Abstract
Aim
This study evaluated serum levels of zinc in patient with CD compare to healthy subjects.
Background
Celiac disease (CD) is characterized by small intestinal malabsorption of nutrients as a consequence of ingestion of wheat gluten. Zinc is an essential trace element that it has vital biological functions.
Patients and methods
Sera of 30 celiac cases and 30 healthy normal cohorts as control group were obtained. Atomic absorption spectrophotometer was employed for estimating serum zinc level.
Results
Zinc concentrations in patients diagnosed with CD were significantly lower than healthy subjects (75.97±12 compared with 92.83±18, P-value < 0.0001).
Conclusion
The result of this study shows that serum zinc concentration is decreased in celiac patients compare to healthy controls. Serum zinc may thus be a marker of CD in adults presenting with gastrointestinal symptoms.
Keywords: Celiac disease, Zinc, Atomic absorption spectrophotometer
Introduction
Celiac disease (CD) is a multifactorial disorder with both genetic and environmental factors implicated in its pathogenesis (1). Gluten, a protein complex formed by glutenin and gliadin proteins, is responsible for immune activation in susceptible individuals (2). In genetically susceptible individuals, CD is triggered by the ingestion of wheat gluten or related rye and barley proteins (3, 4). CD was considered a rare disease in Iran, but serological screening studies have shown that it is relatively common, affecting approximately 1% of the population in Europe and North America and Iran (5, 6). A gluten free diet is used to treat CD.
The mineral zinc is an essential nutrient. It is a key trace element for the growing organism and is found in almost every cell of the body. High levels occur in specific organs such as the brain, middle ear, eye, skin, hair, and nails. It plays vital role in the activity of approximately 100 enzymes, and supports a healthy immune system. This micronutrient is involved in wound healing, DNA synthesis, energy metabolism, hemoglobin production, carbon dioxide transport, prostaglandin function, synthesis of collagen, protein synthesis, and vitamin A metabolism (7). It supports normal growth and development during pregnancy, childhood, and adolescence. Several symptoms such as retardation of physical growth, poor appetite, impairment of sexual maturation and impaired taste acuity have been illustrated in zinc-deficient individual (8, 9).
Zinc is absorbed throughout the small intestine. The small intestine has a fundamental role in maintaining zinc homeostasis. CD significantly affects the proximal small intestine. So, zinc deficiency in patient suffering from CD may result from a cumulative loss of insoluble zinc complexes with fat and phosphate, exudation of zinc protein complexes into the intestinal lumen and massive loss of intestinal secretions or impaired zinc absorption because of damaged intestinal epithelial cell membrane (10). Some symptoms of CD (e.g. anorexia and reduced growth rate) also occur in zinc deficiency (11).
The aim of this study was to investigate, prospectively, the level of zinc in serum from patients with CD and compare it with healthy subjects.
Patients and Methods
Measurement of serum Zinc Levels
Serum was obtained from 30 patients, previously diagnosed with CD (14 males and16 females with mean age of 33.6 ± SD 11.3 years). The diagnosis of CD was based on clinical, serological, and histological criteria. The blood samples (5 ml) were collected in zinc-free vials under aseptic conditions in fasting patients. Then, they were centrifuged to isolate serum and stored at -20oC until further analysis. Serum was diluted in 1:5 ratio with distilled water. For standardization and calibration, standard solutions (Sigma Aldrich) containing 25, 50 and 100 mg. dl–1 of zinc were used. Acetylene gas was employed for the burner. Monochromatic light (wavelength of 213.9nm and slit-width of 1 mm) was used for zinc. The diluted samples were analyzed in triplicates in serial order. Finally, mean value obtained for each sample was reported. Thirty healthy normal volunteers 15 males and 15 females with mean age of 34.7± standard deviation 12.2 years) served as control group. The results of serum zinc measurements in CD patients were compared with zinc measurements in a control group. Atomic absorption spectrophotometer (PERKLIN ELMER 400) was employed for measurement of serum zinc.
Statistical analysis
The statistical analysis was performed using the Fisher exact test and χ2. A P value of less than 0.05 was considered statistically significant. A one way ANOVA test was used to compare three or more groups or conditions in an experiment.
Results
In this study, zinc concentrations in 30 patients suffering from CD were compared with 30 normal control subjects. For the two cohorts, CD and control, the one way ANOVA was documented (Table 1).
Table 1.
ANOVA Table for two cohorts of CD and healthy subject*
| Source | SS | DF | MS | F | Prob > F |
|---|---|---|---|---|---|
| Columns | 4267.30 | 1 | 4267.27 | 17.55 | 9.63e-005 |
| Error | 14099.10 | 58 | 243.09 | ||
| Total | 18366.40 | 59 |
The Sum of the Squares (SS), Degrees Of Freedom (DF), Mean Square (MS), F ratio(F), The p-value(Prob > F)
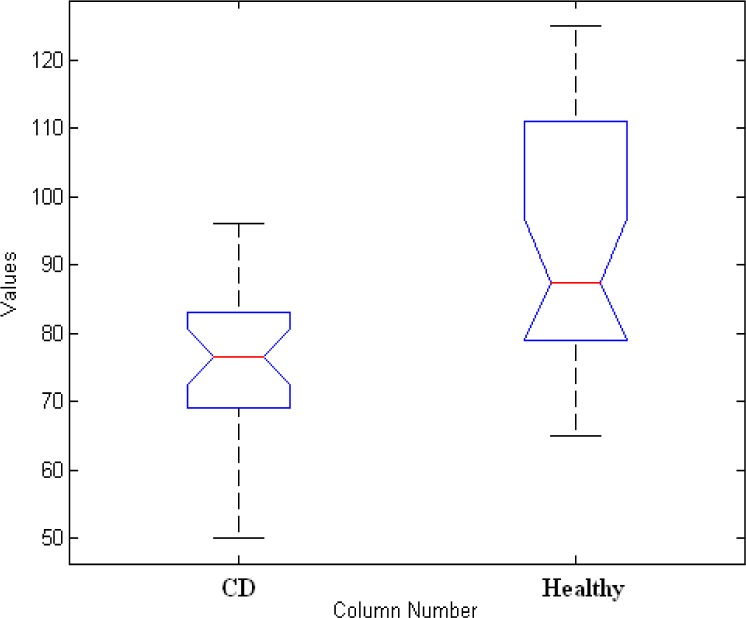
Two types of hypothesis were statistically tested. Firstly, the null hypothesis attempting to show that no variation exists between variables, or that a single variable is no different than zero. Secondly, the hypothesis proposes that statistical significance exists in a set of data. But the data presented in Table 1 shows p-value (P<0.0001). Consequently, the null hypothesis of equal means is the null hypothesis is false, the two mean squares estimate the different quantity and should be of approximately different importance. This difference was statistically significant when we compare the two groups (p< 0.0001). Figure 1 represents box plot for two cohorts. This figure represents the shape of the distribution, its central value, difference between two groups, and variability using in exploratory data analysis. As Figure 1 shows, large difference in the center lines of the boxes correspond to great values of F and small values of p-values.
Figure 1.
Box plot for 30 patients and 30 healthy subjects
The mean concentration of zinc in celiac patients compare to healthy control was 75.97±12 μg/l and 92.83±18μg/l, respectively.
Discussion
Zinc is a vital trace element that the role of this element is as a component in metallo proteins for a substantial number of human metabolic functions. The importance of zinc concentration changes in CD patients reported in previous studies (11–13).
Rawal and co-authors evaluated the effects of GFD with or without zinc supplementation on plasma zinc levels in diagnosed CD patients with low plasma zinc levels (12). Plasma zinc levels were measured in both groups after 4 weeks and compared. Also they showed that zinc levels rise with GFD irrespective of zinc supplementation.
Altuntas et al. indicated that zinc deficiency is an important problem in CD children with short stature (11). Hogberg et al. related the serum concentration of zinc to the morphology of the small bowel mucosa in 58 children, all under 4 years of age and under investigation for CD(13).
As a result of intestinal malabsorption and increased fecal zinc loss, zinc deficiency has been documented in CD and inflammatory bowel disease (14). In this study, 30 patients in the CD group and 30 in the control group had compared plasma zinc levels (P< 0.0001). Mean serum zinc concentrations were lower in patients with CD than the control group. Probably the results of several mechanisms are the reason low serum zinc concentrations in CD. The first is reason reduced intake. Other reasons include malabsorption of zinc due to the proximal small bowel enteropathy, a low serum protein-binding capacity, desaturation of plasma zinc binding sites and at last losses through damaged intestinal epithelium(13).
Small intestinal biopsy histology score has been correlated with low plasma concentrations of zinc in screening cases with CD (15). It is worthy of mention, that zinc metabolism is disturbed in chronic liver disease, because the liver plays an important role in the metabolism of zinc (16).
In conclusion, this study shows that serum zinc concentration is decreased in untreated CD. Thus in CD with gastrointestinal symptoms, Serum zinc may thus be a marker of coeliac enteropathy (10). However further studies are required to identify theoretical role of zinc in the pathogenesis and treatment of CD.
Acknowledgements
The authors would like to express their thanks to Gastroenterology and Liver Diseases Research Center and school of paramedical science, Shahid Beheshti University of Medical Sciences, for their valuable collaboration in this study. This paper is resulted from PhD thesis of Fariba Fathi.
(Please site as: Fathi F, Ektefa F, Tafazzoli M, Rostami K, Rostami Nejad M, Fathi M, et al. A concentration of serum zinc in celiac patients compare to healthy subject in Tehran. Gastroenterol Hepatol Bed Bench 2013;6(2):92-95).
References
- 1.Schuppan D. Current concepts of celiac disease pathogenesis. Gastroenterology. 2000;119:234–42. doi: 10.1053/gast.2000.8521. [DOI] [PubMed] [Google Scholar]
- 2.Koning F. Celiac disease: caught between a rock and a hard place. Gastroenterology. 2005;129:1294–301. doi: 10.1053/j.gastro.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 3.Farrell RJ, Kelly CP. Celiac sprue. N Engl J Med. 2002;346:180–88. doi: 10.1056/NEJMra010852. [DOI] [PubMed] [Google Scholar]
- 4.Green PH, Jabri B. Coeliac disease. Lancet. 2003;362:383–91. doi: 10.1016/S0140-6736(03)14027-5. [DOI] [PubMed] [Google Scholar]
- 5.Rostami Nejad M, Rostami K, Emami MH, Zali MR, Malekzadeh R. Epidemiology of celiac disease in iran; a review. Middle East Journal of Digestive Diseases. 2011;3:74–77. [PMC free article] [PubMed] [Google Scholar]
- 6.Bertini I, Calabro A, Carli VD, Luchinat C, Nepi S, Porfirio B, et al. The Metabonomic signature of celiac disease. J Proteome Res. 2009;8:170–77. doi: 10.1021/pr800548z. [DOI] [PubMed] [Google Scholar]
- 7.Tapiero H, Tew KD. Trace elements in human physiology and pathology: zinc and metallothioneins. Biomed Pharmacother. 2003;57:399–411. doi: 10.1016/s0753-3322(03)00081-7. [DOI] [PubMed] [Google Scholar]
- 8.Hambidge KM, Hambidge C, Jacob M, Baum JD. Low levels of zinc in hair, anorexia, poor growth and hypogeusia in children. Pediatr Res. 1972;6:868–74. doi: 10.1203/00006450-197212000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Sandstead HH, Prasad AS, Schulent AR. Human zinc deficiency, endocrine manifestations and response to treatment. Am J Clin Nutr. 1967;20:422–42. doi: 10.1093/ajcn/20.5.422. [DOI] [PubMed] [Google Scholar]
- 10.Singhal N, Alam S, Sherwani R, Musarrat J. Serum zinc levels in celiac disease. Indian Pediatr. 2008;45:319–21. [PubMed] [Google Scholar]
- 11.Altuntaş B, Filik B, Ensari A, Zorlu P, Teziç T. Can zinc deficiency be used as a marker for the diagnosis of celiac disease in Turkish children with short stature? Pediatr Int. 2000;42:682–4. doi: 10.1046/j.1442-200x.2000.01313.x. [DOI] [PubMed] [Google Scholar]
- 12.Rawal P, Thapa BR, Prasad R, Prasad KK, Nain CK, Singh K. Zinc supplementation to patients with celiac disease—is it required? J Trop Pediatr. 2010;56:391–97. doi: 10.1093/tropej/fmq011. [DOI] [PubMed] [Google Scholar]
- 13.Hogberg L, Danielsson L, Jarleman S, Sundqvist T, Stenhammar L. Serum zinc in small children with coeliac disease. Acta Paediatrica. 2009;98:343–5. doi: 10.1111/j.1651-2227.2008.01085.x. [DOI] [PubMed] [Google Scholar]
- 14.Hambidge KM. Zinc and diarrhea. Acta Paediatrica. 1992;381:82–86. doi: 10.1111/j.1651-2227.1992.tb12377.x. [DOI] [PubMed] [Google Scholar]
- 15.Hoffenberg EJ, Emery LM, Barriga KJ, Bao F, Taylor J, Eisenbarth GS. Clinical features of children with screening-identified evidence of celiac disease. Pediatrics. 2004;113:1254–59. doi: 10.1542/peds.113.5.1254. [DOI] [PubMed] [Google Scholar]
- 16.Faa G, Nurchi VM, Ravarino A, Fanni D, Nemolato S, Gerosa C, et al. Zinc in gastrointestinal and liver disease. Coordination Chemistry Reviews. 2008;252:1257–69. [Google Scholar]