Abstract
Aim
In this study we co-administered melittin along with HBsAg/alum vaccine to investigate if it helps elicitation of Th1/Th2 response.
Background
Hepatitis B virus (HBV) infection is a life-threatening liver infection, which can lead to chronic liver disease. Vigorous T cell responses are stimulated at acute, self-limiting HBV infection, while chronic HBV infection elicits very weak T cell responses. The prevalence of HBV infection has been decreased by the approved vaccination approach using recombinant HBs antigen (HBsAg) and alum i.e. HBV vaccine. Alum, a strong Th2 stimulator, is usually used as adjuvant to increase HBsAg immunogenicity. The present vaccine does not induce protective and/or prophylactic immune response in some groups. Melittin, major active component in the venom of honeybee, induces Th1 development.
Patients and methods
Experimental mice were immunized with melittin plus hepatitis B vaccine on day 0 following by two booster doses with the same injections. Lymphocyte proliferation, IFN-γ, and IL-4 level, total antibody and isotyping of IgG1, IgG2a IgG2b, and IgM were measured using ELISA.
Results
Administration of melittin and HBV vaccine had no effect on lymphoproliferation and total antibody responses, but increased IFN-γ response and induced Th1 response.
Conclusion
The present study proposed that administration of melittin along with conventional vaccine shifts T cell responses towards Th1/Th2 dominated with Th1 response. The resultant immune response leads to activation of both cell-mediated and humoral immune responses, both of which required for clearance of HBV infection.
Keywords: Hepatitis B vaccine, Melittin, Adjuvant, IFN-γ, Th1 response
Introduction
Hepatitis B virus (HBV) infection is the life-threatening liver infection, which can lead to chronic liver disease and put people at the danger of liver cirrhosis, liver cancer or other severe illnesses or death (1). The World Health Organization has recommended national immunization programs since 1991, decreasing the prevalence of HBV infection among people in many nations (2). This success has been achieved by approved vaccination approach using recombinant HBs antigen (HBsAg).
HBV is non-cytopathic and Th1 responses seem to be involved in the clearance of HBV from hepatocytes (3, 4). Vigorous T cell responses are stimulated at acute, self-limiting HBV infection, while chronic HBV infection elicits very weak T cell responses (5). In addition, the present vaccine containing recombinant HBsAg does not induce protective and/or prophylactic immune response in some groups, known as non-responders, such as some healthy individuals, hemodialysis patients, and hepatitis B carrier mothers neonates (6). Alum is usually used as adjuvant along with recombinant HBsAg vaccine, as HBsAg has weak immunogenicity. Several ways of increasing vaccine efficacy have been previously proposed, including changes in vaccine composition and vaccine delivery route. Most of the therapeutic HBV vaccines designed to date have used envelope proteins as the target antigen to develop both the prevention and treatment of hepatitis B (7, 8). Alum is a strong Th2 stimulator, and poorly stimulates Th1 immunity (1). It seems that adding an appropriate adjuvant may induce Th1/Th2 immune response to help subjects with chronic HBV infection and non-responders to the vaccine.
Melittin is the main component of bee venom with a molecular weight of 2850Da; it contains 26 amino acids, with a hydrophobic amino-terminal and hydrophilic carboxyl-terminal regions (9–11). This peptide can bind to cell membranes and insertion at threshold levels of bound peptides (12). Melittin has hemolytic activity and is the major component of bee venom, which constitutes 50% of dry bee venom (13–15). The lytic property of melittin is thought to be partly due to its detergent-like sequence and cationic nature with hydrophobic amino acids at the N-termini (residues 1-20) plus hydrophilic amino acids at the C-termini (residues 21-26). The peptide has three lysine and two arginine residues giving a net positive charge of z = 5+ 14. Melittin has been proposed as a possible absorption-enhancing agent. Immunomodulatory effect of melittin (17) prompted us to investigate if it can stimulate Th1/Th2 response, when administered with the present HBV vaccine.
Patients and Methods
Melittin isolation with HPLC
Venom collection was performed based on electric shock. Melittin purification was carried out using Reverse Phase-High Performance Liquid Chromatography (RP-HPLC) using KNAUER instrument (Dr. Ing. Herbert Knauer GmbH, Berlin, Germany). The apparatus was composed of a column with C18 packing (250 x 4.6 mm), pore size: 100A, HPLC PUMP K-1001, detector (UV K-2501), column oven, manual injector with 40 µm loop and Chrom gate computer software.
A gradient system of two solvents including (C) Trifluoroacetic acid (TFA) /H2O (1:1000) and (D) TFA/Acetonitrile (1:1000) were used during the experiment. The constant flow rate of 1 mLmin-1 with a 214nm UV source wavelength was selected as column elution rate within 70 min (melittin isolation in 42-47 minutes). The details are depicted in Table 1.
Table 1.
The gradient system of RP-HPLC
| Time (minute) | 0-5 | 5-60 | 60-65 | 65-70 |
|---|---|---|---|---|
| Solution D: TFA 0.05% in ACN | 0 | 60 | 90 | 0 |
| Solution C: TFA 0.05% in H2O | 100 | 40 | 10 | 100 |
In order to test the honey bee venom, 4mg was weighed, dissolved in 100µl of HPLC grade water, and centrifuged at 13000rpm for 10min. The gradient system of RP-HPLC: Incremental linear gradient chromatography in 60% solution of TFA 0.05% in ACN along with TFA 0.05% in H2O solution was applied for 70 minutes. In the 5 initial and final minutes, the mobile phase of TFA 0.05% in H2O was used.
Then 40µl of the supernatant was injected to the loop of HPLC instrument using a hamilton syringe (data not shown).
Determination of protein concentration by Bicinchoninic acid (BCA) method
The protein concentration was determined using BCA method. To do so, 10µl of melittin and 10µl of working solution were added to a well of a microplate, incubated for 30min at 37 °C, and OD was read at 562nm wavelength. Analysis was based on BSA standard curve.
SDS-PAGE
Purity of the purified melittin was evaluated using SDS-PAGE was done. Fifteen percent gel with pH = 8.8 and 5% gel with pH = 6.8 were used as resolving and stacking gels, respectively Four microgram of the venom and melittin were taken on the gel in separate wells. After the run, the gels were stained with coomassie dyes overnight, bleached with acetic acid and methanol, and the 2.8 KD melittin band was visualized on the gel (data not shown).
Hemolytic test
To check the bioactivity of melittin, hemolytic test was done. For this purpose, first 2 ml of fresh human blood was collected and heparin was used as anticoagulation agent. The blood sample was then incubated for 10 minutes at 37°C, diluted with PBS (1/5), and centrifuged at 2500rpm for 10 min. The supernatant was discarded and 2% Red Blood Cell (RBC 2%) was prepared. A microplate containing 8 dilutions of melittin in the first row, 1% triton in the second row (as positive control), and PBS in the third row (negative control) was prepared. An equal volume of RBC 2% was added to each well of the three rows. The plate was incubated for 90 minutes at 37°C and then centrifuged at 3000rpm for 15min. The supernatant was transferred to another microplate and optical density was read at 540nm.
Animals
Six to eight-weeks old inbred female Balb/C mice were purchased from Pasteur Institute (Karaj, Iran). Mice were housed for one week before the experiment, given free access to food and water and maintained in a light/dark cycle (12hrs/12hrs) and 20-22°C temperature. All experiments were in accordance with the animal care and use protocol of Pasteur Institute of Iran.
Experimental groups and immunization
Experimental mice groups were immunized intraperitoneally with 2.5µg, 5µg, and 10µg of melittin plus hepatitis B vaccine (10 µg/dose) (n = 6) on day 0 in a total volume of 500 µl. On days 14 and 28, mice were boosted with the same injections. Three control groups were considered including PBS, hepatitis B vaccine, and melittin. Each control group were injected with 500µl of sterile PBS (n = 6), hepatitis B vaccine (n = 6), and 2.5µg, 5µg and 10µg melittin alone (n = 6), respectively. Immunologic parameters were evaluated two weeks after final immunization. Laboratory personnel towards experiment and 3 control groups of mice were blinded.
Lymphocyte proliferation assay
Under sterile conditions, the spleen of mice were removed and suspended in cold PBS containing 2% FBS. RBCs were lysed with lysis buffer and single-cell suspension was adjusted to 3×106 cell/ml in RPMI 1640 (Gibco) supplemented with 10% FBS, 4mM L-glutamine, 0.1mM non-essential amino acid, 1mM sodium pyruvate, 50 µm 2ME, 100µg/ml streptomycin and 100 IU/ml penicillin. One hundred microliters of cell suspensions was dispensed into 96-well flat-bottom culture plates (Nunc) and stimulated with 5µg/ml of HbsAg. Phytohemagglutinin-A (5 µg/ml; Gibco) was used as a positive control and un-stimulated wells used as negative controls. After incubating for 72hrs at 37°C in 5% CO2 humid incubator, the cells were pulsed with 20µl BrdU per well and incubation continued for 18hrs and then the plates were centrifuged at 300g for 10min and the supernatant from each well was carefully aspirated and dried under warm air flow and denatured with fixation/denaturation buffer for 30min and then 100µl of anti-BrdU was added for 2hrs and washed with PBS for 5 times. Afterwards TMB substrate was added to each well for 5min in the dark and reaction was stopped with adding 100µl of H2SO4 2N. The absorbance of each well was then determined at wavelength of 450nm. Stimulation Index was calculated according to the following formula:
Stimulation Index = OD of the wells stimulated with antigen/OD of the negative control wells
IL-4 and IFN-γ Cytokine assay
Two weeks after the second boosting, a total number of 3 × 106 spleen cells were re-suspended in complete RPMI 1640, placed in each well of 24-well plate, stimulated with 5µg/ml of HBsAg, and incubated at 37°C in 5% CO2 for 72hrs. The supernatants were then collected and analyzed to detect the presence of IFN-γ and IL-4 using ELISA Kit (Quantikine, R&D Systems, USA) according to the manufacturer's instruction. The concentration of each sample (pg/ml) was calculated according to the standard curve.
ELISA of specific total antibodies and isotypes
Specific antibodies were evaluated using indirect ELISA method. Briefly, 100µl of 5µg/ml of HBsAg in PBS were coated into 96-well ELISA maxisorp plates (Nunc, Naperville, IL) by overnight incubation at 4°C. The wells were washed with PBS containing 0.05% tween 20 (washing buffer) and blocked 1hrs at 37°C with 5% BSA (blocking buffer). Serial dilutions of sera from 1/100 to 1/204800 were prepared. After washing the wells, 100µl of each dilution was added to each well and incubated at 37°C for 2 hrs. Then the wells were washed five times with washing buffer and incubated for 2hrs. Again, the wells were washed five times with washing buffer, 100µl of 1/7000 dilution of anti-mouse conjugated to HRP (sigma) was added, and incubated for 2hrs at 37°C. The wells were washed five more times with washing buffer, 100µl of TMB substrate was added, and incubated for 30 min in the dark. The reaction was stopped with 2N H2SO4 and color density was measured at A450nm with ELISA plate reader. To detect specific IgG1, IgG2a, IgG2b and IgM subclasses, goat anti-mouse IgG1, IgG2a, IgG2b and IgM secondary antibodies (sigma) were used.
Statistical Analysis
Non-parametric Mann-Whitney U test was used for comparison of statistical significance between the experimental groups. Values of P< 0.05 were considered to represent statistically significant differences. Results are presented as mean ± S.D.
Results
Melittin purification and Hemolytic assay
Result of SDS-PAGE electrophoresis of HPLC-purified melittin shows a band at 2.8KD and confirmed validity of the melittin purification process. In order to detect the bioactivity of melittin, hemolytic assay was carried out on human red blood cells. Result shows that melittin had 85% hemolytic activity in concentration of 2.5µg.
Lymphocyte proliferation assay
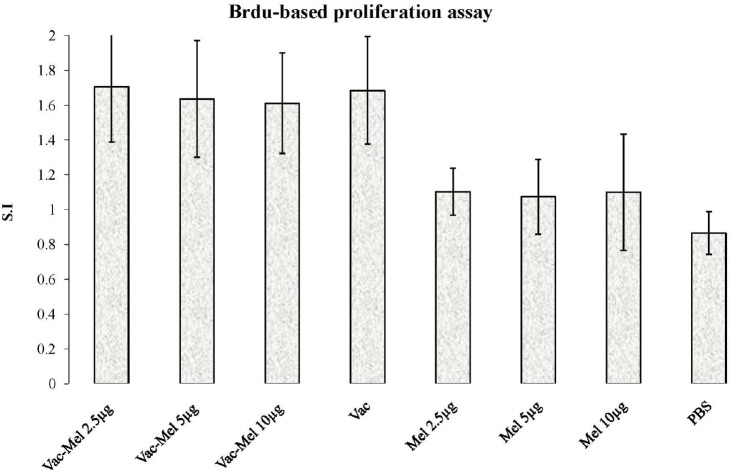
To evaluate the lymphocyte proliferative response, splenocytes were re-stimulated with 5µg/ml of HBsAg in vitro for 72hrs and proliferation assay was performed using BrdU method. Immunization of mice by HBV vaccine without or plus melittin (2.5µg, 5µg and 10µg) significantly increased lymphocyte proliferation in comparison to PBS control group (P<0.019). Immunization of mice with HBV vaccine plus melittin (2.5µg, 5µg, and 10µg) did not significantly increase lymphocyte proliferation in comparison to HBV vaccine-immunized group (P>0.715). In addition, there was no significant difference among the groups immunized with 2.5µg, 5µg, and 10µg of HBsAg plus melittin (P>0.715). There was no significant difference among control groups (p > 0.05) (Figure 1).
Figure 1.
Lymphoproliferative response. Spleen cells were re-stimulated with stimulated 5µg/ml of HbsAg in the presence of BrdU. Results are shown as S.I. and represent the mean±SD
IFN-γ Cytokine ELISA assay
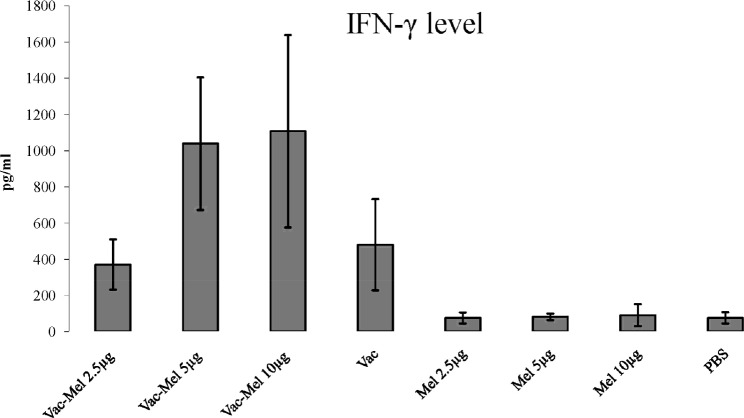
IFN-γ cytokine was evaluated using quantitative ELISA method. Immunization of mice with HBsAg vaccine with or without melittin (2.5µg, 5µg, 10µg) significantly increased IFN-γ secretion in comparison to control groups (P<0.01). Immunization of mice with HBsAg plus 2.5µg melittin did not significantly increased IFN-γ secretion in comparison to HBsAg vaccine-immunized group (P=0.522). Immunization of mice with HBsAg plus 5µg or 10µg melittin significantly increased IFN-γ secretion in comparison to animals immunized with HBsAg vaccine (P<0.037). Also, immunization of mice with HBsAg plus 5µg or 10µg melittin significantly increased IFN-γ secretion in comparison to animals vaccinated with HBsAg plus 2.5µg of melittin (P=0.004) (Figure 2).
Figure 2.
IFN-γ response in hepatitis B vaccine and hepatitis B vaccine plus melittin vaccinated animals. ELISA determined the level of response after two weeks of the last immunization. Spleen cells were stimulated with 5µg/ml of HBsAg in RPMI 1640 10% FBS for 72 hrs. Vaccination of mice with HBsAg plus 5µg or 10µg melittin significantly increased IFN-γ secretion in comparison to animals immunized with HBsAg vaccine. Immunization of mice with HBsAg plus 5µg or 10µg melittin significantly increased IFN-γ secretion in comparison to animals vaccinated with HBsAg plus 2.5µg of melittin.
IL-4 Cytokine ELISA assay
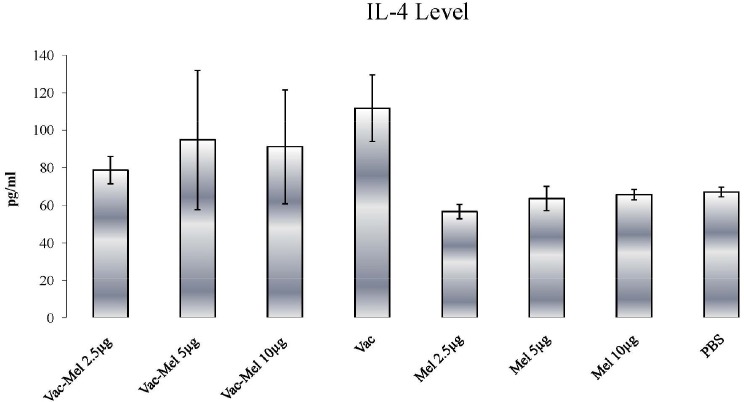
A significant increase in IL-4 release was found in animals immunized with HBsAg vaccine or HBsAg vaccine plus 2.5µg, 5µg, or 10µg melittin in comparison to PBS and melittin control groups (P<0.045). In addition, IL-4 response in animals immunized with HBsAg vaccine plus 2.5µg, 5µg, or 10µg melittin was lower than the HBsAg vaccine-immunized animals. However, only the difference between HBsAg vaccine + 2.5µg melittin- and HBsAg vaccine-immunized mice was statistically significant (P=0.004) (Figure 3).
Figure 3.
IL-4 response in hepatitis B vaccine and hepatitis B vaccine plus melittin vaccinated animals. The protocol was similar to that of explained in Figure 4. IL-4 release significantly increases in animals immunized with HBsAg vaccine or HBsAg vaccine plus 2.5µg, 5µg, or 10µg melittin in comparison to PBS and melittin control groups. IL-4 response in animals immunized with HBsAg vaccine plus 2.5µg, 5µg, or 10µg melittin was lower than the HBsAg vaccine-immunized animals. The difference between HBsAg vaccine + 2.5µg melittin- and HBsAg vaccine-immunized mice was statistically significant (pg/ml = pictogram/milliliter).
Humoral immune response and isotyping
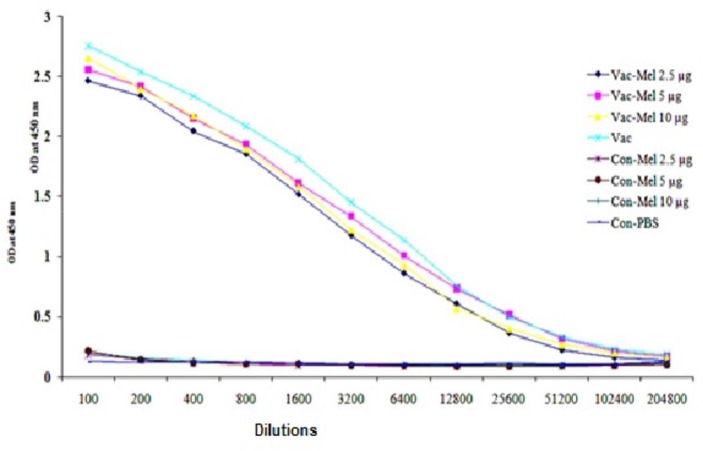
Specific humoral immune responses were monitored with an optimized indirect ELISA method. For this purpose, initially, 12 serial dilutions from all sera were prepared (1/100 to1/204800) and then total antibody was evaluated. Results show that immunization of mice with HBsAg vaccine plus 2.5µg, 5µg, or 10µg melittin or HBsAg vaccine alone significantly increased total antibody titers in comparison to PBS and melittin control groups (P<0.05, except 1/102400 and 1/204800 dilutions). However, no significant difference between HBsAg vaccine plus 2.5µg, 5µg, or 10µg melittin-immunized group was observed in comparison to animals immunized with HBsAg vaccine in all dilutions (P>0.05) (Figure 4).
Figure 4.
Analysis of total antibody: HBV vaccine plus melittin- and HBV vaccine-injected animals are significantly different compared to control groups. However, there was no significant difference seen in any of the mentioned activities in groups treated with HBV vaccine compared to groups treated with vaccine plus the aforementioned melittin doses.
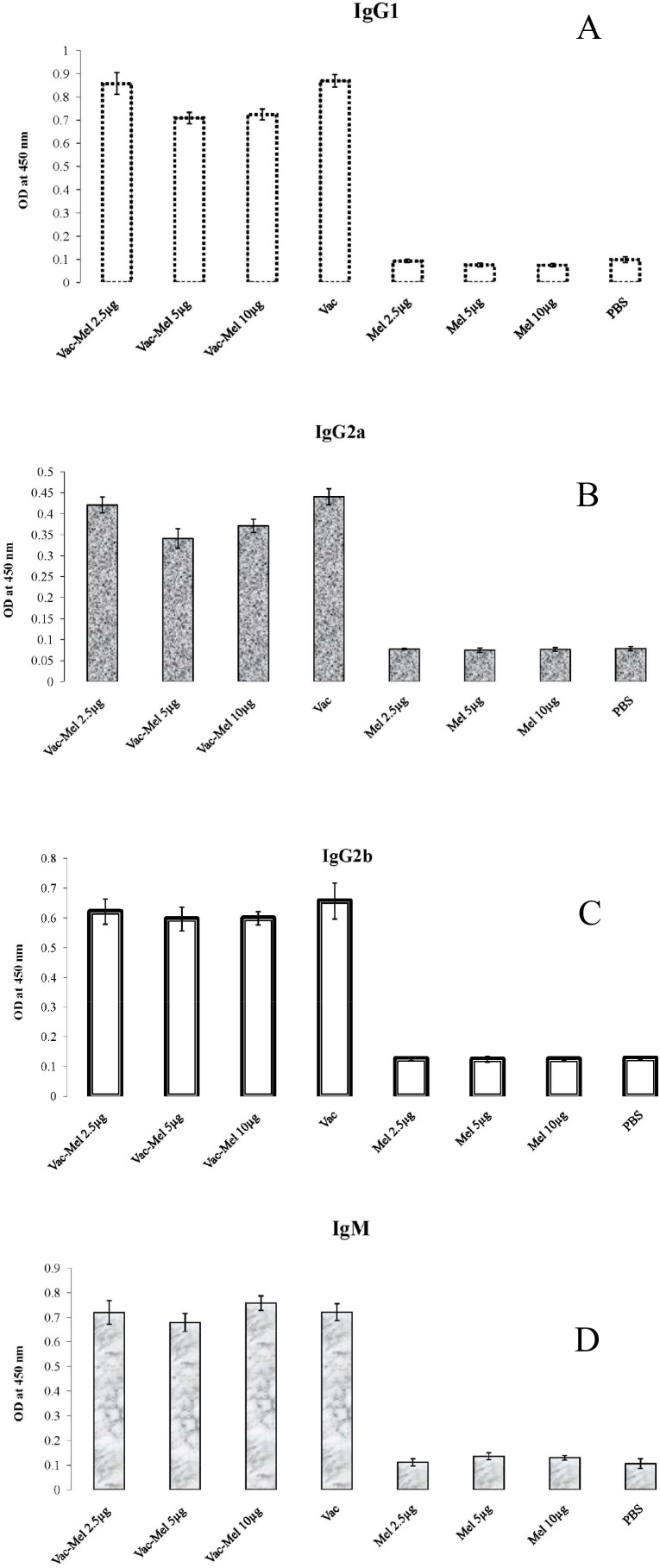
Results of isotyping showed that immunization with HBsAg vaccine alone or HBsAg vaccine plus 2.5µg, 5µg, or 10µg melittin increased specific IgG1, IgG2a, IgG2b and IgM in comparison to PBS and melittin control groups (P≤0.001). IgG1 analysis revealed that there was no significant difference between animals immunized with HBsAg vaccine plus 2.5µg melittin in comparison to HBsAg vaccine-immunized group (P=0.793). However, immunization with HBsAg vaccine plus 5µg or 10µg melittin significantly decreased IgG1 titer in comparison to animals immunized with HBsAg vaccine (P=0.001) or HBsAg vaccine plus 2.5µg of melittin (P=0.001) (Figure 5A). Results of IgG2a show that immunization of mice with HBsAg vaccine plus 5µg or 10µg melittin decreased IgG2a titer in comparison to HBsAg vaccine-immunized group (P=0.093 and P=0.001, respectively).
Figure 5.
Results of antibody isotyping. Sera were collected 2 weeks after the last immunization and serial dilutions of sera from 1/100 to 1/204800 were used. A) Significant decrease in IgG1 titer appeared to be in order of: HBV vaccine ∼ HBV vaccine plus 2.5µg of melittin > HBV vaccine plus 5µg or 10µg melittin (∼ means no significant difference). B) Immunization of mice with HBV vaccine plus 2.5µg, 5µg, or 10µg melittin decreased IgG2a titer in comparison to HBV vaccine-immunized group. The significant decrease in IgG2a titer appeared to be in order of: HBV vaccine > HBV vaccine plus 2.5µg melittin > HBV vaccine plus10µg melittin > HBV vaccine plus 5 µg melittin. C) IgG2b titer was not significantly different among HBV vaccine plus 2.5µg, 5µg, or 10µg melittin and HBV vaccine-immunized groups. D) Immunization of mice with HBV vaccine plus 10µg melittin significantly increased IgM titer in comparison to HBV vaccine-immunized group.
In addition, IgG2a titer was significantly decreased when immunization was made with 5µg or 10µg melittin plus HBsAg vaccine compared to that of 2.5µg melittin plus HBsAg (p = 0.001). However, IgG2a level in 10µg melittin plus HBsAg vaccine-immunized group was significantly more than that of 5µg melittin plus HBsAg vaccine- immunized group (P=0.014) (Figure 5B). There were no significant differences in IgG2b titer among animals immunized with HBsAg vaccine plus 2.5µg, 5µg, or 10µg melittin and HBsAg vaccine-immunized group (P=0.091) (Figure 5C). Results of specific IgM evaluation show that immunization of mice only with 10µg melittin and HBsAg vaccine significantly increased IgM titer in comparison to HBsAg vaccine-immunized group (P=0.036) (Figure 5D).
Discussion
Different type of immune response is displayed during HBV infection, depending on whether patients naturally resolve viral infection or develop chronic infection. Patients with self-limited acute hepatitis B infection display specific CD4 T-cell responses, with the secretion of antiviral cytokines and the production of anti-HBV antibodies. In contrast, patients with chronic hepatitis B infection have very weak or functionally impaired immune responses. Two strategies have been adopted towards HBV infection, including therapeutic and preventive vaccination (17, 18). The treatment of chronic HBV infection is based on antiviral agents that fail to eradicate infection and may cause toxicity and intolerance in long-term treatment. Importantly, these rarely lead to the long-term immunological control of HBV infection (19, 20). Therefore, with regard to acute form of HBV infection in which protective immunity develops after elimination of HBV, it is appealing to devise new therapeutic vaccines to stimulate the patient's immune responses.
Preventive vaccination has reduced the global incidence of hepatitis. Since 1982, a vaccine against hepatitis B has been available. It is 95% prophylactically effective preventing HBV infection and its chronic consequences (7), while 5% are regarded as non-responders (6). Moreover, preventive vaccines are not good candidates for therapeutic vaccination. Several ways of increasing vaccine efficacy have been previously proposed, including changes in vaccine composition and vaccine delivery route (8). Most of the therapeutic HBV vaccines designed to date have used envelope proteins as the target antigen to develop both the prevention and treatment of hepatitis B. Th2 immune response, represented by IL-4 cytokine, just raises humoral immune response neutralizing new attacking and/or viruses and does not eradicate previously infected cells with HBV. In contrast, Th1 response, represented by IFN-γ raising cytotoxic response, targeting, and killing infected cells with HBV, is required for clearance of HBV infection (17–20). In this study we investigated the effect of commercial HBV vaccine containing HBsAg + alum on the type of immune response when administered with melittin.
Studies performed by Shaposhnikova and colleagues revealed that melittin promotes thymocyte proliferation at low concentrations (below 5µg/ml), while it causes death in thymocytes by induction of initial necrosis at high concentrations (14). Orsolic and colleagues also demonstrated that bee venom has anti-tumor activity in vivo and in vitro, depending on concentration and route of administration (21). In this study, results of proliferative response showed that melittin alone at 2.5µg, 5µg, and 10µg concentrations formulated in vaccine increases proliferative response, in comparison to PBS-immunized group. However, our result did not indicate a dependency between melittin dose and the proliferative response, which may be due to different experimental setting. In addition, melittin did not increase proliferative response when administered along with HBsAg + alum, in comparison with HBsAg + alum-immunized group. This is consistent with the previous study in which melittin + alum decreased proliferative response when administered intraperitoneally (22). Nam and colleagues demonstrated that bee venom can affect T helper cell dichotomy and promotes Th1 development from CD4+ T cells indicated by increased expression of IFN-γ (23). IFN-γ cytokine is an important cytokine in the induction of immune responses against HBsAg. A number of healthy individuals, hemodialysis patients and hepatitis B carrier mothers’ neonates could not respond to hepatitis B vaccine (24–27). The mechanism causing non-responsiveness to hepatitis B vaccines in humans is still unknown. However, another study revealed that the content of IFN-γ produced by PBMCs after PHA and HBsAg stimulation was obviously lower in non-responders compared with responder individuals. Therefore, Immunologic non-responsiveness to HBsAg after vaccination might be related to IFN-γ hypo-secretion in PBMCs (28) and induction of IFN-γ response against HBsAg may abrogate the un-responsiveness in these populations. In the present study, cytokine pattern results showed that melittin at doses of 5µg and 10µg is able to strongly enhance IFN-γ expression. Consistent with other studies, our experiments indicate that melittin acts as adjuvant and promotes Th1 cytokine pattern, in a dose-dependent manner (29). Melittin has one dominant T-cell epitope (30), which presumably favors Th1 response and IFN-γ expression. Since IFN-γ has beneficial role in the resolution of acute HBV infection (31–33), administration of melittin with HBsAg + alum may improve the situation in non-responders to the vaccine and in chronic HBV infection.
On the other hand, Bramwell and colleagues (29) reported that melittin stimulates humoral immune responses and increases antibody secretion. Our result demonstrated that melittin had no effect on the humoral immune response. The difference between our result with that of Bramwell and colleagues may be concerned with the type of antigen and route of administration. They used diphtheria and tetanus vaccine, while we used HBsAg vaccine. In addition, they administered the antigen intranasally, while in this study antigen was administered intraperitoneally. This suggests that melittin can act as a good adjuvant when intranasally administered, but its adjuvant activity decreases when administered intraperitoneally.
Several studies have shown the importance of the route of immunization on the efficacy of immune responses (34). Oršolic and colleagues demonstrated that bee venom significantly reduces lung metastases when administered intravenously injection. However, subcutaneous injection of bee venom did not show such an impact, suggesting the dependence of bee venom effect on the route of administration (35).
A considerable notion of this study was decrease in IgG1 level when melittin was administered with HBsAg + alum. This is consistent with the previous study in which melittin + alum decreased IgG1 when administered intraperitoneally (22). In addition, administration of melittin with HBsAg + alum resulted in lower IgG2a level than that of HBsAg + alum. This may appear inconsistent with increase IFN-γ level following melittin + HBsAg + alum administration. It is noteworthy that many articles have proposed that antibody isotype and cytokine profiles do not always match (36–38). Therefore, decreased IgG2a level presented in this study does not violate increase of IFN-γ level.
In summary, administration of melittin along with conventional vaccine shifts T cell responses towards Th1/Th2 dominated with Th1 response. The resultant immune response leads to activation of both cell-mediated and humoral immune responses, both of which required for clearance of HBV infection.
Effect of melittin administration along with HBsAg and alum is suggested for future investigation in chronic HBV infection. More research is required to elucidate other immunological aspect of this approach, such as its effect on regulatory T cells and cytotoxicity.
Acknowledgements
This work was supported in part by a grant from Pasteur Institute of Iran
(Please cite as: Taghizadeh Dezfuli H, Shahbazzadeh D, Eidi A, Pooshang Bagheri K, Pakravan N, Amini S, et al. Induction of IFN-γ cytokine response against Hepatitis B surface antigen using melittin. Gastroenterol Hepatol Bed Bench 2014;7(2):108-117).
References
- 1.Zhang X, He P, Hu Z, Wang X, Liang Z. Enhanced specific immune responses by CpG DNA in mice immunized with recombinant hepatitis B surface antigen and HB vaccine. Virol J. 2011;8:78–83. doi: 10.1186/1743-422X-8-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current current and emerging prevention and control measures. J Viral Hepat. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 3.Thimme R, Wieland S, Steiger C. CD8+ T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77:68–76. doi: 10.1128/JVI.77.1.68-76.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chisari FV. Hepatitis B virus immunopathogenesis. Ann Rev Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 5.Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, et al. Characterization of hepatitis B virus (HBV)-specific T cell dysfunction in chronic HBV infection. J Virol. 2007;81:4215–25. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sönmez E, Sönmez AS, Bayindir Y, Coskun D, Aritürk S. Antihepatitis B response to hepatitis B vaccine administered simultaneously with tetanus toxoid in nonresponder individuals. Vaccine. 2002;21:243–46. doi: 10.1016/s0264-410x(02)00452-8. [DOI] [PubMed] [Google Scholar]
- 7.Chen DS. Hepatitis B vaccination: the key towards elimination and eradication of hepatitis B. J Hepatol. 2009;50:805–16. doi: 10.1016/j.jhep.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Xu DZ, Zhao K, Guo LM, Li LJ, Xie Q, Ren H, et al. A randomized controlled phase IIb trial of antigen-antibody immunogenic complex therapeutic vaccine in chronic hepatitis B patients. PLoS One. 2008;3:e 2565. doi: 10.1371/journal.pone.0002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Habermann E. Bee and wasp venoms. Science. 1972;177:314–22. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- 10.Park JH, Kim KH, Lee WR, Han SM, Park KK. Protective effect of melittin on inflammation and apoptosis in acute liver failure. Apoptosis. 2012;17:61–69. doi: 10.1007/s10495-011-0659-0. [DOI] [PubMed] [Google Scholar]
- 11.Dotimas EM, Hider RC. Honey bee venom. Bee World. 1987;68:51–70. [Google Scholar]
- 12.van den Bogaart G, Guzmán JV, Mika JT, Poolman B. On the mechanism of pore formation by melittin. J Biol Chem. 2008;283:33854–57. doi: 10.1074/jbc.M805171200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussein AA, Nabil ZI, Zalat SM, Rakha MK. Comparative study of the venoms from three species of bees: effects on heart activity and blood. J Nat Toxins. 2001;10:343–57. [PubMed] [Google Scholar]
- 14.Shaposhnikova VV, Egorova MV, Kudryavtsev AA, Levitman MKh, Korystov YuN. The effect of melittin on proliferation and death of thymocytes. FEBS Lett. 1997;410:285–88. doi: 10.1016/s0014-5793(97)00578-4. [DOI] [PubMed] [Google Scholar]
- 15.Attia W, Gabry M, El-Shaikh K, Othman G. Melittin, An active ingredient of honeybee venom (Apis mellifera), as a potent inhibitor of tumor growth in mice. Egyp J Natural Toxins. 2009;6:33–58. [Google Scholar]
- 16.Terwilliger TC, Eisenberg D. The structure of melittin. II. Interpretation of the structure. J Biol Chem. 1982;257:6016–22. [PubMed] [Google Scholar]
- 17.Chang JJ, Lewin SR. Immunopathogenesis of hepatitis B virus infection. Immunol Cell Biol. 2007;85:16–23. doi: 10.1038/sj.icb.7100009. [DOI] [PubMed] [Google Scholar]
- 18.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–29. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 19.Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359:1486–500. doi: 10.1056/NEJMra0801644. [DOI] [PubMed] [Google Scholar]
- 20.Zoulim F, Locarnini S. Hepatitis B virus resistance to nucleos(t)ide analogues. Gastroenterology. 2009;137:1593–608. doi: 10.1053/j.gastro.2009.08.063. [DOI] [PubMed] [Google Scholar]
- 21.Orsolic N, Sver L, Bendelia K, Basic I. Antitumor activity of bee venom. J Period Biol. 2001;103:49–54. [Google Scholar]
- 22.King TP, Lu G, Agosto H. Antibody responses to bee melittin (Api m 4) and hornet antigen 5 (Dol m 5) in mice treated with the dominant T-cell epitope peptides. J Allergy Clin Immunol. 1998;101:397–403. doi: 10.1016/S0091-6749(98)70254-4. [DOI] [PubMed] [Google Scholar]
- 23.Nam S, Ko E, Park SK, Ko S, Jun CY, Shin MK, et al. Bee venom modulates murine Th1/Th2 lineage development. Int Immunopharmacol. 2005;5:1406–14. doi: 10.1016/j.intimp.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Barnaba V, Franco A, Alberti A, Benvenuto R, Balsano F. Selective killing of hepatitis B envelope antigen-specific B cells by class I- restricted, exogenous antigen-specific Tlymphocytes. Nature. 1990;345:258–60. doi: 10.1038/345258a0. [DOI] [PubMed] [Google Scholar]
- 25.del Canho R, Grosheide PM, Schalm SW, de Vries RR, Heijtink RA. Failure of neonatal hepatitis B carrier mothers and HLAantigens in neonates. J Hepatol. 1994;20:483–86. doi: 10.1016/s0168-8278(05)80494-5. [DOI] [PubMed] [Google Scholar]
- 26.George J, John GT, Jacob CK, Shastry JC. Active immunization against hepatitis B infection of a haemodialisis population. Natl Med J India. 1994;7:115–16. [PubMed] [Google Scholar]
- 27.Struve J, Aronsson B, Frenning B, Forsgren M, Weiland O. Seroconversion after additional vaccine doses to non-responders to three doses of intradermally or intramuscularly administered recombinant hepatitis B vaccine. Scand J Infect Dis. 1994;26:468–70. doi: 10.3109/00365549409008621. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Tan D, Liu H, Li K. CD4(+) CD25(+) FoxP3(+) T regulatory cells in subjects responsive or unresponsive tohepatitis B vaccination. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2011;36:1046–51. doi: 10.3969/j.issn.1672-7347.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Bramwell VW, Somavarapu S, Outschoorn I, Alpar HO. Adjuvant action of melittin following intranasal immunisation with tetanus and diphtheria toxoids. J Drug Target. 2003;11:525–30. doi: 10.1080/10611860410001670080. [DOI] [PubMed] [Google Scholar]
- 30.Fehlner PF, Berg R, Tam JP, King TP. Murine T cell responses to melittin and its analogs. J Immunol. 1991;146:799–806. [PubMed] [Google Scholar]
- 31.Zou Z, Li B, Xu D, Zhang Z, Zhao JM, Zhou G, et al. Imbalanced intrahepatic cytokine expression of interferon-gamma, tumor necrosis factor-alpha, and interleukin-10 in patients with acute-on-chronic liver failure associated with hepatitis B virus infection. J Clin Gastroenterol. 2009;43:182–90. doi: 10.1097/MCG.0b013e3181624464. [DOI] [PubMed] [Google Scholar]
- 32.Visnjić D, Batinić D, Banfić H. Arachidonic acid mediates interferon-gamma-induced sphingomyelin hydrolysis and monocytic marker expression in HL-60 cell line. Blood. 1997;89:81–91. [PubMed] [Google Scholar]
- 33.Paul S, Tabassum S, Islam MN. A study on interferon-gamma (IFN-gamma) response by T cells stimulated by hepatitis B virus core antigen. Bangladesh Med Res Counc Bull. 2004;30:9–15. [PubMed] [Google Scholar]
- 34.De Rose R, Tennent J, McWaters P, Chaplin PJ, Wood PR, Kimpton W, et al. Efficacy of DNA vaccination by different rout of immunization in sheep. Vet Immunol Immunopathol. 2002;90:55–63. doi: 10.1016/s0165-2427(02)00221-0. [DOI] [PubMed] [Google Scholar]
- 35.Orsolić N, Sver L, Verstovsek S, Terzić S, Basić I. Inhibition of mammary carcinoma cell proliferation in vitro and tumor growth in vivo by bee venom. Toxicon. 2003;41:861–70. doi: 10.1016/s0041-0101(03)00045-x. [DOI] [PubMed] [Google Scholar]
- 36.Martin RM, Lew AM. Is IgG2a a good Th1 marker in mice? Immunol Today. 1998;19:49. doi: 10.1016/s0167-5699(97)87499-3. [DOI] [PubMed] [Google Scholar]
- 37.Mizel SB, Bates JT. Flagellin as an adjuvant: cellular mechanisms and potential. J Immunol. 2010;185:5677–82. doi: 10.4049/jimmunol.1002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thatte J, Rath S, Bal V. Analysis of immunization route-related variation in the immune response to heat-killed Salmonella typhimurium in mice. Infect Immun. 1995;63:99–103. doi: 10.1128/iai.63.1.99-103.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]