Abstract
Aim
The present study aimed to investigate the influence of histological factors on survival of patients with esophageal cancer.
Background
Esophageal cancer is almost the common form of malignancy in the eastern world.
Patients and methods
Through a retrospective cohort study a consecutive series of 134 patients with definite diagnosis of esophageal cancer who had been hospitalized at the Towhid hospital, Sanandaj city, Kurdistan province western Iran during a five-year period from 2006 onward were recruited. The survival time of patients stratified by this grouping method were analyzed by Kaplan-Meier analysis and Cox regression.
Results
Overall, 127 males (55.1%), with a mean age of 65.38 ±11.62 years were included. Based on histological type of tumor, 23 patients (18.1%) had adenocarcinoma (AC) and 94 patients (74.0%) had squamous cell carcinoma (SCC). Gender was not significantly associated with survival (Log rank =0.480). Location of tumor (log rank =0.014), histological type (log rank ≤0.001) and grade of tumor (log rank =0.008) had significantly influenced the survival rates variation. For patients at initial stages of the disease, the overall one-year, two years and five years survival rates were 73.2%, 52.8% and 31.2% respectively. For advanced stages, the survival ranged from 46.3% in the first year to 8.2% in the five years. The five-year survival rates (by year) were estimated to be 49%, 27%, 24%, 22% and 19% respectively.
Conclusion
Tumor grade, tumor deferential, clinical staging and location of the tumor were the prognostic factors for survival in patients with esophageal cancer.
Keywords: Adenocarcinoma, Esophageal cancer, Squamous cell carcinoma, Survival
Introduction
Esophageal cancer is the sixth cause of cancer death worldwide (1), with an estimated 500,000 new cases each year (2). Esophageal cancer is a relatively common form of cancer in the eastern world (3). The disease is highly lethal, with overall five-year survival rates less than 10%. The high mortality is due to the late onset of symptoms (4). Histologically, there are two main types; one type grows in the inside layer of the lining of the esophagus squamous cell carcinoma (SCC) a second cancer that starts in gland cells are called adenocarcinoma (AC) (5).
A worldwide increasing incidence for AC but not for SCC has been reported in North America, Europe and Japan (6). In Kerman province in Iran, an increased incidence of adenocarcinoma of the esophagus 1991 to 2002 has been reported while the incidence of esophageal SCC hardly increased. (7). In Ardabil province in 2007, the one- and five-years survival rates in patients with upper gastrointestinal cancer were estimated to be 40.5% and 0.8% respectively (8). Geographic variations in Iran show that the incidence and mortality of gastric cancer is higher in west and North West regions and in the Kurdistan province in particular.
Previous survival studies in Iran have focused on esophageal cancer, indicating the importance of gender, age, residence (rural and urban) and risk factors whereas inconsistent results have been observed for education, and income (8–10). Factors such as histological type and grade, location and stage of tumor, surgical treatments have not been studied in relation to survival. Therefore, the present study aimed to investigate the effects of histological factors on the survival of patients with esophageal cancer.
Patients and Methods
Data were sourced mainly from the patient reports of pathology laboratories and hospital database record. Through a retrospective cohort study using censes method; all eligible patients with esophageal cancer (134 gastric cancers) who had been hospitalized at the Towhid hospital, Sanandaj city, Kurdistan province western Iran were recruited. Inclusion criteria were patients with definite diagnosis of esophageal cancer during a five-year period from 2006 onward. Samples were coded under the direct supervision of clinical pathologists according to the International Classification of Diseases for Oncology. Clinical data such as practice treatment were obtained through a structured questionnaire and the patients’ clinical records. Vital status and date of death were determined through the by official death certificates, with a maximum follow-up of 90 months. Survival time (in months) was calculated from the date of diagnosis through the date of death or last follow-up. A failure was defined as death by any cause during the follow-up period and patients alive at the end of the follow-up period were censored. 7 patients were excluded from the analyses according to exclusion criteria (4 patients lost follow-up, 2 illegible data, and 1 patient due to migration). Overall, 127 patients with esophageal cancer were enrolled. Clinical and pathologic variables, which were sub-layered into age, gender, setting, histological type of tumor and practice treatment were entered into parametric regression models (by considering and not considering heterogeneity) for multivariate analysis in order to assess the relationships between the characteristics and prognostic factors for survivors. Ilam University of Medical Sciences, Ethics Committee on considering of publication data result in general approved the study (Code No: 91002, Date: 22.08.2012).
Statistical analysis
The Kaplan Meier and Log rank statistic methods were used to compare survival rates in different subgroups. Using life table, survival rates and survival density function was assessed at year intervals. The Breslow (generalized Wilcoxon) statistics was used to compare median survival time in three age groups. The Cox hazards regression analysis was also used to investigate the effect of the variables and adjusting the effect of age. The graphical (diagram Log (S) t vs. time) and analytical (time-varying covariate) methods was applied to test the appropriateness of Cox's proportional hazard (11).
The Multivariate Cox regression analysis was used to identify independent predictors for patient survival using a backward stepwise approach with an entry limit of p < 0.1 and a removal limit of p < 0.05. The survival time of patients stratified by this grouping method were analyzed by the Kaplan-Meier analysis and Cox regression as described earlier. All statistical analyses were performed using SPSS16.0.
Results
A total number of 127 patients (55.1% males) with esophageal cancer were recruited. Mean age ±standard deviation was 65.38 ±11.62 years with a range of between 35 to 86 years. Based on histological type of tumor, 23 (18.1%) had AC type, 94 patients (74.0%) had SCC and other histological type 10 (7.9%). For 44 patients (36.6%), the tumors were located in the lower third of the esophagus. Meanwhile, the tumors were located in the middle third and upper third of the esophagus for 28 (22.0%) and 48 patients (37.8%) respectively. There were also overlapping lesions in 7 patients (5.5%). The tumors were well differentiated for 42 patients (48.8%), moderately differentiated for 35 patients (27.6%), poorly differentiated in 16 patients (12.6%) and unknown differentiated for 14 patients (11.0%).
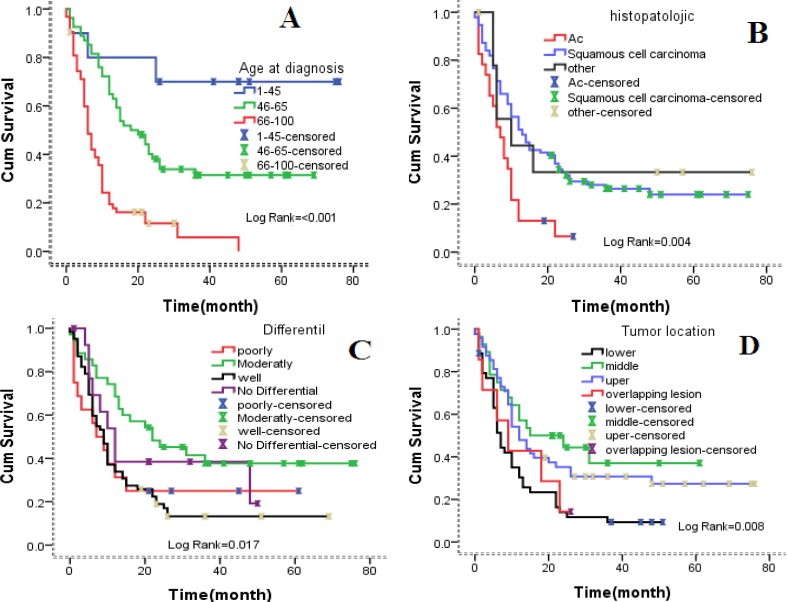
Gender and setting of patients had no significant effects on survival rate variation in univariate analysis. Age, location of tumor, histological type, tumor grade, stage of diagnosis and practice treatment showed a significant association on the survival rates variation (Table 1). The Kaplan-Meier analysis revealed significant differences in five-year survival rate and age of diagnosis (log-rank=< 0.001) (Figure 1A), histological type (log-rank = 0.004) (Figure 1B), tumor grade (log-rank=0.017) (Figure 1C), and tumor locate (log-rank=0.008) (Figure 1D).
Table 1.
Clinic-pathological characteristic of the patients with esophageal cancer
| Factors | Patients, n (%) | Median of survival (CI 95%) | Pvalue |
|---|---|---|---|
| Gender | 0.480 | ||
| Male | 70(55.1) | 10(7.97-12.03) | |
| Female | 57(44.9) | 12(6.46-17.54) | |
| Age | <0.001 | ||
| 45 > | 11(8.7) | 25(10.94-31.33) | |
| 46-65 | 54(42.5) | 18(10.94-25.06) | |
| 66 < | 42(48.8) | 6(4.6-7.4) | |
| Setting | 0.148 | ||
| City | 82(64.6) | 10(8.05-11.95) | |
| Village | 45(35.4) | 14(10.05-17.95) | |
| Histological type | <0.001 | ||
| AC | 23(18.1) | 7(4.18-9.82) | |
| SCC | 94(74.0) | 13(9.34-16.66) | |
| Indeterminate | 10(7.9) | 10(7.93-12.07) | |
| Location of tumor | 0.014 | ||
| Lower | 44(34.6) | 7(5.40-8.60) | |
| Middle | 28(22.0) | 18(10.94-25.06) | |
| Upper | 48(37.8) | 12(8.60-15.40) | |
| Overlapping lesion | 7(5.5) | 9(3.92-16.68) | |
| Histology grade | 0.008 | ||
| Poorly | 16(12.6) | 7(1.12-12.88) | |
| Moderately | 35(27.6) | 22(6.06-37.94) | |
| Well | 42(48.8) | 9(6.80-11.20) | |
| No Differential | 14(11.0) | 12(7.41-16.59) | |
| Stage | <0.001 | ||
| Localized | 15(11.8) | 25(12.02-35.98) | |
| Regional | 32(25.2) | 24(13.01-34.99) | |
| Distant | 32(25.2) | 10(6.32-13.68) | |
| Unknown | 38(37.08) | 5(3.88-6.12) | |
| Practice treatment | <0.001 | ||
| surgery | 15(11.8) | 12(4.42-19.57) | |
| chemotropic | 30(23.6) | 5(3.92-6.07) | |
| radiotherapy | 7(5.5) | 5(2.91-7.09) |
Figure 1.
The association between demographic, histological factors and survival in esophageal cancer patients. (A) By age of diagnosis (B) by histology of tumor (C) by histology grade (D) by location of tumor.
Cox-regression analysis using histological factors 45> year as reference revealed that patients whose 46-65 year at diagnosis (HR= 3.43, 95% CI = 1.03-11.41, p= 0.044), 66< year (HR =9.78, 95% CI = 2.93-32.64, p = < 0.001) had an increased risk for disease progression and death. Cox-regression analysis for poor grade tumors as reference revealed that patients whose tumors with moderately differentiate (HR= 0.52, 95% CI = 0.25-1.07, p= 0.078) and well differentiate (HR =0.98, 95% CI = 0.51-1.85, p= 0.951) had a decreased risk for death from esophageal cancer.
Similar results were obtained for location of the tumor. Cox regression coefficient (β) analysis shows patient with tumor located in middle of esophaus (β = -0.91) and upper esophagus (β =-0.13) have lower death rates compression to tumors that are located in the lower esophagus. Similar results were obtained for tumor grade (Table 2F).
Table 2.
Multivariate Cox regression analyses; F: Cox regression analysis for age, gender, setting, stage at diagnosis and practice treatment; E: Cox-regression analysis for age, gender, setting, histology grade and location of tumor
| F Characteristics | β | HR(95% CI) | P-value |
|---|---|---|---|
| Age | - | Overall | <0.001 |
| 45>(n = 10) | Ref | 1 | Ref |
| 46-65(n = 54) | 0.628 | 1.87(0.56-6.24) | 0.307 |
| <65(n = 63) | 1.59 | 4.92(1.46-16.53) | 0.010 |
| Practice treatment | - | Overall | <0.001 |
| Surgery(n = 15) | Ref | 1 | Ref |
| Chemotherapy(n = 30) | 1.02 | 2.79(1.35-5.73) | 0.005 |
| Radiotherapy(n = 2) | 1.98 | 7.27(1.53-34.39) | 0.012 |
| surgery and chemotherapy(n = 19) | -0.74 | 0.47(0.19-1.12) | 0.089 |
| chemotherapy and radiotherapy(n = 15) | -.60 | 0.54(0.21-1.37) | 0.199 |
| surgery, chemotherapy and radiotherapy(n = 16) | -1.01 | 0.36(0.13-0.95) | 0.040 |
| no treatment(n = 30) | 1.12 | 3.08(1.50-6.34) | 0.002 |
| E Characteristics | β | HR (95% CI) | P-value |
|---|---|---|---|
| Age | - | Overall | <0.001 |
| 45 > | Ref | 1 | Ref |
| 46-65 | 1.23 | 3.43 (1.03-11.41) |
0.044 |
| <65 | 2.28 | 9.78 (2.93-32.64) |
<0.001 |
| Histology grade | - | Overall | 0.085 |
| poorly | Ref | 1 | Ref |
| Moderately | -0.64 | 0.52 (0.25-1.07) |
0.078 |
| well | -0.02 | 0.98 (0.51-1.85) |
0.951 |
| Location of tumor | - | Overall | 0.009 |
| Lower | Ref | 1 | Ref |
| Middle | -0.91 | 0.40 (0.22-0.73) |
0.003 |
| Upper | -0.13 | 0.53 (0.33-0.85) |
0.009 |
Stage of tumor and practice treatment in step 2 of Cox-regression analysis flowing demographic characteristic in step 1 using treatment surgery as reference revealed that patients who only had chemotherapy in process of treatment (HR = 2.79, 95% CI = 1.35-5.73, p = 0.005), and patients who only had radiotherapy (HR = 7.27, 95% CI = 1.53-34.39, p = 0.012) had an increased risk of deaths from esophageal cancer. Coefficient value β analysis shows patient who had only chemotherapy (β =1.98) have increase death rates in comparison to patients who had surgical operation only. Similar results were obtained for patient who only had chemotherapy (Table 2F).
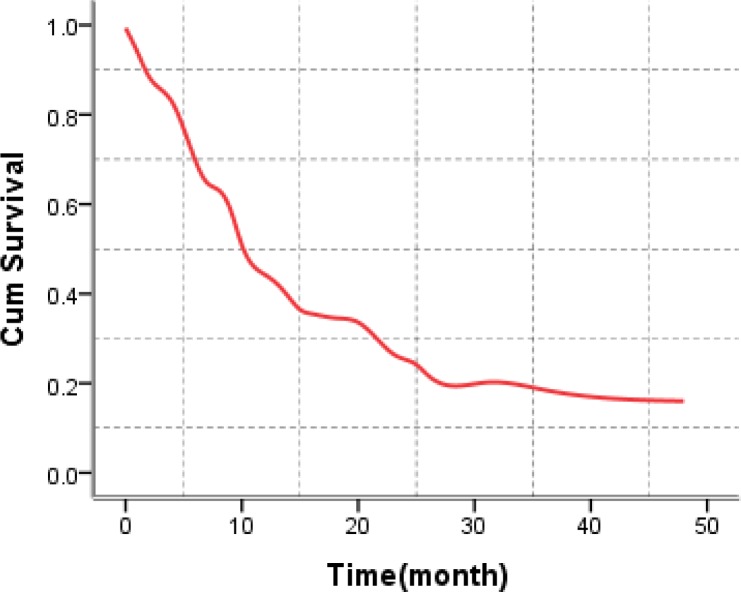
The five-year survival rates (by year) were 49%, 27%, 24%, 22% and 19% respectively. Overall Five-year survival in the cases of esophageal cancer in the present study was 49.0%, with survival rates being higher in the group of women compared to the men (19.7% versus 13.8%) (p = 0.48), higher in clinical localized stages (41.2%) compared to regional stages (14.3%) (p =< 0.001), one survival rate higher in cases submitted to curative surgery (53.2%) compared to those submitted to chemotherapy care (13.5), (p =< 0.001), as demonstrated in Table 3 and Figure 2.
Table 3.
The 1-5 year survival rates and med time survival in patients with esophageal cancer
| Survival variables | Survival rates (%) | Med time Survival by month | ||||
|---|---|---|---|---|---|---|
| 1year | 2year | 3year | 4year | 5year | ||
| Gender | ||||||
| Male | 46.1 | 26.3 | 19.1 | 19.3 | 13.8 | 11.7 |
| Female | 52.8 | 29.6 | 24.5 | 18.2 | 19.7 | 13.4 |
| Age | ||||||
| 45 > | 80.2 | 80.1 | 68.5 | 68.3 | 68.3 | 60 |
| 46-65 | 72.1 | 38.8 | 31.3 | 27.8 | 27.2 | 20.2 |
| 66 < | 24.8 | 8.3 | 4.2 | 4.3 | - | 7.9 |
| Histological type | ||||||
| AC | 21.3 | 19.7 | 5.1 | 1.3 | - | 7.6 |
| SCC | 56.3 | 32.8 | 25.3 | 22.8 | 19.6 | 15.3 |
| Location of tumor | ||||||
| Lower | 34.5 | 28.3 | 23.7 | 15.1 | 15.3 | 9.3 |
| Middle | 64.2 | 44.2 | 31.8 | 25.3 | 21.7 | 22.3 |
| Upper | 54.3 | 34.9 | 29.2 | 28.4 | 24.2 | 14.2 |
| Histology grade | ||||||
| Poorly | 31.3 | 28.7 | 18.3 | 18.9 | 16.3 | 9.6 |
| Moderately | 74.2 | 47.6 | 39.7 | 33.8 | 25.3 | 23.13 |
| Well | 37.6 | 17.5 | 13.5 | 11.7 | 11.2 | 9.5 |
| Stage | ||||||
| Localized | 73.2 | 52.8 | 45.3 | 45.6 | 31.2 | 30 |
| Regional | 62.5 | 27.3 | 2.03 | 20.5 | 14.3 | 26 |
| Distant | 46.3 | 24.6 | 24.02 | 12.5 | 8.2 | 11.3 |
| Practice treatment | ||||||
| Surgery | 53.2 | 30.6 | 21.3 | 21.3 | - | 13.9 |
| Chemotropic | 13.5 | - | - | - | - | 6.9 |
Figure 2.
Cumulative survival rate in patients with esophageal cancer
Discussion
In a recent review of survival analyses, it was found that many studies have indicated clinical and pathologic characteristics of patients as explanatory variables with respect to survival (12). Age of diagnosis was an independent covariate for survival in patients in this study and better prognosis observed in >45 year age group. The reasons for this are likely to include a combination of better general health, more effective response to treatment and earlier diagnosis in younger people. Differences in underlying tumor biology may also play a part. Previous report indicated better survival in young patients (13). On the other hand, some other studies do not have the same report (14, 15). The present results showed that patients’ gender had no significant impact on survival rate (p = 0.48). Median survival time for man and women was (10.0±1.03) and (12.0±2.83), respectively. Sex was not an independent prognostic factor in either Chinese patients (p = 0.23) or white patients living in the United States (p = 0.28). Overall survival was significantly worse only in male white compared with Chinese patients (median survival time, 12.4 versus 14.5 months, respectively; p < 0.01), (8). However, some studies have found better survival rate for women (10).
The SCC cancer is a significant factor that had impact on the survival probability of patients in univariate analysis, which is similar to some other studies. This result reinforced SCC and AC are distinct malignancies of the esophagus, with different risk factors and different natural histories. In this study patient with SCC histology had a better survival, although in previous studies ACs generally have a slightly better prognosis (outlook) in overall(12).
Stage of diagnosis was strongly associated with prognosis in our study, which is a finding repeated in several other studies (4, 10). Overall, the five-year survival rate of people with esophageal cancer is about 17%. However, survival rates depend on several factors, including the stage (or extent) of the cancer at the time of diagnosis. Fortunately, new treatment approaches have lowered the death rate from esophagus cancer, which at one time killed 95% of those diagnosed with the disease. Today, about 16% of all esophageal cancer patients will survive five years or more (13). As with most types of cancer, survival odds increase when the disease is caught and treated early (16). The five-year survival rate of people with cancer located only in the esophagus is about 41.2%. The five-year rate for those with disease that has spread regionally is 14.3%, if it has spread to distant organs, about 8.2% (Table 2).
Surgical results in treatment of esophageal cancer have improved significantly over recent years. However, medical centers now report that patients undergoing surgery alone have median survival rates between 13 and 19 months, 2-year survival rates between 35% and 42%, and 5-year survival rates of 15% to 24% (17). In present study median of survival and 4-year survival rate in this cases was (12±3.86 month) and (21.3), respectively. In patients that radiation therapy was the only optionfor treatment the mean survival rate was (5±1.06 month). Radiation therapy has been used in the past as a single-modality approach with curative intent. However, except for those with very early-stage disease, radiation has had little impact on long-term survival. Chemotherapy has been given preoperatively, postoperatively, or both median survival rates were (5±0.548 month). Multimodality treatment approaches have evolved over recent years in response to the frequent loco regional and distant recurrences identified after surgery or radiation therapy alone.
There are limitations with the present study in which the survival rate unable to predict future events for a particular person. Meanwhile, it was not possible to consider changes in characteristics after diagnosis, which may have affected survival. Hospital series often provide more optimistic data. They are of limited value because of unavoidable selection bias, in particular in case selection and patient's characteristics.
In conclusions, the present study showed that age of diagnosis, histological grade, and location of tumor was prognostic factors for survival in patients with esophageal cancer. It can be concluded the early detection of patients at younger age and in primary stages and histological grade may have positive effect on patients with esophageal cancer and be important to increase the survival time.
Acknowledgements
Ilam University of Medical Sciences, Ilam, Iran, supported this study financially.
(Please cite as: Delpisheh A, Veisani Y, Sayehmiri K, Rahimi E. Esophageal carcinoma: long-term survival in consecutive series of patients through a retrospective cohort study. Gastroenterol Hepatol Bed Bench 2014;7(2):101-107).
References
- 1.Scarpa M, Valente S, Alfieri R, Cagol M, Diamantis G, Ancona E, et al. Systematic review of health-related quality of life after esophagectomy for esophageal cancer. World J Gastroenterol. 2011;17:4660–74. doi: 10.3748/wjg.v17.i42.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin H-R, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008. Int J Cancer. 2008;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Seminars in Radiation Oncology. 2007;17:2–9. doi: 10.1016/j.semradonc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Rasouli M, Ghadimi MR, Mahmoodi M, Mohammad K, Zeraati H, Hosseini M. Survival analysis of patients with esophageal cancer using parametric cure model. Asian Pac J Cancer Prev. 2012;12:2359–63. [PubMed] [Google Scholar]
- 5.Bashash M, Shah A, Hislop G, Brooks-Wilson A, Le N, Bajdik C. Incidence and survival for gastric and esophageal cancer diagnosed in British Columbia, 1990 to 1999. Can J Gastroenterol. 2008;22:143–48. doi: 10.1155/2008/645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong R, Malthaner R. Esophageal cancer: a systematic review. Curr Probl Cancer. 2000;24:297–373. doi: 10.1016/s0147-0272(00)80002-1. [DOI] [PubMed] [Google Scholar]
- 7.Haghdoost AA, Hosseini H, Chamani G, Zarei MR, Rad M, Hashemipoor M, et al. Rising incidence of adenocarcinoma of the esophagus in Kerman. Iran. Arch Iran Med. 2008;11:364–70. [PubMed] [Google Scholar]
- 8.Samadi F, Babaei M, Yazdanbod A, Fallah M, Nouraie M, Nasrollahzadeh D, et al. Survival rate of gastric and esophageal cancers in Ardabil province, North-West of Iran. Arch Iran Med. 2007;10:32–37. [PubMed] [Google Scholar]
- 9.Ghadimi MR, Mahmoodi M, Mohammad K, Rasouli M, Zeraati H, Fotouhi A. Factors affecting survival of patients with oesophageal cancer: a study using inverse Gaussian frailty models. Singapore Med J. 2012;53:336–43. [PubMed] [Google Scholar]
- 10.Hajian-Tilaki KO. Factors affecting the survival of patients with oesophageal carcinoma under radiotherapy in the north of Iran. Br J Cancer. 2001;85:1671–74. doi: 10.1054/bjoc.2001.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daivid G, Klein B, editors. Survival analysis. 2nd ed. New York: Springer; 2005. [Google Scholar]
- 12.Zhu HP, Xia X, Yu CH, Adnan A, Liu SF, Du YK. Application of Weibull model for survival of patients with gastric cancer. BMC Gastroenterol. 2011;11:1. doi: 10.1186/1471-230X-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haugstvedt TK, Viste A, Eide GE, Soreide O. Norwegian multicentre study of survival and prognostic factors in patients undergoing curative resection for gastric carcinoma. The Norwegian Stomach Cancer Trial. Br J Surg. 1993;80:475–78. doi: 10.1002/bjs.1800800423. [DOI] [PubMed] [Google Scholar]
- 14.Orsenigo E, Carlucci M, Braga M, Tomajer V, Di Palo S, Tamburini A, et al. Prognostic factors of gastric neoplasms: experience with 1,074 cases undergoing surgical treatment at a single center. Suppl Tumori. 2005;4:86–87. [PubMed] [Google Scholar]
- 15.Coburn NG, Swallow CJ, Kiss A, Law C. Significant regional variation in adequacy of lymph node assessment and survival in gastric cancer. Cancer. 2006;1:2143–51. doi: 10.1002/cncr.22229. [DOI] [PubMed] [Google Scholar]
- 16.Dikken JL, Lemmens VE, Wouters MW, Wijnhoven BP, Siersema PD, Nieuwenhuijzen GA, et al. Increased incidence and survival for oesophageal cancer but not for gastric cardia cancer in the Netherlands. Eur J Cancer. 2012;48:1624–32. doi: 10.1016/j.ejca.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Zhang HL, Liu RL, Shi YT, Wang ZC, Wang BH, Li YJ. Analysis of the survival in patients after surgical resection of thoracic esophageal cancer. Zhonghua Zhong Liu Za Zhi. 2009;31:541–45. [In Chinese] [PubMed] [Google Scholar]