Abstract
Problem
To determine if down-regulation of TIMP3 expression promotes TACE expression and increases in TNFα production by placental trophoblast cells.
Method of study
Placental expression of TIMP3 and TACE was examined by immunostaining and Western blot. Effects of TIMP3 on TACE expression and TNFα production were assessed by transfection of TIMP3 siRNA into trophoblasts isolated from normal placentas. Effects of oxidative stress on trophoblast TIMP3 expression and TNFα production were also determined. Trophoblast production of TIMP3, TACE, and TNFα were measured by ELISA.
Results
TIMP3 expression was markedly reduced in preeclamptic placentas compared to normal placentas; Oxidative stress down-regulated trophoblast TIMP3 expression and production, p<0.01. Down-regulation of TIMP3 expression by TIMP3 siRNA resulted in significant increases in TACE expression and TNFα production, p<0.01.
Conclusion
Since TIMP3 is an endogenous TACE inhibitor, down-regulation of trophoblast TIMP3 expression/activity could result in increased TACE expression and subsequently lead to increased TNFα production in preeclamptic placentas.
Keywords: TIMP3, TACE, TNFα, trophoblast, preeclampsia
Introduction
Tumor necrosis factor-alpha (TNFα) is a pleiotropic inflammatory cytokine. It has a wide spectrum of bioactivity in both physiological and pathophysiology conditions. In the placenta, TNFα could provoke multitude of biological responses. It plays an important role in regulating physiological balance of trophoblast turnover and renewal 1. It not only induces trophoblast apoptosis and cytotoxicity, but also inhibits trophoblast fusion and invasion 1-3. In preeclampsia, placental production and expression of TNFα significantly increased 4, and the increased TNFα production is likely to contribute to placental and endothelial injury in this pregnancy disorder 4-7. The involvement of TNFα in the development of placental atherosclerotic lesions further indicates the importance of TNFα deregulation in placental dysfunction in preeclampsia 8
TNFα is expressed as a membrane-bounded molecule and is released from the cell surface by proteolytic cleavage. The major metalloproteinase responsible for TNFα cleavage is a disintegrin and metalloproteinase domain-containing protein 17 (ADAM17), which is also named as TNFα converting enzyme (TACE). We recently found that TACE expression is increased in preeclamptic placentas 9. We further found that hypoxia/oxidative stress promoted TACE expression and subsequently increased TNFα production by placental trophoblasts 9. These data demonstrated the role of TACE in the regulation of TNFα production in the placenta 9.
TACE activity is regulated by a group of endogenous physiological inhibitors including the tissue inhibitors of matrix metalloproteases (TIMPs). To date, four members of TIMPs (TIMP-1, -2, -3, and -4) have been found in the mammalian cells 10. Purified TIMPs from mouse myeloma cells showed that TIMP-1 and TIMP-3 have the ability to inhibit the protease activity of ADAM10 11, while only TIMP-3 has the ability to inhibit TACE 12. Although TACE expression is increased in preeclampsia placenta 9, little is known about TIMP3 expression and activity in the human placentas. In the present study, we sought to determine if TIMP3 expression was decreased in preeclamptic placentas and whether inhibition of TIMP3 expression could promote TACE expression and increase TNFα production in placental trophoblasts.
Materials and Methods
Placenta collection
Placentas delivered by normal and preeclamptic pregnant women were collected immediately after delivery, which was approved by the Institutional Review Board for human research at Louisiana State University Health Sciences Center-Shreveport, LA. A total of 33 placentas, 21 from normal and 12 from preeclamptic pregnancies, were used in this study. Among them, tissue pieces from 24 placentas (12 from normal and 12 from preeclamptic) were either fixed with 10% formalin for immunohistochemistry or snap frozen for total tissue protein expression; and 9 normal term placentas were used for trophoblast isolation. Normal pregnancy is defined as pregnancy with normal blood pressure (<140/90mmHg) without proteinuria and absence of obstetrical and medical complications. Preeclampsia is defined as blood pressure ≥ 140/90 mmHg on two separate readings and proteinuria >1+ on dipstick or ≥ 300mg in 24hrs urine. No patient had signs of infection. Smokers were excluded.
Immunohistochemistry
Expression for TIMP3 and TACE was examined by immunohistochemistry in paraffin embedded placental tissue sections. A standard immunohistochemistry procedure was performed as previously described 13. TIMP3 antibody was obtained from Santa Cruz (San Diego, CA) and TACE antibody was obtained from Abcam Inc. (Cambridge, MA). Tissue sections stained with isotype IgG or without primary antibody served as negative controls. Slides stained with the same antibody were all stained at the same time. Stained slides were then reviewed under microscope and images were captured with PictureFrame computer software (Uptronics Inc., Sunnyvale, CA) and recorded to a microscope linked PC computer.
Trophoblast isolation and culture
Trophoblasts were isolated from freshly obtained placentas immediately after delivery from normal pregnancies as previously described 9. Briefly, trophoblasts were isolated by trypsin digestion (0.125 % trypsin solution containing 0.1mg/ml DNase I and 5mM MgCl2) in Dulbecco's Modified Eagle Medium (DMEM) at 37°C for 90 min. Isolated trophoblasts were further purified by Percoll gradient centrifugation. Freshly isolated trophoblasts were then incubated with DMEM with 5% fetal bovine serum (FBS) in 6-well plate (5×106cells/well). Isolated trophoblasts were either treated with hypoxic mimicking agent CoCl2 to determine effects of oxidative stress on TIMP3 expression and production or transfected with TIMP3 siRNA to determine effects of TIMP3 inhibition on TACE expression and TNFα production.
TIMP3 siRNA transfection assay
To determine if TIMP3 regulates TACE expression and TNFα production in placental trophoblasts, TIMP3 siRNA transfection assay was performed. TIMP3 siRNA was purchased from Thermo Scientific (Rockford, IL) and scrambled siRNA was purchased from Santa Cruz (San Diego, CA). Briefly, 30nM of TIMP3 siRNA were transfected into primary isolated trophoblasts (5×106 cells/well) 24hrs after seeding using Lipofectamine™ RNAiMAX transfection agent (Invitrogen, Carlsbad, CA). Cells transfected with scrambled siRNA were used as control. Culture medium and total cellular protein was collected 48 hours after transfection. Medium levels of TIMP3, TACE, and TNFα were measured by enzyme-link immunoassay (ELISA). Protein expression of TIMP3 and TACE were determined by Western blot.
Protein expression for TIMP3 and TACE
Placental tissue and trophoblast expression of TIMP3 and TACE were determined by Western blot. For tissue expression, total protein was extracted from snap frozen tissues. For trophoblast expression, total cellular protein was extracted immediately after cell culture experiment. An aliquot of total protein of 15μg per sample was used for electrophoresis (Bio-Rad, Hercules, CA) and then transferred to Hybond-protein transfer membranes (Amersham Corp, Arlington Heights, Ill). The membranes were probed with antibodies against TIMP3 or TACE. TIMP3 and TACE expression were detected by an enhanced chemiluminescent detection kit (Amersham). The same antibodies were used for the immunostaining assay described above. β-actin expression was determined to verify the protein loading consistency for each sample.
Measurement of TIMP3, TACE, and TNFα production
ELISA kits for measuring TIMP3, TACE, and TNFα were purchased from R&D systems, Inc. (Minneapolis, MN). All assays were performed according to the manufacturer's instructions. All samples were tested in duplicate. The range of standard curve was 3.9 to 4,000pg/ml for TIMP3, 9.8 to 10,000pg/ml for TACE, and 0.98 to 1,000pg/ml for TNFα. Within- and between-assay variations were < 8% for all assays.
Statistical analysis
Data are presented as mean ± SE. Statistical analysis was performed with ANOVA or paired t-test by computer software program StatView. Student-Newman-Keuls test was used as post hoc tests. A probability level less than 0.05 was considered statistically significant.
Results
Decreased TIMP3 expression and increased TACE expression in preeclamptic placentas
Figure 1 shows TIMP3 and TACE expression examined by immunostaining and Western blot. Representative images for TIMP3 and TACE expression by immunostaining are shown in Figure 1A. TIMP3 and TACE are mainly expressed in the syncytiotrophoblast layer. In normal placenta, TIMP3 is also stained in villous stromal cells. Compared to normal placentas, TIMP3 expression is reduced in preeclamptic placentas. In contrast, TACE expression is strongly stained in preeclamptic placentas compared to normal placentas. Relative tissue protein expression of TIMP3 and TACE are shown in Figure 1B. Consistent with immunostaining results, Relative tissue TIMP3 expression was significantly reduced and TACE expression was significantly increased in preeclamptic placentas compared to normal placentas, TIMP3: 0.26 ± 0.07 vs. 0.72 ± 0.18, p<0.05, and TACE: 0.75 ± 0.19 vs. 0.32 ± 0.07, p<0.05.
Figure 1.
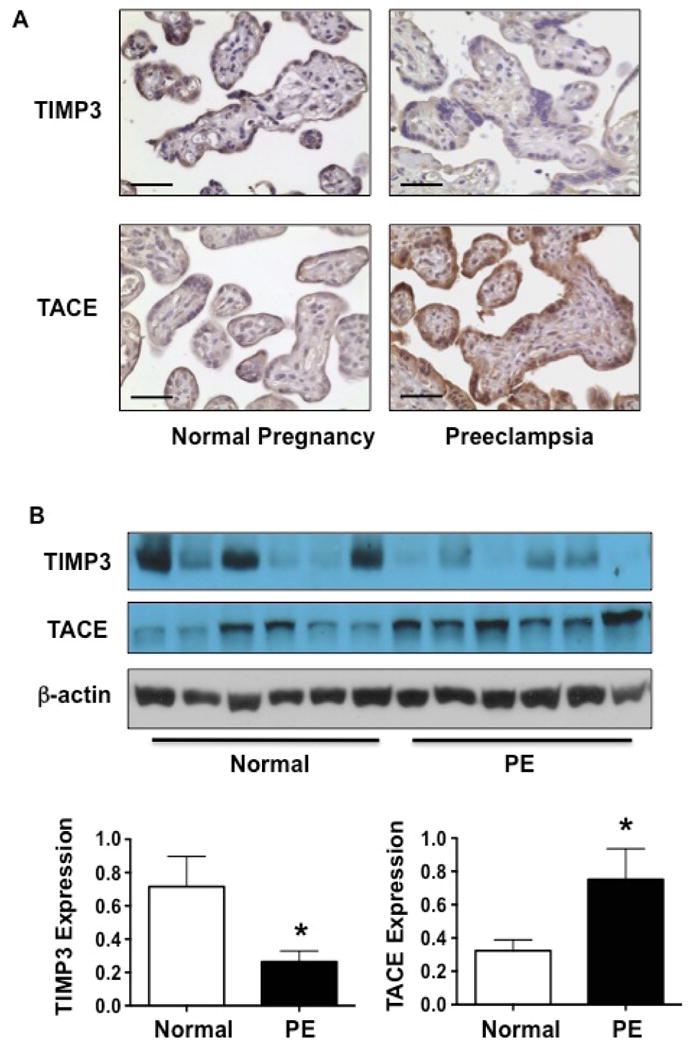
Expression of TIMP3 and TACE in normal and preeclamptic placentas. A: Representative immunostaining of TIMP3 and TACE in villous tissues from normal and preeclamptic placentas. TIMP3 expression was decreased and TACE expression was increased in syncytiotrophoblasts of preeclamptic placentas compared to normal placentas. Bar=50micron. B: Total protein expression of TIMP3 and TACE in normal and preeclamptic placentas by Western blot. Consistent with immunostaining results, TIMP3 expression was significantly reduced and TACE expression was significantly increased in preeclamptic placentas compared to normal placentas, * p<0.05.
Inhibition of TIMP3 expression results in increased TACE expression and production by placental trophoblasts
TIMP3 inhibits TACE activity in mouse myeloma cells 12. To determine if TIMP3 regulates TACE expression and production in placental trophoblasts, TIMP3 siRNA was used. In this experiment, TIMP3 siRNA were transfected into trophoblasts isolated from normal term placentas. TIMP3 and TACE expression and production were then determined. As shown in Figure 2A, TIMP3 expression was significantly reduced in cells transfected with TIMP3 siRNA. In contrast, TACE expression was markedly increased in cells transfected with TIMP3 siRNA. Consistent with protein expression, TIMP3 production was also significantly reduced and TACE production was significantly increased in trophoblasts transfected with TIMP3 siRNA compared to cells transfected with scrambled siRNA, TIMP3: 1.20 ± 0.28 vs. 1.8 ± 0.25ng/5×106 cells/ml, p<0.01, and TACE: 152.54 ± 9.82 vs. 85.08 ± 15.75pg/5×106 cells/ml (Figure 2B). Data are means from 5 independent experiments.
Figure 2.
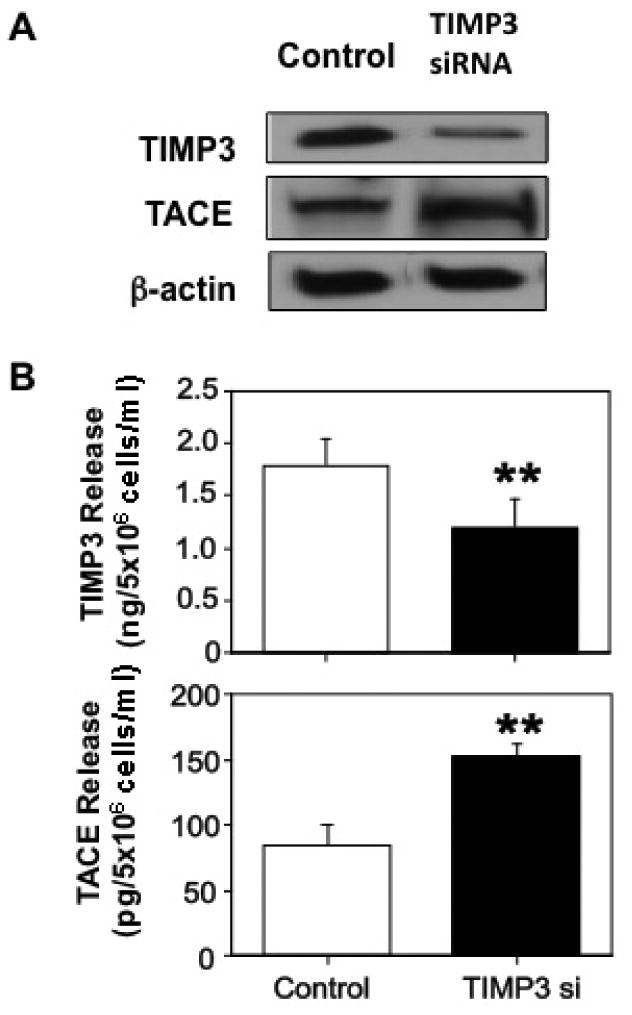
Effects of TIMP3 siRNA on trophoblast TIMP3 and TACE expression and production. Inhibition of TIMP3 expression resulted in an increase in TACE expression (A) that was associated with reduced TIMP3 release and increased TACE release by trophoblasts in culture (B). Data are means from 5 independent siRNA transfection experiments, ** p<0.01, respectively.
Oxidative stress downregulates TIMP3 expression
Increased oxidative stress is an underlying pathophysiology in preeclamptic placentas. Hypoxia/oxidative stress promotes TNFα production 9. To determine if hypoxia affects TIMP3 expression and production, trophoblasts were treated with cobalt (II) chloride (CoCl2) at different concentrations for 48 hours. CoCl2 is a hypoxia mimetic agent, which has been used as a hypoxia/oxidative stress inducer in numerous in vitro cell culture studies including adipocytes, retinal ganglion cells, and trophoblasts 9, 14-16. Our results showed that TIMP3 expression and production were significantly reduced in cells treated with CoCl2 compared to control cells and the oxidative stress-induced down-regulation of TIMP3 expression and production were dose-dependent, Figure 3A. Data are means from 6 independent experiments.
Figure 3.
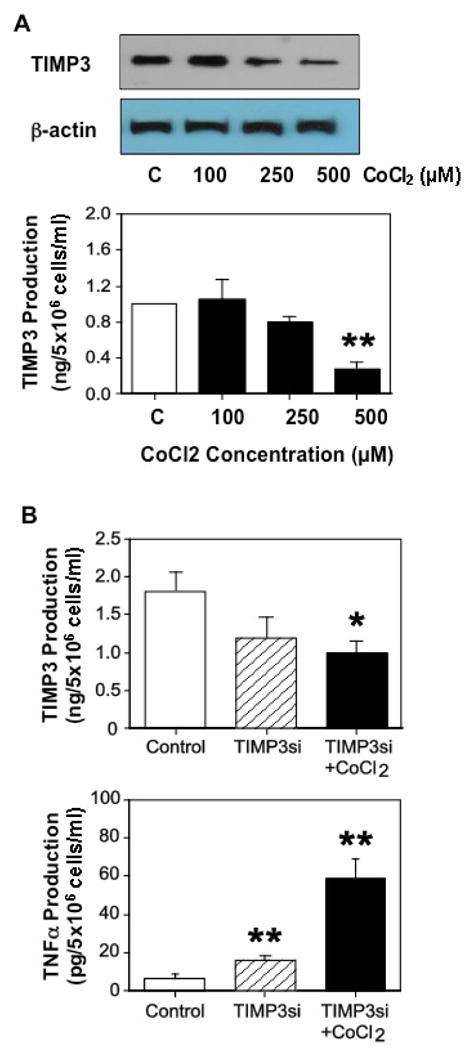
Effects of oxidative stress on TIMP3 expression, and TIMP3 and TNFα production. A: TIMP3 expression and release in trophoblasts treated with CoCl2, a hypoxic mimicking agent. Trophoblasts from normal placentas were treated with different doses of CoCl2 for 48hrs. CoCl2 induced a dose-dependent decrease in TIMP3 expression and TIMP3 release in trophoblasts isolated from normal placentas. Data are means from 3 independent experiments. ** p<0.01: treated vs. untreated control cells (C). B: TIMP3 and TNFα production in trophoblasts transfected with TIMP3 siRNA with or without CoCl2 in culture. Data are means from 5 independent experiments. * p<0.05 and ** p<0.01: treated vs. control cells.
Inhibition of TIMP3 promotes TNFα production under oxidative stress stimulation
To further determine effects of TIMP3 and hypoxia/oxidative stress on TNFα production, we measured TIMP3 and TNFα production in cells transfected with TIMP3 siRNA with or without CoCl2 treatment. As shown in Figure 3B, TIMP3 production was reduced in cells transfected with TIMP3 siRNA, 1.19±0.28ng/5×106 cells/ml, and further reduced in TIMP3 siRNA transfected cells treated with CoCl2, 1.01±0.15ng/5×106 cells/ml, p<0.05, compared to control cells, 1.80±0.25ng/5×106 cells/ml. In contrast, TNFα production was increased in cells transfected with TIMP3 siRNA, 15.35±0.91pg/5×106 cells/ml, and further increased in TIMP3 siRNA transfected cells treated with CoCl2, 58.37±10.55pg/5×106 cells/ml, p<0.01, compared to control cells, 6.16±0.56pg/5×106 cells/ml. In this experiment, CoCl2 at a concentration of 500μM was used. These results indicate that oxidative stress potentiates TIMP3 inhibition-mediated TNFα production. Data are means from 5 independent experiments.
Discussion
In this study, we determined TIMP3 and TACE expression in normal and preeclamptic placentas and investigated the role of TIMP3 in TNFα production by placental trophoblasts. We found that TIMP3 was strongly expressed in the syncytiotrophoblast layer of villous tissue from normal placentas. In contrast, TACE was strongly expressed in the syncytiotrophoblast layer of villous tissues from preeclamptic placentas. TIMP3 is a native inhibitor of TACE (a TNFα converting enzyme). TNFα production was increased in preeclamptic placentas 4. Thus, reduced TIMP3 expression could result in increased TACE expression and subsequently increased TNFα production in preeclamptic placentas.
To investigate if reduced TIMP3 expression could lead to an increase in TACE expression associated with increased TNFα production in placental trophoblasts, TIMP3 siRNA was used. Our results showed that inhibition of TIMP3 expression by TIMP3 siRNA resulted in an increase in TACE expression. The decreased TIMP3 expression was also accompanied by reduced TIMP3 production and increased TACE release by placental trophoblasts. These results prove that TACE is a target of TIMP3 and inhibition of TIMP3 expression could directly affect TACE activity/expression in placental trophoblasts.
Increased oxidative stress is an underlying pathophysiology in preeclamptic placentas. In fact, many pathophysiological phenotypic changes found in preeclamptic placentas are closely associated with increased oxidative stress, such as increased vasoconstrictor thromboxane production, increased inflammatory cytokine IL-6 and IL-8 production, and increased anti-angiogenic factor sFlt-1 and sEng production 13, 17-19. To define if reduced TIMP3 expression found in preeclamptic placentas is associated with increased oxidative stress, we determined TIMP3 expression and production in trophoblasts treated with CoCl2. CoCl2 is a hypoxia mimetic agent and has been used to induce oxidative stress in numerous in vitro cell culture studies including trophoblasts 9. As we expected, CoCl2 induced a dose dependent-decrease in TIMP3 expression and production. We further found that decreased TIMP3 expression and increased TNFα production induced by TIMP3 siRNA could be further potentiated by treating trophoblasts with CoCl2. These results provide convincing evidence that down-regulation of TIMP3 expression is likely a consequence of increased oxidative stress in preeclamptic placentas. Although we did not specifically examine whether reduced placental TIMP3 expression or increased placental TACE expression is associated with the severity of the disease, the findings of CoCl2 induced dose-dependent decreases in TIMP3 expression and production suggest this might be the case, which needs to be further investigated.
TIMP3 is an endogenous negative regulator of TNFα in tissue response to injury and plays important roles in regulating the integrity of extracellular matrix and tissue remodeling. For example, animal studies have shown that TIMP3 could act as an on-and-off switch for myogenic differentiation by regulating autocrine TNFα release 20. On the other hand, TIMP3 deficiency results in an increase in TACE activity and fails to control the release of TNFα production, leading to inappropriate control of systemic inflammation and TNFα-mediated cell death 21, 22. TIMP3 deficiency also contributes significantly to organ dysfunction and systemic vascular diseases. In kidney, loss of TIMP3 enhances interstitial nephritis and fibrosis 23. In diabetic animals, loss of TIMP3 exacerbates nephropathy and promotes vascular inflammation 24, 25. In placental tissues, TIMP3 is strongly expressed in syncytiotrophoblasts in normal placentas. Owing to the specific location of syncytiotrophoblasts at the maternal-fetal interface during pregnancy, there would be no question that the lack or insufficiency of TIMP3 in syncytiotrophoblasts would result in increased TNFα release into maternal circulation and contribute to increased circulating TNFα levels in preeclampsia.
In conclusion, we have made several important findings in this study. First, we demonstrated that TIMP3 expression, the suppressor of TACE, is decreased in placental trophoblasts in preeclampsia. Reduced TIMP3 expression is associated with increased TACE expression. Second, we found that inhibition of TIMP3 expression by TIMP3 siRNA results in increased TACE expression and increased TNFα production by trophoblasts from normal placentas. Last but not least, we proved that hypoxia/oxidative stress not only reduces TIMP3 expression but also potentiates TIMP3 siRNA-induced increased TNFα production by placental trophoblasts. Thus, we believe that increased oxidative stress is likely a causative factor to down-regulate TIMP3 expression and activity in preeclampsia placenta.
Acknowledgments
This study was supported in part by grants from National Institute of Health, RO1 NICHD (HD36822) and RO1 NHLBI (HL65997) to Y.W.
Footnotes
Disclosure: None
References
- 1.Yui J, Garcia-Lloret M, Wegmann TG, Guilbert LJ. Cytotoxicity of tumour necrosis factor-alpha and gamma-interferon against primary human placental trophoblasts. Placenta. 1994;15:819–835. doi: 10.1016/s0143-4004(05)80184-5. [DOI] [PubMed] [Google Scholar]
- 2.Bauer S, Pollheimer J, Hartmann J, Husslein P, Aplin JD, Knofler M. Tumor necrosis factor-alpha inhibits trophoblast migration through elevation of plasminogen activator inhibitor-1 in first-trimester villous explant cultures. J Clin Endocrinol Metab. 2004;89:812–822. doi: 10.1210/jc.2003-031351. [DOI] [PubMed] [Google Scholar]
- 3.Todt JC, Yang Y, Lei J, Lauria MR, Sorokin Y, Cotton DB, Yelian FD. Effects of tumor necrosis factor-alpha on human trophoblast cell adhesion and motility. Am J Reprod Immunol. 1996;36:65–71. doi: 10.1111/j.1600-0897.1996.tb00141.x. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Walsh SW. TNFα concentrations and mRNA expression are increased in preeclamptic placentas. J Reprod Immunol. 1996;32:157–169. doi: 10.1016/s0165-0378(96)00998-9. [DOI] [PubMed] [Google Scholar]
- 5.Benyo DF, Miles TM, Conrad KP. Hypoxia stimulates cytokine production by villous explants from the human placenta. J Clin Endocrinol Metab. 1997;82:1582–1588. doi: 10.1210/jcem.82.5.3916. [DOI] [PubMed] [Google Scholar]
- 6.Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol. 1997;37:240–249. doi: 10.1111/j.1600-0897.1997.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 7.Malek A, Sager R, Schneider H. Effect of hypoxia, oxidative stress and lipopolysaccharides on the release of prostaglandins and cytokines from human term placental explants. Placenta. 2001;22(Suppl A):S45–50. doi: 10.1053/plac.2001.0635. [DOI] [PubMed] [Google Scholar]
- 8.Pijnenborg R, McLaughlin PJ, Vercruysse L, Hanssens M, Johnson PM, Keith JC, Jr, Van Assche FA. Immunolocalization of tumour necrosis factor-alpha (TNF-alpha) in the placental bed of normotensive and hypertensive human pregnancies. Placenta. 1998;19:231–239. doi: 10.1016/s0143-4004(98)90054-6. [DOI] [PubMed] [Google Scholar]
- 9.Ma R, Gu Y, Groome LJ, Wang Y. ADAM17 regulates TNFα production by placental trophoblasts. Placenta. 2011;32:975–980. doi: 10.1016/j.placenta.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pohar N, Godenschwege TA, Buchner E. Invertebrate tissue inhibitor of metalloproteinase: structure and nested gene organization within the synapsin locus is conserved from Drosophila to human. Genomics. 1999;15:293–296. doi: 10.1006/geno.1999.5776. [DOI] [PubMed] [Google Scholar]
- 11.Amour A, Knight CG, Webster A, Slocombe PM, Stephens PE, Knäuper V, Docherty AJ, Murphy G. The in vitro activity of ADAM-10 is inhibited by TIMP-1 and TIMP-3. FEBS Lett. 2000;19:275–279. doi: 10.1016/s0014-5793(00)01528-3. [DOI] [PubMed] [Google Scholar]
- 12.Amour A, Slocombe PM, Webster A, Butler M, Knight CG, Smith BJ, Stephens PE, Shelley C, Hutton M, Knäuper V, Docherty AJ, Murphy G. TNF-alpha converting enzyme (TACE) is inhibited by TIMP-3. FEBS Lett. 1998;435:39–44. doi: 10.1016/s0014-5793(98)01031-x. [DOI] [PubMed] [Google Scholar]
- 13.Gu Y, Lewis DF, Wang Y. Placental productions and expressions of soluble endoglin, soluble fms-like tyrosine kinase receptor-1, and placental growth factor in normal and preeclamptic pregnancies. J Clin Endocrinol Metab. 2008;93:260–266. doi: 10.1210/jc.2007-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamiya T, Hara H, Inagaki N, Adachi T. The effect of hypoxia mimetic cobalt chloride on the expression of EC-SOD in 3T3-L1 adipocytes. Redox Rep. 2010;15:131–134. doi: 10.1179/174329210X12650506623483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashi M, Sakata M, Takeda T, Yamamoto T, Okamoto Y, Sawada K, Kimura A, Minekawa R, Tahara M, Tasaka K, Murata Y. Induction of glucose transporter 1 expression through hypoxia-inducible factor 1alpha under hypoxic conditions in trophoblast-derived cells. J Endocrinol. 2004;183:145–154. doi: 10.1677/joe.1.05599. [DOI] [PubMed] [Google Scholar]
- 16.Tulsawani R, Kelly LS, Fatma N, Chhunchha B, Kubo E, Kumar A, Singh DP. Neuroprotective effect of peroxiredoxin 6 against hypoxia-induced retinal ganglion cell damage. BMC Neurosci. 2010;125:125. doi: 10.1186/1471-2202-11-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowen RS, Zhang Y, Gu Y, Lewis DF, Wang Y. Increased phospholipase A2 and thromboxane but not prostacyclin production by placental trophoblast cells from normal and preeclamptic pregnancies cultured under hypoxia condition. Placenta. 2005;26:402–409. doi: 10.1016/j.placenta.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Bowen RS, Gu Y, Zhang Y, Lewis DF, Wang Y. Hypoxia promotes interleukin-6 and -8 but reduces interleukin-10 production by placental trophoblast cells from preeclamptic pregnancies. J Soc Gynecol Investig. 2005;12:428–432. doi: 10.1016/j.jsgi.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Gu B, Zhang Y, Lewis DF, Wang Y. Hypoxia-induced increase in soluble Flt-1 production correlates with enhanced oxidative stress in trophoblast cells from the human placenta. Placenta. 2005;25:210–217. doi: 10.1016/j.placenta.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Liu H, Chen SE, Jin B, Carson JA, Niu A, Durham W, Lai JY, Li YP. TIMP3: a physiological regulator of adult myogenesis. J Cell Sci. 2010;123:2914–2921. doi: 10.1242/jcs.057620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohammed FF, Smookler DS, Taylor SE, Fingleton B, Kassiri Z, Sanchez OH, English JL, Matrisian LM, Au B, Yeh WC, Khokha R. Abnormal TNF activity in Timp3-/- mice leads to chronic hepatic inflammation and failure of liver regeneration. Nat Genet. 2004;36:969–977. doi: 10.1038/ng1413. [DOI] [PubMed] [Google Scholar]
- 22.Smookler DS, Mohammed FF, Kassiri Z, Duncan GS, Mak TW, Khokha R. Tissue inhibitor of metalloproteinase 3 regulates TNF-dependent systemic inflammation. J Immunol. 2006;176:721–725. doi: 10.4049/jimmunol.176.2.721. [DOI] [PubMed] [Google Scholar]
- 23.Kassiri Z, Oudit GY, Kandalam V, Awad A, Wang X, Ziou X, Maeda N, Herzenberg AM, Scholey JW. Loss of TIMP3 enhances interstitial nephritis and fibrosis. J Am Soc Nephrol. 2009;20:1223–1235. doi: 10.1681/ASN.2008050492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basu R, Lee J, Wang Z, Patel VB, Fan D, Das SK, Liu GC, John R, Scholey JW, Oudit GY, Kassiri Z. Loss of TIMP3 selectively exacerbates diabetic nephropathy. Am J Physiol Renal Physiol. 2012;303:F1341–F1352. doi: 10.1152/ajprenal.00349.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Federici M, Hribal ML, Menghini R, Kanno H, Marchetti V, Porzio O, Sunnarborg SW, Rizza S, Serino M, Cunsolo V, Lauro D, Mauriello A, Smookler DS, Sbraccia P, Sesti G, Lee DC, Khokha R, Accili D, Lauro R. Timp3 deficiency in insulin receptor-haploinsufficient mice promotes diabetes and vascular inflammation via increased TNF-alpha. J Clin Invest. 2005;115:3494–3505. doi: 10.1172/JCI26052. [DOI] [PMC free article] [PubMed] [Google Scholar]


