Abstract
Aim
We aimed to investigate the prevalence of Human Bocavirus isolates among Iranian patients suffering from acute gastroenteritis.
Background
Human Bocavirus is a new parvovirus that has been identified in association with gastroenteritis. Limited data are available about HBoV in Iran.
Patients and methods
Viral DNA was extracted from all 294 stool samples. HBoV DNA was detected in extracted samples by polymerase chain reaction (PCR) amplification of a 354 bp of noncapsid protein 1 (NP1) gene. In addition, all samples were also subjected to a nested PCR to amplify a 455 bp of nonstructural 1 (NS1) gene.
Results
The main clinical symptoms among HBoV positive patients were diarrhea (77.7%), fever (62.9%), vomiting (55.5%), and anorexia (59.2%). NP1 PCR was positive in 8 samples (2.72%), NS1 was positive in 16 patients (5.44%) and 3 samples had positive results in both regions (1.02%).
Conclusion
Our results suggest that HBoV could be considered as one of the important etiologic agents of acute gastroenteritis cases in Iran.
Keywords: Human Bocavirus, Acute gastroenteritis, Iran
Introduction
Viruses, Bacteria and Parasites can cause gastroenteritis. Molecular epidemiology of enteric viruses in patients with acute gastroenteritis was previously investigated in Iran. The results showed that Norovirus, Adenovirus and Sapovirus are responsible for 4 to 9 percent of sporadic gastroenteritis cases (1–4).
Human Bocavirus (HBoV) was firstly detected in 2005 (5). This virus was first discovered in children with respiratory tract illness but also has been detected in stool samples from children with gastroenteritis (5, 6). Recently, several genotypes of this parvovirus, HBoV genotype 2 (HBoV2), genotype 3 (HBoV3) and genotype 4 (HBoV4) were discovered that were closely related to HBoV (7, 8). HBoV2 was firstly detected in stool samples from children with flaccid paralysis in Pakistan (9) and followed in other, HBoV3 was detected in Australia (10) and HBoV4 was identified in stool samples from Nigeria, Tunisia and United States (11). Previous reports are demonstrated that there is a close taxonomic association between HBoV and bovine parvovirus, that is causes gastrointestinal disorders in cattle (12, 13). Bocaviruses like other parvoviruses are predominantly resistant to heat and detergent inactivation and due to this fact they could be stable in stool samples for long times (14). Distribution of HBoV infections is different during various seasons, and the highest prevalence is related to cold seasons (15). Due to the considered persistence of HBoVs to different environmental factors and detergent material (16, 17), It is possible that fecal-oral transmission, in addition to transmission via respiratory droplets, could be important in interpreting the previous observations (6). The aim of this study was to determine the prevalence of HBoV infection and its dominant genotypes among Iranian patients with acute gastroenteritis.
Patients and Methods
From May 2008 to June 2010, 294 stool samples were collected from patients referred to Taleghani Hospital, Shohadaye Tajrish Hospital, Mofid Children's Hospital and Children Clinical Center in Tehran, Iran. Samples were obtained from all age groups comprised of 227 children (<18 years of age, 77.2%, 132 male, 95 female) and 67 adults (>18 years of age, 22.8%, 36 male, 31 female). Informed consent was taken from all children's parents or adult patients and the study protocol was reviewed and approved by the Ethic committee at the Gastroenterology and Liver Diseases Research center. DNA was extracted from stool samples using the QIAamp DNA Mini kit (QIAGEN, Hilden, Germany). HBoV DNA was detected in extracted samples by polymerase chain reaction (PCR) amplification of a 354 bp of noncapsid protein 1 (NP1) gene using primers 188F and 542R as reported previously (5). In addition, all samples were also subjected to a nested PCR as described by Kapoor et al, to amplify a 455 bp of nonstructural 1 (NS1) gene (3). The BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA, USA) was employed for sequencing. All sequences were analyzed bidirectionally. To compare the frequency clinical symptoms between the HBoV positive and negative patients we conducted chi-square tests. Quantitative characteristics of the study population such as Age were compared using Students t test. All statistical analyzes were performed using SPSS version 20 software (IBM SPSS Statistics, Chicago, IL, USA).
Results
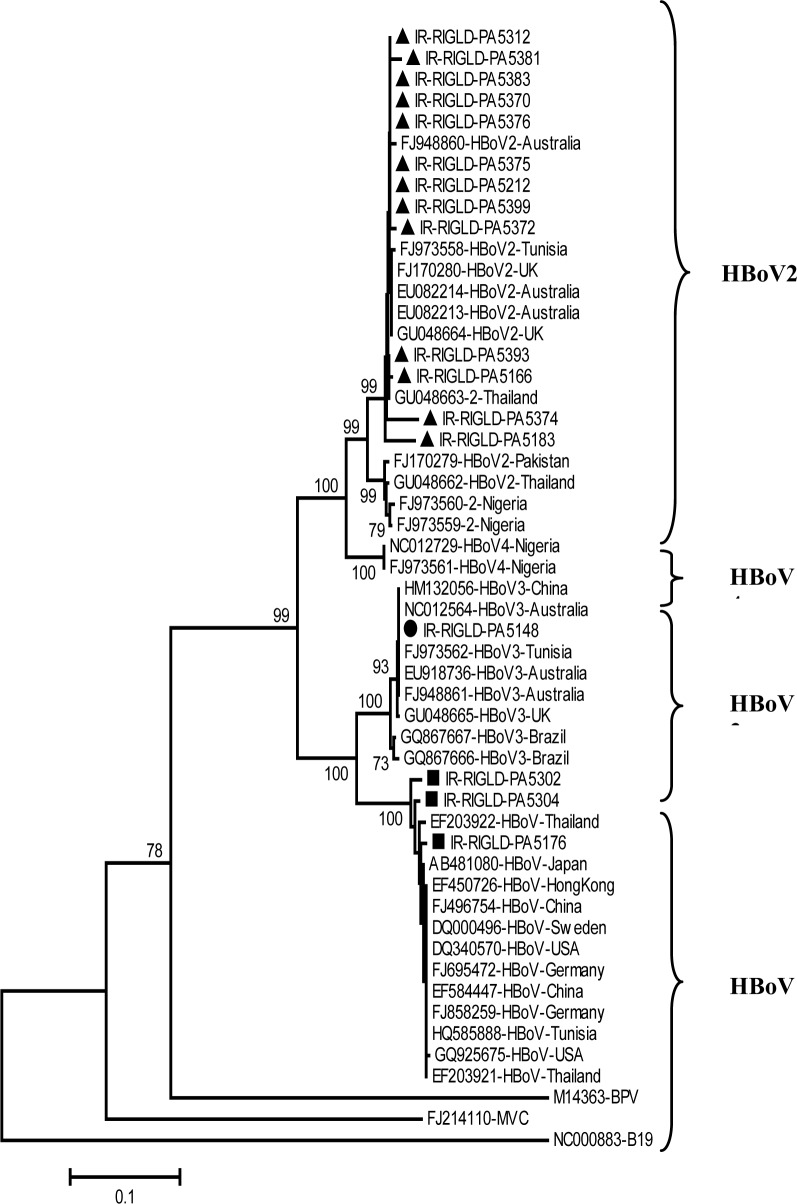
Overall, of the 294 patients tested, 27 samples (9.18%) were positive (13 male and 14 female). Higher rate of positive result was observed in children group (24 of 227, 10.57%) in comparison with adult group (3 of 67, 4.48%) but this difference was not statistically significant (p=0.129 by χ2 test). Higher rate of infection were observed during autumn and winter seasons (16 cases). The main clinical symptoms among HBoV positive patients were diarrhea (77.7%), fever (62.9%), vomiting (55.5%), and anorexia (59.2%). Four HBoV positive patients reported cough and rhinorrhea accompanied with gastroenteritis symptoms (14.8%). No statistically difference in the prevalence of clinical symptoms between HBoV positive and negative patients was observed. NP1 PCR was positive in 8 samples (2.72%), NS1 was positive in 16 patients (5.44%) and 3 samples had positive results in both regions (1.02%). NP1 PCR products of 10 samples and NS1 products of 17 samples were subjected to direct sequencing. Phylogenetic Analysis of NS1 nucleotide sequences revealed that 3 patients infected with HBoV genotype 1 (HBoV), 13 patients infected with HBoV2 and one patient infected with HBoV3 (Figure 1).
Figure 1.
Phylogenetic analysis of the partial nucleotide sequences of non structural protein 1 (NS1) from Iranian isolates in comparison with different human Bocavirus genotypes. Three HBoV sequences (labeled with black squares), thirteen HBoV2 sequences (indicated with black triangles) and one HBoV3 sequence (labeled with black circle) were analyzed with thirty tree HBoV sequences of genotypes 1 to 4 (HBoV to HBoV4) available from GenBank as reference genes. Nucleotide sequences were aligned using CLUSTAL X software (www.clustal.org) and compared with corresponding regions of reference sequences from the GenBank database, genetic distances were calculated by a Kimura two parameter algorithms and phylogenetic trees were constructed by neighbor joining (NJ) method. These analyses were performed using the Molecular Evolutionary Genetics Analysis (MEGA) program, version 3.1 (www.megasoftware.net). The reliability of phylogenetic trees was confirmed by the bootstrap resampling test (n=1000). Bootstrap values >70% are presented at the branching points. BPV: Bovine parvovirus; MVC: Minute virus of canines; B19: human parvovirus B19. B19 was used as the out group. Sequences generated in the present study were submitted to GenBank under accession numbers: JF507199-JF705208 for (NP1) and JN091176-NJ091191 for (NS1).
NP1 and NS1 nucleotide sequences were highly conserved and no differences in nucleotide sequences of NP1 gene (354 bp) were observed between Iranian isolates, except one isolate. Nucleotide identity of Iranian HBoV NS1 sequences (435 bp) with other HBoV reference sequences (accession numbers were indicated in the Figure 1) was 99%–100%. On the other hand, HBoV2 NS1 sequences share 97.4%–98.2% nucleotide sequence identity and 98.5%–99.2% deduced amino acid sequences identity with other HBoV2 reference isolates and 79.6%–81.5% nucleotide identity and 87.2%–91.8% deduced amino acid sequence identity with HBoV prototypes. Only one isolate was classified as HBoV3 based on phylogenetic analysis of NS1 region and this isolate showed 99.6%, 90.2% and 80% nucleotide sequence identity and 100%, 96.5% and 89.5% deduced amino acid sequence identity with HBoV3, HBoV and HBoV2 reference sequences, respectively.
Discussion
Human Bocavirus (HBoV) is considered as major agent of several respiratory tract diseases. However, there are several reports that showed relationship between HBoV and gastrointestinal disorders.
To clear this relationship and due to the fact that the prevalence and molecular characteristics of HBoV in Iranian patients with acute gastroenteritis has not been well studied, we carried out a molecular detection and phylogenetic study of HBoV in Iran.
The present study identified a 9.18% HBoV DNA detection rate in children and adults with acute diarrhea in Tehran, Iran, during May 2009 to June 2010. According to results of present study, Human Bocavirus (HBoV) was detected in 27 (9.18%) of 294 patients with acute gastroenteritis. Nucleotide sequences of NP1 and NS1 genes were highly conserved between Iranian isolates. The study population was comprised of children and adult groups but the number of adult patients was lower than children group (67 versus 227 patients). The number of HBoV positive patients in the adult group was also less than children (4.48% vs. 10.57%). This may be due to fewer adults refer to hospitals or clinical centers especially when they don't face with severe symptoms of gastroenteritis. Therefore, the study may underestimate the rate of HBoV infection among Iranian adult patients with acute gastroenteritis and it is necessary to perform further studies with larger adult populations to determine more accurate rate of HBoV infection among them.
Previous reports of HBoV prevalence around the world show a variation from 1.5% to 19% (8, 18–21). In Iran, there were only two reports about HBoV infection. Naghipour et al. was studied on 261 Iranian children with acute respiratory illnesses under 5 years of age in Rasht (a city located in north of Iran) during winter 2003 to 2004 and found HBoV positivity in 8% of studied children (22). Another study with lower sample size was performed by Nadji and his colleagues on 50 exacerbated asthma cases, 83 cases with acute respiratory illnesses and 47 patients with acute gastroenteritis and they reported 6%, 7.2% and 12.8% HBoV positivity in the studied groups, respectively (23).
We found some data from prevalence of other enteric viruses among Iranian patients with gastroenteritis. Nadji et al. in 2010 performed a small scale study on 47 stool samples of patients with gastroenteritis and reported that Norovirus and Sapovirus are found in 21.3% and 2.1% of samples (24). Our previous work has demonstrated that two genotypes (GI and GII) of Norovirus are involved in 9.8% of acute gastroenteritis cases (4). In other study among Iranian patients with acute gastroenteritis, we demonstrated that Sapoviruses could be responsible for 11.9% of acute gastroenteritis cases (2).
Higher rate of HBoV infection was detected in cold seasons in comparison with warm seasons of the year but this difference was not statistically significant. Although, Susanna et al. reported the seasonal epidemiological profile of HBoV with the highest incidence in fall and winter (25) but some other studies couldn't find any obvious seasonal pattern (18, 23). The difference between results could be due to different sample collection periods and the age groups studied. The most common clinical symptoms of the disease in HBoV positive patients were diarrhea, fever, vomiting and anorexia. No significant differences in the rate of gastroenteritis symptoms were found between HBoV positive or negative patients, as well as between HBoV positive children and adults groups. HBoV nucleotide sequences of NP1 and NS1 genes are highly conserved and very low nucleotide or deduced amino acid sequence variability was observed between Iranian isolates, especially within HBoV1. Previous studies also reported low nucleotide sequence variability among HBoV1 isolates from different parts of the world. Kapoor et al proposed that HBoV1 evolved more recently than other HBoV genotypes (11). It seems that different genotypes of HBoV are widespread in the world.
Our results suggest that different HBoV genotypes are circulating among Iranian patients and they most be considered as one of the etiologic agents of acute gastroenteritis cases in Iran. To confirm pathogenicity of HBoV in acute gastroenteritis further studies on mucosa biopsy samples from patients with disease, are suggested.
Acknowledgements
This work was supported by gastrointestinal and liver diseases research center, Shahid Beheshti University of medical sciences, Tehran, Iran. Authors appreciate Mrs Seyedeh Mina Seyedi and Mrs Leila Kashi for their collaboration.
(Please cite as: Romani S, Mohebbi SR, Khanyaghma M, Azimzadeh P, Majidizadeh Bozorgi S, Damavand B, et al. Detection of human Bocavirus 1, 2 and 3 from patients with acute gastroenteritis. Gastroenterol Hepatol Bed Bench 2013;6(Suppl. 1):S77–S81).
References
- 1.Damavand B, Azimzadeh P, Mohebbi SR, Romani S, Majidizadeh Bozorgi S, Jadali F, et al. Prevalence of adenovirus infection and the dominant serotype among patients with acute gastroenteritis in Tehran between May 2008 and May 2009. Medical Science Journal of Islamic Azad Univesity - Tehran Medical Branch. 2013;23:59–63. [In Persian] [Google Scholar]
- 2.Romani S, Azimzadeh P, Mohebbi SR, Majidizadeh Bozorgi S, Zali N, Jadali F. Prevalence of sapovirus infection among infant and adult patients with acute gastroenteritis in Tehran, Iran. Gastroenterol Hepatol Bed Bench. 2012;5:43–48. [PMC free article] [PubMed] [Google Scholar]
- 3.Romani S, Mohebbi SR, Hosseini SM, Azimzadeh P, Vahedi M, Derakhshan F, et al. Prevalence of norovirus infection in children and adults with acute gastroenteritis, Tehran, Iran, 2008–2009. Food Environ Virol. 2012;4:1–5. doi: 10.1007/s12560-011-9071-8. [DOI] [PubMed] [Google Scholar]
- 4.Romani S, Mohebbi SR, Hosseini SM, Azimzadeh P, Vahedi M, Derakhshan F, et al. Prevalence of Norovirus Infection in Children and Adults with Acute Gastroenteritis, Tehran, Iran, 2008–2009. Food Environ Virol. 2012;4:1–5. doi: 10.1007/s12560-011-9071-8. [DOI] [PubMed] [Google Scholar]
- 5.Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12891–96. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vicente D, Cilla G, Montes M, Perez-Yarza EG, Perez-Trallero E. Human bocavirus, a respiratory and enteric virus. Emerg Infect Dis. 2007;13:636–67. doi: 10.3201/eid1304.061501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santos N, Peret TC, Humphrey CD, Albuquerque MC, Silva RC, Benati FJ, et al. Human bocavirus species 2 and 3 in Brazil. J Clin Virol. 2010;48:127–30. doi: 10.1016/j.jcv.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Gonzalez R, Zhou H, Li J, Li Y, Paranhos-Baccala G, et al. Detection of human bocavirus 3 in China. Eur J Clin Microbiol Infect Dis. 2011;30:799–805. doi: 10.1007/s10096-011-1159-4. [DOI] [PubMed] [Google Scholar]
- 9.Kapoor A, Slikas E, Simmonds P, Chieochansin T, Naeem A, Shaukat S, et al. A newly identified bocavirus species in human stool. J Infect Dis. 2009;199:196–200. doi: 10.1086/595831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arthur JL, Higgins GD, Davidson GP, Givney RC, Ratcliff RM. A novel bocavirus associated with acute gastroenteritis in Australian children. PLoS Pathog. 2009;5:17. doi: 10.1371/journal.ppat.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapoor A, Simmonds P, Slikas E, Li L, Bodhidatta L, Sethabutr O, et al. Human Bocaviruses Are Highly Diverse, Dispersed, Recombination Prone, and Prevalent in Enteric Infections. J Infect Dis. 2010;201:1633–43. doi: 10.1086/652416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durham PJ, Johnson RH, Isles H, Parker RJ, Holroyd RG, Goodchild I. Epidemiological studies of parvovirus infections in calves on endemically infected properties. Research in Veterinary Science. 1985;38:234–40. doi: 10.1016/S0034-5288(18)31832-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durham PJ, Lax A, Johnson RH. Pathological and virological studies of experimental parvoviral enteritis in calves. Research in Veterinary Science. 1985;38:209–19. [PubMed] [Google Scholar]
- 14.Sauerbrei A, Wutzler P. Testing thermal resistance of viruses. Arch Virol. 2009;154:115–19. doi: 10.1007/s00705-008-0264-x. [DOI] [PubMed] [Google Scholar]
- 15.Szomor KN, Kapusinszky B, Rigo Z, Kis Z, Rozsa M, Farkas A, et al. Detection of human bocavirus from fecal samples of Hungarian children with acute gastroenteritis. Intervirology. 2009;52:17–21. doi: 10.1159/000210834. [DOI] [PubMed] [Google Scholar]
- 16.Bonvicini F, Gallinella G, Gentilomi GA, Ambretti S, Musiani M, Zerbini M. Prevention of iatrogenic transmission of B19 infection: different approaches to detect, remove or inactivate virus contamination. Clin Lab. 2006;52:263–68. [PubMed] [Google Scholar]
- 17.Brauniger S, Peters J, Borchers U, Kao M. Further studies on thermal resistance of bovine parvovirus against moist and dry heat. Int J Hyg Environ Health. 2000;203:71–75. doi: 10.1078/S1438-4639(04)70010-3. [DOI] [PubMed] [Google Scholar]
- 18.Bastien N, Brandt K, Dust K, Ward D, Li Y. Human Bocavirus infection, Canada. Emerg Infect Dis. 2006;12:848–50. doi: 10.3201/eid1205.051424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan NM, Dove W, Abu-Zeid AF, Shamoon HE, Abd-Eldayem SA, Hart CA. Human bocavirus infection among children, Jordan. Emerg Infect Dis. 2006;12:1418–20. doi: 10.3201/eid1209.060417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pham NT, Trinh QD, Chan-It W, Khamrin P, Nishimura S, Sugita K, et al. Human bocavirus infection in children with acute gastroenteritis in Japan and Thailand. J Med Virol. 2011;83:286–90. doi: 10.1002/jmv.21876. [DOI] [PubMed] [Google Scholar]
- 21.Smuts H, Hardie D. Human bocavirus in hospitalized children, South Africa. Emerg Infect Dis. 2006;12:1457–58. doi: 10.3201/eid1209.051616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naghipour M, Cuevas LE, Bakhshinejad T, Dove W, Hart CA. Human bocavirus in Iranian children with acute respiratory infections. J Med Virol. 2007;79:539–43. doi: 10.1002/jmv.20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nadji SA, Poos-Ashkan L, Khalilzadeh S, Baghaie N, Shiraghaei MJ, Hassanzad M, et al. Phylogenetic analysis of human bocavirus isolated from children with acute respiratory illnesses and gastroenteritis in Iran. Scand J Infect Dis. 2010;42:598–603. doi: 10.3109/00365540903582442. [DOI] [PubMed] [Google Scholar]
- 24.Fazeli Z, Baghaie N, Khavarinejad RA, Khoramdel M, Sigaroodi A, Nadji SA. Hospital based study of prevalence and genotyping of Noroviruses and Sapoviruses isolated from children with acute gastroenteritis referred to Masih Daneshvari hospital. Gastroenterol Hepatol Bed Bench. 2010;3:91–97. [Google Scholar]
- 25.Lau SK, Yip CC, Que TL, Lee RA, Au-Yeung RK, Zhou B, et al. Clinical and molecular epidemiology of human bocavirus in respiratory and fecal samples from children in Hong Kong. J Infect Dis. 2007;196:986–93. doi: 10.1086/521310. [DOI] [PMC free article] [PubMed] [Google Scholar]