rate of JC virus in adult healthy individuals who had attended Fajr hospital and Farjam clinical laboratory.
Patients and Methods
Sample collection: 133 samples of urine from healthy individuals (excluding criteria were Progressive Multifocal Leukoencephalopathy (PML), cancer and chronic kidney infection), who were above 18 years old and attended the Fajr hospital and Farjam clinical laboratory for annual checkup from June to October 2010. These samples were kept in -20 degree Celsius until the DNA extraction.
DNA extraction from urine: Extraction was performed according to Agostini and colleagues method (23). In the first phase urine samples were concentrated through centrifuging. Then the extraction was performed by using the QIAamp viral RNA kit (QIAGEN, Germany). All the stages were performed according to the kit manual. The extracted DNA was kept in -20 degree Celsius until PCR test.
PCR: Since the viral load in urine samples was low, we utilized two separate amplification processes. In the first PCR, we used Ryschkewitsch method for amplification, which produced a 215 bp fragment. PCR's details has been described somewhere else (24). We used the generunner program for designing the primers who were targeted for VP1 gene. The second PCR's product length was 1110 bp. 5 micro liters of extracted DNA used as the template, other materials included: 1X PCR buffer, MgCl2 1.5mM, 0.2 mM of dNTPs, 10 pM of primers, 2 Units of Taq DNA polymerase. The sequence of primers is listed in the Table 1.
Table 1.
Primer sequence
| Forward | 60°C |
| 5’-AGC AAT CAA AGC AAT AGC AATC-3’ | |
| Reverse | |
| 5’-CAG CCT CAG AAA CAG TAG CAAC-3’ |
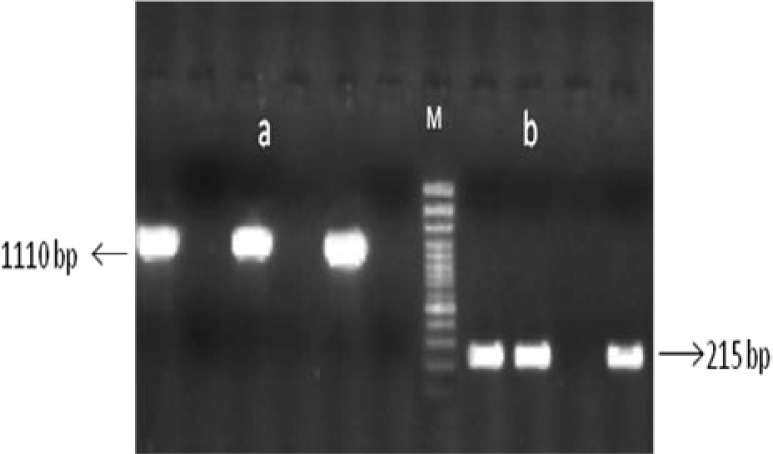
The PCR conditions were as follow: 95°C for 4 min, 35 cycles of 95°C for 45 sec, annealing for 1 min at 60°C, extension for 1 min at 72°C and a final extension step at 72°C for 10 min. The PCR products were visualized by electrophoresis on 1% agarose gel. The bands were visible under UV illuminator. The products of these PCR are shown in Figure 1.
Figure 1.
Panel a: product of R1 reaction, Panel b: the product of JLP. M DNA molecular weight marker (gene ruler DNA ladder 100 bp, Fermentas, Latvia)
The results of tests and the demographics of patients were entered in SPSS 19.0 program and were analyzed. Pearson's chi square was used for analyzing the independence of discrete variables and Mann-Whitney test was used for analyzing the non-discrete variables. Continuous variables are represented here as mean± standard deviation and other parameters as frequency and percentage. P-value of 0.05 or less was considered statistically significant and all reported p-values were two sided.
Results
Total number of 133 urine samples from healthy individuals was investigated in this study. Mean age of the studied population was 42.2±13.5. Seventy one subjects (53.3%) were male with age range from 20 to 83 years, and sixty two subjects (46.7%) were female with age range of 20 to 84 years old. Mean age of females was 44.8±14.5 and the mean age of males was 41.7±12.3. There was no significant difference in two sexes in regards to age (p= 0.18).
Fifty one subjects (38.3%) were positive for JC virus presence and 70 subjects (52.6%) were negative. Both PCR tests confirmed these results. From 51 positive subjects, 31 (60.8%) individuals were male and 20 (39.2%) were female. While 29.9% of women were positive in regards to presence of JC virus, 47 percent of male subjects were positive. There was a statistically significant difference between two sexes in regards to their positivity condition (p = 0.04). Male subjects have higher chance of infection in comparison to female subjects (OR = 2.08: 95%CI = 1.02-4.24).
Mean age of JC virus positive subjects was 44.6±13, whereas the mean age of negative subjects was 42.4±13.4. The difference between the mean age of these two groups was not statistically significant (p = 0.3). After dividing these subjects in three groups according to their age (Table 1), we observed that in the younger subjects the prevalence rate was lower 36.5% compare to older subjects 52.6%. There was no significant differences in these three age groups in regards to JC virus positivity (p = 0.67).
Table 2.
JC virus prevalence according to age groups*
| Age groups (years) | Subjects | JC virus positive |
|---|---|---|
| <40 | 63 (47.4)† | 23 (36.5) |
| 40-60 | 51 (38.3) | 18 (35.3) |
| >60 | 19 (14.3) | 10 (52.6) |
| Total | 133 (100) | 51 (100) |
p= 0.67
Number (percent)
Discussion
This study shows that the rate of JC virus presence among healthy individuals in Tehran is moderate. This virus is mostly attributed to PML and other diseases in immune-compromised individuals like AIDS patients and patients who had bone marrow or kidney transplants. However this virus is shed in normal healthy individuals in their urine, too. In a study performed by Sundsfjord and colleagues, researchers found out that in 20 percent of healthy control individuals JC virus will shed through urine (viruria) (25). In another study performed in Italy, Pagani and colleagues found out that the 46% of healthy individuals have viruria (26). In a study performed by Behbahani and colleagues in Shiraz, Iran, researchers observed that 33 percent of healthy individuals had JC virus, while in this rate in HIV positive patients was 82.5% (22)
We observed in this study that men had higher JC positive cases than women- 47% compare to 29.9%, respectively. Gender has a significant relationship in regards to JC virus presence status (p= 0.042). Men had two fold risk of viruria compare to women subjects (OR= 2.081 CI= 1.021-4.244). This observation is in accordance with other studies in different parts of the world (26–29). The reason for this observation is not clear, but some researchers have attributed it to the physiological differences between male and female subjects.
We did not find a statistically significant relationship between age and JC virus presence status. This is in contradiction with results obtained in Rodrigues (23, 27, 29). However we observed that the rate of JC virus positivity was higher in older subjects compare to younger individuals. The reason that we did not saw the significant relationship might be ascribed to the differences between Iranian population and those mentioned above. These researchers ascribed the significant relation between age and JC virus to demising the immune system of subjects.
As mentioned earlier in this discussion, there is a difference in the rate of JC virus among different population. Obtained rate of JC virus prevalence in this study was 38.3%, which is in accordance with prevalence rate reported from India 42% (30) and Philippine 47% (31) and is much lower from china 62-80% (30) and higher than Korea 20% (29). The rate of JC virus prevalence is also diverse in Europe. The reported rates include: Portugal 23.9% (27), Ireland 20.7% (32), Italy 40.2% and Spain 41.2% (34). The rate of JC virus excretion reported in a study in America is 41.2% (28), which is also in accordance with our findings.
Since the AIDS incidence in Iran is increasing and PML is one cause of mortality among AIDS patients, further studies about prevalence of this virus in other cities of Iran is of great importance. In addition, this virus is a candidate in etiology of some forms of cancers, like colorectal cancer and gastric cancer. These facts highlight the need for public health officials to investigate more deeply the presence of this virus in Iranian population and also its causal relationship with different diseases.
Gastrointestinal cancers and especially gastric and colorectal cancers are among the most important cancers with high incidence in Iranian population (35). Currently the infectious agents are in the center of researchers’ attention, because we can prevent some of them and with a simple test diagnose them and in some cases cure them. The JC virus is one of ubiquitous pathogens which almost half of the world population is infected with it. This virus has carcinogenic characteristics have been proved in recent years (12, 13). The role of this virus in esophageal, gastric and colorectal cancers has been shown to the world (10, 14, 15). This observation necessitates the more extended study on the prevalence of this virus and also its relation with gastrointestinal cancers in Iranian population.
(Please cite as: Majidizadeh Bozorgi S, Tahaei SME, Mohebbi SR, Sahba N, Damavand B, Romani S, et al. Molecular prevalence of JC virus in Tehran, Iran. Gastroenterol Hepatol Bed Bench 2012;5(2):84-89.)
References
- 1.Gardner S, Knowels W. Human polyomaviruses. 3rd edn. John Wiley; 1995. [Google Scholar]
- 2.Abend JR, Jiang M, Imperiale MJ. BK virus and human cancer: innocent until proven guilty. Semin Cancer Biol. 2009;19:252–60. doi: 10.1016/j.semcancer.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkin SJ, Griffin BE, Dilworth SM. Polyoma virus and simian virus 40 as cancer models: history and perspectives. Semin Cancer Biol. 2009;19:211–17. doi: 10.1016/j.semcancer.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Padgett BL, Zurhein GM, Walker DL, Eckroade RJ, Dessel BH. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971;297:1257–60. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- 5.Bofill-Mas S, Girones R. Excretion and transmission of JCV in human populations. J NeuroVirol. 2001;7:345–49. doi: 10.1080/13550280152537210. [DOI] [PubMed] [Google Scholar]
- 6.Dörries K. Molecular biology and pathogenesis of human polyomavirus infections. Dev Biol Stand. 1998;94:71. [PubMed] [Google Scholar]
- 7.Brew BJ, Davies NWS, Cinque P, Clifford DB, Nath A. Progressive multifocal leukoencephalopathy and other forms of JC virus disease. Nat Rev Neurol. 2010;6:667–79. doi: 10.1038/nrneurol.2010.164. [DOI] [PubMed] [Google Scholar]
- 8.Khalili K, Del Valle L, Otte J, Weaver M, Gordon J. Human neurotropic polyomavirus, JCV, and its role in carcinogenesis. Oncogene. 2003;22:5181–91. doi: 10.1038/sj.onc.1206559. [DOI] [PubMed] [Google Scholar]
- 9.Laghi L, Randolph AE, Chauhan DP, Marra G, Major EO, Neel JV, et al. JC virus DNA is present in the mucosa of the human colon and in colorectal cancers. Proc Natl Acad Sci U S A. 1999;96:7484–89. doi: 10.1073/pnas.96.13.7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enam S, Del Valle L, Lara C, Gan DD, Ortiz-Hidalgo C, Palazzo JP, et al. Association of human polyomavirus JCV with colon cancer: evidence for interaction of viral T-antigen and beta-catenin. Cancer Res. 2002;62:7093–101. [PubMed] [Google Scholar]
- 11.Yamaoka S, Yamamoto H, Nosho K, Taniguchi H, Adachi Y, Sasaki S, et al. Genetic and epigenetic characteristics of gastric cancers with JC virus T-antigen. World J Gastroenterol. 2009;15:5579–85. doi: 10.3748/wjg.15.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selgrad M, Malfertheiner P, Fini L, Goel A, Boland CR, Ricciardiello L. The role of viral and bacterial pathogens in gastrointestinal cancer. J Cell Physiol. 2008;216:378–88. doi: 10.1002/jcp.21427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins D, Hogan AM, Winter DC. Microbial and viral pathogens in colorectal cancer. Lancet Oncol. 2011;12:504–12. doi: 10.1016/S1470-2045(10)70186-8. [DOI] [PubMed] [Google Scholar]
- 14.Del Valle L, White MK, Enam S, Pina Oviedo S, Bromer MQ, Thomas RM, et al. Detection of JC virus DNA sequences and expression of viral T antigen and agnoprotein in esophageal carcinoma. Cancer. 2005;103:516–27. doi: 10.1002/cncr.20806. [DOI] [PubMed] [Google Scholar]
- 15.Shin SK, Li MS, Fuerst F, Hotchkiss E, Meyer R, Kim IT, et al. Oncogenic T-antigen of JC virus is present frequently in human gastric cancers. Cancer. 2006;107:481–88. doi: 10.1002/cncr.22028. [DOI] [PubMed] [Google Scholar]
- 16.Murai Y, Zheng HC, Abdel Aziz HO, Mei H, Kutsuna T, Nakanishi Y, et al. High JC virus load in gastric cancer and adjacent non-cancerous mucosa. Cancer Sci. 2007;98:25–31. doi: 10.1111/j.1349-7006.2006.00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goel A, Arnold CN, Niedzwiecki D, Chang DK, Ricciardiello L, Carethers JM, et al. Characterization of sporadic colon cancer by patterns of genomic instability. Cancer Res. 2003;63:1608–14. [PubMed] [Google Scholar]
- 18.Ricciardiello L, Laghi L, Ramamirtham P, Chang CL, Chang DK, Randolph AE, et al. JC virus DNA sequences are frequently present in the human upper and lower gastrointestinal tract. Gastroenterology. 2000;119:1228–35. doi: 10.1053/gast.2000.19269. [DOI] [PubMed] [Google Scholar]
- 19.Knowles WA, Pipkin P, Andrews N, Vyse A, Minor P, Brown DWG, et al. Population based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol. 2003;71:115–23. doi: 10.1002/jmv.10450. [DOI] [PubMed] [Google Scholar]
- 20.Padgett B, Walker D. New human papovaviruses. Prog Med Virol. 1976;22:1–35. [PubMed] [Google Scholar]
- 21.Knowles WA. Discovery and epidemiology of the human polyomaviruses BK virus (BKV) and JC virus (JCV) Adv Exp Med Biol. 2006;577:19–45. doi: 10.1007/0-387-32957-9_2. [DOI] [PubMed] [Google Scholar]
- 22.Behzad-Behbahani A, Klapper PE, Vallely PJ, Cleator GM, Khoo SH. Detection of BK virus and JC virus DNA in urine samples from immunocompromised (HIV-infected) and immunocompetent (HIV-non-infected) patients using polymerase chain reaction and microplate hybridisation. J Clin Virol. 2004;29:224–29. doi: 10.1016/S1386-6532(03)00155-0. [DOI] [PubMed] [Google Scholar]
- 23.Agostini HT, Deckhut A, Jobes DV, Girones R, Schlunck G, Prost MG, et al. Genotypes of JC virus in east, central and southwest Europe. J Genl Virol. 2001;82:1221. doi: 10.1099/0022-1317-82-5-1221. [DOI] [PubMed] [Google Scholar]
- 24.Ryschkewitsch CF, Friedlaender JS, Mgone CS, Jobes DV, Agostini HT, Chima SC, et al. Human polyomavirus JC variants in Papua New Guinea and Guam reflect ancient population settlement and viral evolution. Microb Infect. 2000;2:987–96. doi: 10.1016/s1286-4579(00)01252-1. [DOI] [PubMed] [Google Scholar]
- 25.Sundsfjord A, Flaegstad T, Flo R, Spein AR, Pedersen M, Permin H, et al. BK and JC viruses in human immunodeficiency virus type 1-infected persons: prevalence, excretion, viremia, and viral regulatory regions. J Infect Dis. 1994;169:485–90. doi: 10.1093/infdis/169.3.485. [DOI] [PubMed] [Google Scholar]
- 26.Pagani E, Delbue S, Mancuso R, Borghi E, Tarantini L, Ferrante P. Molecular analysis of JC virus genotypes circulating among the Italian healthy population. J Neurovirol. 2003;9:559–66. doi: 10.1080/13550280390241269. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigues C, Pinto D, Medeiros R. Molecular epidemiology characterization of the urinary excretion of polyomavirus in healthy individuals from Portugal--a Southern European population. J Med Virol. 2007;79:1194–98. doi: 10.1002/jmv.20907. [DOI] [PubMed] [Google Scholar]
- 28.Agostini HT, Ryschkewitsch CF, Stoner GL. Genotype profile of human polyomavirus JC excreted in urine of immunocompetent individuals. J Clin Microbiol. 1996;34:159–64. doi: 10.1128/jcm.34.1.159-164.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeong BH, Lee KH, Choi EK, Kim K, Kim YS. Genotyping of the JC virus in urine samples of healthy Korean individuals. J Med Virol. 2004;72:281–89. doi: 10.1002/jmv.10568. [DOI] [PubMed] [Google Scholar]
- 30.Cui X, Wang JC, Deckhut A, Joseph BC, Eberwein P, Cubitt CL, et al. Chinese strains (Type 7) of JC virus are afro-asiatic in origin but are phylogenetically distinct from the Mongolian and Indian strains (Type 2D) and the Korean and Japanese strains (Type 2A) J Mol Evol. 2004;58:568–83. doi: 10.1007/s00239-003-2579-2. [DOI] [PubMed] [Google Scholar]
- 31.Miranda JJ, Sugimoto C, Paraguison R, Takasaka T, Zheng HY, Yogo Y. Genetic diversity of JC virus in the modern Filipino population: implications for the peopling of the Philippines. Am J Phys Anthropol. 2003;120:125–32. doi: 10.1002/ajpa.10155. [DOI] [PubMed] [Google Scholar]
- 32.Schaffer K, Sheehy N, Coughlan S, Bergin C, Hall WW. JC virus in the Irish population: significant increase of genotype 2 in immunocompromised individuals. J Neurovirol. 2006;12:39–46. doi: 10.1080/13550280600614965. [DOI] [PubMed] [Google Scholar]
- 33.Rossi A, Delbue S, Mazziotti R, Valli M, Borghi E, Mancuso R, Calvo MG, et al. Presence, quantitation and characterization of JC virus in the urine of Italian immunocompetent subjects. J Med Virol. 2007;79:408–12. doi: 10.1002/jmv.20829. [DOI] [PubMed] [Google Scholar]
- 34.Polo C, Perez JL, Mielnichuck A, Fedele CG, Niubo J, Tenorio A. Prevalence and patterns of polyomavirus urinary excretion in immunocompetent adults and children. Clin Microbiol Infect. 2004;10:640–44. doi: 10.1111/j.1469-0691.2004.00882.x. [DOI] [PubMed] [Google Scholar]
- 35.Islamic Republic of Iran Ministry of Health and Medical Education Office of Deputy Minister for Health Center for disease control, cancer office; 2007. Iranian annual National Cancer Registration Report, 2006-2007. [Google Scholar]