Abstract
Tyrosine hydroxylase (TH) is the key enzyme for synthesizing dopamine (DA) in dopaminergic neurons and its terminals. Emerging experimental and clinical evidence support the hypothesis of a disturbance in dopamine neurotransmission following traumatic brain injury (TBI). However, the effect of controlled cortical impact (CCI) injury on TH in the nigrostriatal system is currently unknown. To determine if there is an alteration in TH after CCI injury, we examined TH levels at 1 day, 7 days, and 28 days post-injury by utilizing a commercially available antibody specific to TH. Rats were anesthetized and surgically prepared for CCI injury (4 m/sec, 3.2 mm) or sham surgery. Injured (n=6) and sham animals (n=6) were sacrificed and coronally sectioned (35 μm thick) through the striatum and substantia nigra (SN) for immunohistochemistry. Additionally, semiquantitative measurements of TH protein in striatal and SN homogenates from injured (n=6) and sham (n=6) rats sacrificed at the appropriate time post-surgery were assessed using Western blot analysis. TH protein is bilaterally increased at 28 days post-injury in nigrostriatal system revealed by immunohistochemistry in injured rats compared to sham controls. Western blot analysis confirms the findings of immunohistochemistry in both striatum and SN. We speculate that the increase in TH in the nigrostriatal system may reflect a compensatory response of dopaminergic neurons to upregulate their synthesizing capacity and a delayed increase in the efficiency of DA neurotransmission after TBI.
Keywords: Immunohistochemistry, Nigrostriatal, Rat, Traumatic brain injury (TBI), Tyrosine hydroxylase (TH), Western blot
1. Introduction
While the neurological and cognitive consequences of traumatic brain injury (TBI) are many and complex, the most persistent include memory impairment and difficulties in attention and concentration (Ragnarsson, 2002), functions that are mediated by multiple brain structures. The hippocampus, amygdala, and dorsal striatum (caudate-putamen) each perform functions, both independently and collaboratively, in the acquisition and expression of learned behavior (White and McDonald, 2002). Focal damage to the striatum in non-human primates causes difficulty with spatial working memory tasks (Divac et al., 1967; Miyoshi et al., 2002). Studies investigating dementia in Parkinson's (Muller et al., 2000; Rinne et al., 2000) and Huntington's (Backman et al., 1997) disease indicate the striatum is part of a neuronal network mediating prefrontal executive functions.
Evidence that dopamine systems are altered in humans following TBI is based on reports that neurostimulants, including dopamine agonists, can be beneficial in attenuating cognitive deficits (Goldstein, 2003; McAllister et al., 2004) and data showing altered dopamine transporter binding (Donnemiller et al., 2000). After experimental TBI, alterations in the catecholamine systems have been found in various brain regions and have been shown to be time-dependent (Dunn-Meynell et al., 1994; McIntosh et al., 1994). Recently, striatal evoked DA overflow was found to be reduced in the striatum ipsilateral to the site of injury (Wagner et al., 2005). Furthermore, decreased DA transporter expression has been observed after TBI (Wagner et al., 2005; Wilson et al., 2005).
The detection of Tyrosine hydroxylase (TH), a specific enzyme or a key enzyme in the synthesis of catecholamines, demonstrates the presence of DA, as well as noradrenaline (NA) and adrenaline. Previous studies have shown that soma and axon terminals of NA-containing neurons cannot always be easily demonstrated using TH immunocytochemistry (Hokfelt et al., 1976, 1977; Joh and Reis, 1975; Pickel et al., 1975). However, the striatum contains only very low levels of NA (Bertler and Rosengren, 1959; Carlsson, 1959), and TH-positive structures represent, in fact, the axons and axon terminals of DA neurons (Anden et al., 1964; Hokfelt and Ungerstedt, 1969; Von Voigtlander and Moore, 1971).
Our previous study demonstrates that TBI produced an increase in TH protein in rat frontal cortex that was detectable by both immunohistochemistry and Western blot at 28 days post-injury (Yan et al., 2001). In contrast, dopamine beta hydroxylase (DBH) protein levels were not altered following TBI suggesting that the increase in TH occurred predominantly in dopaminergic axons (Yan et al., 2001). The effect of controlled cortical impact (CCI) injury on TH in the nigrostriatal system is currently unknown. To determine if there is an alteration in TH after CCI injury, we examined TH levels at 1 day, 7 days, and 28 days post-surgery by utilizing a commercially available antibody specific to TH.
2. Result
2.1. Immunohistochemistry
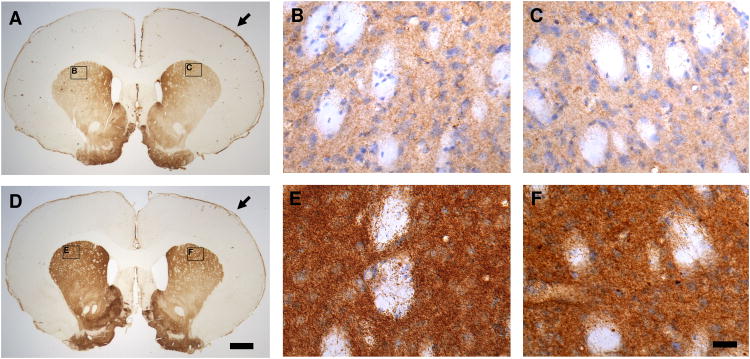
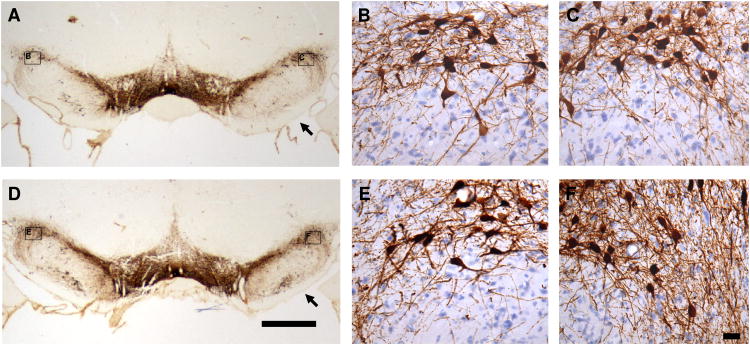
Traumatic brain injury results in a chronic increase in TH levels by immunohistochemistry in the rat striatum and substantia nigra in injured rats compared to sham controls at four weeks after TBI in rat CCI model. A bilateral increase in TH immunoreactivity (TH-IR) was observed in the striatum and substantia nigra in TBI rats compared to sham controls 28 days after surgery. No significant changes in TH-IR were observed in the rat striatum and substantia nigra at 1 day, or 7 days, after TBI compared to the sham controls. The pattern of the TH-IR distribution observed in the sham groups was similar to that previously reported (Rosin et al., 1992). Figure 1 illustrates the representative TH immunostaining of coronal sections from the ipsilateral (Fig. 1C and 1F) and the contralateral striatum (Fig. 1B and 1E) at 28 days after sham surgery (Fig. 1A, 1B and 1C) or TBI (Fig. 1D, 1E and 1F). An increased expression of TH-IR was observed in the TBI group compared to the sham controls. A dense plexus of TH-IR fibers and punctate vesicles were observed in both sham and injured groups, but was more pronounced in the latter. Interestingly, although the increased expression of TH protein in the striatum (Fig. 1) was observed bilaterally at four weeks after TBI in CCI model, this increased expression was more pronounced in the contralateral than the ipsilateral side as revealed by immunohistochemistry. Figure 2 illustrates the representative TH immunostaining of coronal sections from the ipsilateral (Fig. 2C and 2F) and the contralateral substantia nigra (Fig. 2B and 2E). An increased expression of TH was shown at four weeks after injury (Fig. 2D, 2E and 2F) compared to sham control animals (Fig. 2A, 2B and 2C). Furthermore, although the increase of TH-IR was observed in the bilateral substantia nigra, it was more pronounced in the ipsilateral than the contralateral substantia nigra (Fig. 2F vs. 2E).
Fig. 1.
Representative tyrosine hydroxylase (TH) immunohistochemical staining of coronal sections from the striatum at 28 days after traumatic brain injury (TBI; D, E & F) revealed a general increase in TH immunoreactivity (TH-IR) with a dense plexus of immunoreactive fibers and punctate vesicles compared to the sham controls (A, B & C). The increase of TH-IR is bilateral but more evident in the contralateral striatum (B & E) compared to the ipsilateral striatum (C & F). A (sham) and D (TBI) are the lower magnified microscopic view (1 × objective lens) of TH immunostaining. B & C (sham) and E & F (TBI) are the sister sections of A and D in the high magnified microscopic view (40 × DIC objective lens) of TH immunostaining counterstained with hematoxylin. B & C and E & F are the close-up views of the areas indicated in panels A and D. Arrow Point: ipsilateral side (in A & D). Bars: A = D = 1,000 μm (1 mm); B = C = E = F = 25 μm.
Fig. 2.
Representative tyrosine hydroxylase (TH) immunohistochemical staining of coronal sections from the substantia nigra at 28 days after traumatic brain injury (TBI; D, E & F) revealed a general increase in TH immunoreactivity (TH-IR) with a dense plexus of immunoreactive fibers and punctate vesicles compared to the sham controls (A, B & C). The increase of TH-IR is bilateral but more evident in the ipsilateral substantia nigra (C & F) compared to the contralateral substantia nigra (B & E). A (sham) and D (TBI) are the lower magnified microscopic view (1 × objective lens) of TH immunostaining. B & C (sham) and E & F (TBI) are the sister sections of A and D in the high magnified microscopic view (40 × DIC objective lens) of TH immunostaining counterstained with hematoxylin. B & C and E & F are the close-up views of the areas indicated in panels A and D. Arrow Point: ipsilateral side (in A & D). Bars: A = D = 1,000 μm (1 mm); B = C = E = F = 25 μm.
2.2. Western blot
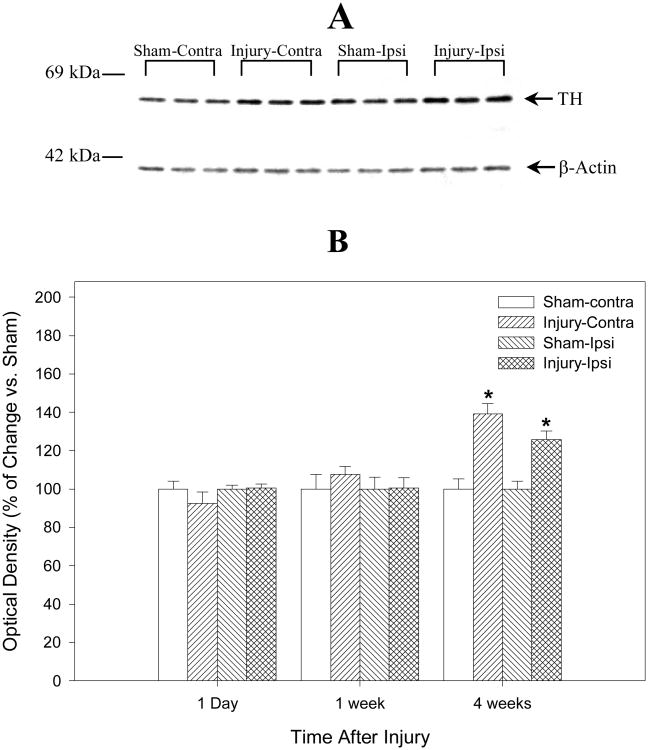
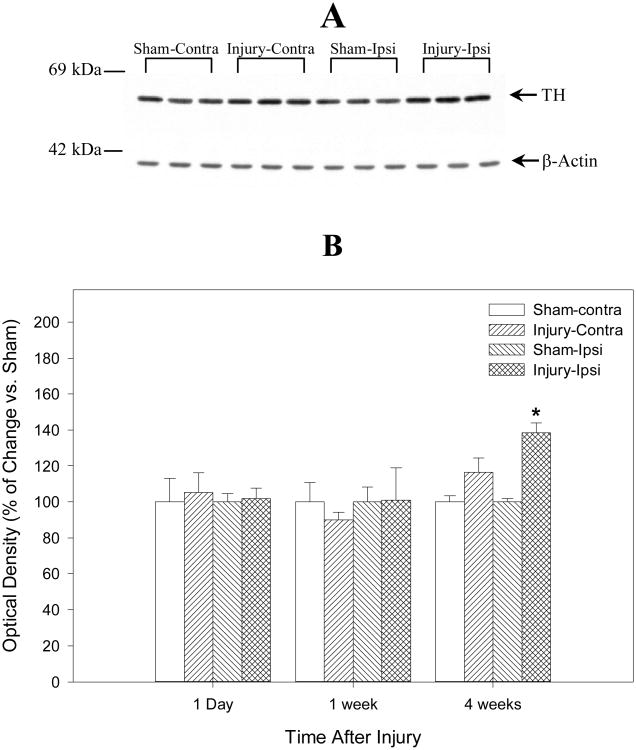
Western blot analysis was performed to evaluate the expression of TH protein in the striatum and the substantia nigra in injured rats at 1 day, 7 days and 28 days after TBI using a TH-specific monoclonal antibody. Specifically, the TH signal was seen as a single band of approximately 59 kDa (Fig. 3A and 4A) and is consistent with previous studies (Soriano et al., 1997; Yan et al., 2001). Consistent with the immunohistochemistry findings using this TH antibody (Fig. 1), Figure 3B shows quantitative evaluation of TH Western blot data from ipsilateral and contralateral striatum homogenates at 1 day, 7 days, and 28 days after TBI or sham operation (N = 6) and representative gel bands of TH at 28 days after TBI or sham operation (N = 3, Fig. 3A). TH is increased bilaterally (compared to control samples) in the striatum 28 days after injury, and this increase is even more pronounced in the contralateral than the ipsilateral side (Fig. 3B, 119.3 ± 4.7 % for the ipsilateral and 132 ± 4.4 % for the contralateral striatum, P < 0.05, respectively) compared with the sham controls at 28 days after TBI. Figure 4B shows a similar analysis of TH at the level of substantia nigra and representative gel bands of TH at 28 days after TBI or sham operation (N = 3, Fig. 4A). TBI causes a delayed increase in TH protein levels in the substantia nigra at 28 days after severe TBI (N = 6). TH is increased bilaterally compared to control samples in the substantia nigra (Fig. 4B, 118.7 ± 5.1 % for the ipsilateral and 111.5 ± 11.0 % for the contralateral substantia nigra). However, in contrast to our observations in the striatum, a statistically significant difference in levels of TH protein between injured animals and sham control at 28 days after TBI could only be measured in the ipsilateral substantia nigra (P < 0.05). No statistically significant changes of TH in the striatum and substantia nigra were observed in the injured animals compared with the sham controls at earlier time points (1 day, or 7 days). Also, no statistically significant differences were found by normalizing TH optical densities to β-actin or not.
Fig. 3.
Summarizes the semiquantitative measurements of tyrosine hydroxylase (TH) protein in rat striatum after traumatic brain injury (TBI) or sham operation. The lower Panel (B) is the histograms of Western blot data from the ipsilateral (Ipsi) and the contralateral (Contra) striatum homogenates at 1 day, 7 days, and 28 days after TBI or sham operation and demonstrates that TH protein is increased bilaterally but more evident in the contralateral side at 28 days after TBI (N = 6, * = p < 0.05). The upper panel (A) is the representative gel bands (N = 3) at 28 days after TBI or sham operation. There is no significant statistical difference in TH optical densities with or without normalizing to β-actin. The number on left of immunoblot indicates the position of the molecular weight marker (A).
Fig. 4.
Summarizes the semiquantitative measurements of tyrosine hydroxylase (TH) protein in rat substantia nigra after traumatic brain injury (TBI) or sham operation. The lower Panel (B) is the histograms of Western blot data from the ipsilateral (Ipsi) and the contralateral (Contra) substantia nigra homogenates at 1 day, 7 days, and 28 days after TBI or sham operation and demonstrates that TH protein is increased bilaterally at 28 days after TBI (N = 6). However, only the ipsilateral substantia nigra showed the statistical difference (* = p < 0.05). The upper panel (A) is the representative gel bands (N = 3) at 28 days after TBI or sham operation. There is no significant statistical difference in TH optical densities with or without normalizing to β-actin. The number on left of immunoblot indicates the position of the molecular weight marker (A).
3. Discussion
The present study demonstrates that experimental TBI in rat produces a delayed increase in TH protein expression in the nigrostriatal system that is detectable at 28 days post-injury using both immunohistochemistry and Western blot techniques. To our knowledge, this is the first time that the expression of the TH protein levels has been quantified in the nigrostriatal system after TBI. The present finding is further evidence that the dopamine system is chronically altered after TBI.
TBI can result in the disturbance of cognitive, behavior, emotional, and physical functioning. Brain-injured patients often display cognitive deficits included socially inappropriate behaviors, impairments in judgment, reduced attention, and working memory dysfunction (Levin, 1998). Evidence that dopamine systems are altered in humans following TBI is based on imaging studies and reports that neurostimulants, including dopamine agonists, can be beneficial in attenuating cognitive deficits (Dunn-Meynell et al., 1994; Katzman et al., 1971; McIntosh et al., 1994). Our previous report confirms the clinical observation that delayed and chronic treatment with the D2 receptor agonist bromocriptine attenuates both working memory and spatial learning acquisition deficits following TBI insult in rats (Kline et al., 2002). Recently, Kobori and Dash (2006) demonstrate that experimental TBI produces a long-lasting working memory impairment that is associated with increased levels of the GABA-synthesizing enzyme glutamic acid decarboxylase 67 (GAD67) in the medial prefrontal cortex for up to 1 month after injury. A single administration of dopamine D1 antagonists at 14 d after injury is sufficient to decrease GAD67 levels and restore working memory (Kobori and Dash, 2006). The present data demonstrates an associative, but not causal relationship between TH up-regulation and behavioral dysfunction after TBI.
The striatum is functionally important for a variety of cognitive and motor behavior. It is known that the striatum is vulnerable to damage from TBI, but few experimental TBI studies have focused on this region. The known effects of TBI on the striatum include in axonal degeneration (Ding et al., 2001), neuronal cell loss (Dunn-Meynell and Levin, 1997), and ischemia (Dietrich et al., 1998). The striatum receives dopaminergic input from the substantia nigra pars compacta, a pathway that is disrupted in Parkinson's disease (PD). Long known to be crucial for motor function, striatal circuitry can regulate cognitive function as well. The possibility that head injury may lead to neurodegenerative movement disorders such as PD has long been the topic of investigation (Doder et al., 1999; Friedman, 1989; Guterman and Smith, 1987). While few epidemiological studies have investigated the frequency of posttraumatic movement disorders, one study reported that 50 out of 221 patients (22.6%) with severe head injury developed movement disorders (Friedman, 1989).
Aspects of TBI-induced alterations in TH expression appear to be similar to that seen in patients with PD. For example, despite a reduction in TH mRNA-positive neurons, higher levels of TH messenger RNA per neuron were observed in PD patients compared to aged controls (Joyce et al., 1997). Thus, surviving neurons in PD exhibit higher levels of TH mRNA than control neurons. The present immunohistochemical studies suggest that TBI did not decrease in the number of TH-positive neurons in the substantia nigra area, a hallmark of PD models. However, the increased appearance of TH in the substantia nigra after TBI is consistent with the clinical observation in PD patients (Joyce et al., 1997) and may reflect increased TH synthesis.
The delayed increase of nigrostriatal TH after TBI in the nigrostriatal system we reported here is consistent with data from other studies addressing lesions of dopaminergic systems. Evidence is accumulating which suggests that grafting also stimulates the activity of residual host dopaminergic neurons (Bankiewicz et al., 1990; Fiandaca et al., 1988; Hirsch et al., 1990; Howells et al., 1993; Jaeger et al., 1983; Plunkett et al., 1990; Przedborski et al., 1991). Animal models of Parkinsonism with surgically lesioned striata, but no grafts, have shown clinical improvement (Fiandaca et al., 1988; Plunkett et al., 1990; Przedborski et al., 1991); however detailed neurochemical correlates are still lacking. Reports have shown increases in striatal presynaptic dopamine uptake site density in rodents (Howells et al., 1993; Przedborski et al., 1991), and increased host TH-IR after grafting (Bankiewicz et al., 1990) or striatal damage in primates (Fiandaca et al., 1988). Increases in TH expression have also been observed following partial lesions of the dopaminergic nigrostriatal system in rats (Blanchard et al., 1995).
Trauma-induced elevations in TH protein may occur through several mechanisms. One possible mechanism is that there may be delayed transcriptional changes that occur after TBI leading to altered protein expression. Tyrosine hydroxylase protein upregulation may reflect a compensatory response of dopaminergic neurons to upregulate their synthesizing capacity and increase the efficiency of DA neurotransmission after TBI. Symptoms in PD do not emerge until the loss of nigrostriatal dopaminergic neurons has led to an 80% decrease in striatal DA (Bernheimer et al., 1973). This suggests the existence of compensatory mechanisms in the remaining dopaminergic neurons (Hornykiewicz, 1993).
It is not known whether morphological reorganization of the dopaminergic nigrostriatal pathway contributes to the compensatory mechanisms. In that respect, regrowth or collateral spouting of catecholaminergic axons have already been demonstrated in experimentally induced lesions of adult central neurons (Fritschy and Grzanna, 1992; Gilad and Reis, 1979; Hirsch et al., 1990; Katzman et al, 1971). Moreover, both in PD patients and in animals with experimental Parkinsonism, neural transplants or the supply of neurotrophic factors may promote regrowth of dopaminergic fibers in the striatum and reverse lesion-induced behavioral deficits (Kopin et al., 1993; Kordower et al., 1991; Tomac et al., 1995). These results indicate a capacity of the dopaminergic neurons in adult brain to exhibit trophic support-induced plastic changes. Trophic factors that promote regeneration of nigrostriatal dopaminergic fibers, such as basic fibroblast growth factor or glial cell line-derived neurotrophic factor, may have been involved in this phenomenon (Otto and Unsicker, 1990; Tomac et al., 1995). The occurrence of spontaneous regrowth of dopaminergic fibers after partial nigrostriatal denervation has already been suggested (Onn et al., 1986). A long-term increase in the amount of striatal TH has been observed after 6-OHDA injection in the SN pars compacta (Blanchard et al., 1995; Dravid et al., 1984; Pasinetti et al., 1992) and might be associated with the sprouting of dopaminergic fibers (Blanchard et al., 1996). It is thus possible that the TBI-induced expression increase of TH in the nigrostriatal system might share the similar mechanisms. However, further studies such as DA turnover, TH activity, DOPAC/DA and DA/TH in the nigrostriatal system (Pifl and Hornykiewicz, 2006) after TBI are needed to confirm whether there is such compensation mechanisms after TBI insult and to answer the question why the increase in TH levels is higher in the contralateral striatum as compared to the ipsilateral striatum at 28 days after TBI.
The other mechanism is that the capacity of surviving dopaminergic neurons to modify their rate of DA metabolism may be attributable, in part, to long-term changes in protein level by induction of gene transcription factors. Alternatively, changes in protein degradation may occur after trauma. The increase in TH protein may indicate increased stability of the protein at later time points after trauma. While the molecular and cellular mechanisms involved in the degradation of TH are not yet known, the possibility that the ubiquitin-proteasome pathway could play a role in its degradation has been suggested (Sasajima et al., 2002). While experimental TBI can alter elements of the ubiquitin-proteasome pathway (Lotocki et al., 2003; Williams et al., 2006; Yao et al., 2006), it is unknown if this is sufficient to prevent TH degradation. Another possibility is that a post-translational modification may render TH less sensitive to degradation or alter its activity via phosphorylation (Kobori and Dash, 2006). Lastly, while it is possible that tissue shrinkage may affect TH protein, this appeared unlikely in the present study given that a greater increase in TH expression was detected in the contralateral striatum, which had no tissue loss relative to the ipsilateral striatum.
The present study found that TH is increased at 28 days after experimental TBI in rat nigrostriatal system and offers additional evidence that the dopaminergic system is disturbed following TBI. Although overt recovery from brain injury has been extensively documented, the mechanisms underlying this process remain to be elucidated. The present study is the first to characterize a delayed, progressive TH change in the nigrostriatal system dopaminergic protein after TBI. Reorganization to enhance neurotransmission probably also plays a role in outcome, and the data reported here point to new targets for therapeutic intervention. Further studies, such as investigating the dynamic changes in the phosphorylation of TH and other biochemical changes occurring after TBI, will help elucidate the systems and mechanisms involved in neuronal survival and behavioral recovery.
In summary, TBI produced an increase in TH protein in rat nigrostriatal system that was detectable by both immunohistochemistry and Western blot at 28 days post-injury. The increase in TH expression may indicate, at least partially, nigrostriatal system reorganization taking place over time following injury as a means of enhancing DA neurotransmission.
4. Experimental Procedure
4.1. Animals
Seventy-two male Sprague-Dawley rats weighing 250-275 grams on the day of surgery were used. Rats were group-housed (2 per cage) in standard steel/wire mesh cages at room temperature (22 ± 2°C) under standard 12-h light/dark cycles (light on at 07:00 o'clock AM) with free access to food and water. After injury, rats were housed in separate cages from uninjured animals. All studies carefully conformed to the guidelines outlined in the Guide for the Care and Use of Laboratory Animals from the U.S. Department of Health and Human Services and were approved by the University of Pittsburgh Medical Center Institutional Animal Care and Use Committee.
4.2. Injury Device
The controlled cortical impact injury (CCI) device (Dixon et al., 1991) consisted of a small (1.975 cm) bore, double-acting, stroke-constrained, pneumatic cylinder with a 5.0 cm stroke. The cylinder was rigidly mounted in a vertical position on a crossbar, which could be precisely adjusted in the vertical axis. The lower rod end had an impactor tip (6 mm diameter) attached (i.e., the part of the shaft that comes into contact with the exposed dura mater). The upper rod end was attached to the transducer core of a linear velocity displacement transducer (LVDT). The velocity of the impactor shaft was controlled by gas pressure. Impact velocity was measured directly by the LVDT (Shaevitz Model 500 HR; Macro Sensors, Pennsauken, NJ), which produced an analog signal that was recorded by a PC-based data acquisition system (Axoscope; Axon Instruments, Inc., Foster City, CA) for analysis of time/displacement parameters of the impactor. The morphopathological features of cortical impact injury have been extensively characterized (Kampfl et al., 1996; Newcomb et al., 1997; Posmantur et al., 1994, 1996).
4.3. Surgical Procedures for Injury
All rats were anesthetized initially with 4% isoflurane with a 2:1 N2O/O2 mixture in a vented anesthesia chamber. Following endotracheal intubation, rats were ventilated mechanically with a 1-1.5% isoflurane mixture. Animals were mounted in a stereotaxic frame on the injury device in a supine position secured by ear and incisor bars. The head was held in a horizontal plane with respect to the interaural line. A midline incision was made, the soft tissues reflected, and a 7 mm craniotomy was made between lambda and bregma and centered 5 mm lateral of the central suture. The animals received a cortical impact through the right craniotomy at a velocity of 4 meters/sec. The injury device was set to produce a tissue deformation of 3.2 mm. Sham rats underwent identical surgical procedures but did not receive a TBI. The sham rats were used to control for nonspecific methodological effects (such as those due to handling, anesthesia and surgery). Core body temperature was monitored continuously by a rectal thermistor probe and maintained at 37 ± 0.5° C with a heating pad (Yan et al., 2000).
4.4. Immunohistochemistry
Thirty-six deeply anesthetized rats were sacrificed for TH immunohistochemistry at 1 day, 7 days, and 28 days after CCI injury and sham surgery (n = 6 in each group for each time point). These time points were selected on basis of previous studies of DA systems after TBI (Yan et al., 2001, 2002). Tissue preparation and immunohistochemistry procedures were as previously reported (Yan et al., 2001). Briefly, animals were deeply anesthetized with pentobarbital (Nembutal, 80-100 mg/kg; Abbott Laboratories, North Chicago, IL) and were transcardially perfused with 100 ml 0.1 M phosphate buffered saline (PBS) with 50 U/ml heparin pH 7.4, followed by 500 ml 4% paraformaldehyde with 15% saturated picric acid in 0.1 M phosphate buffer pH 7.4. After perfusion, the brain was removed and placed into the same fixative for 30 minutes, then immersed in 4% paraformaldehyde in 0.1 M phosphate buffer pH 7.4 at 4°C overnight. The brain was transferred to 15% sucrose in 0.1 M phosphate buffer pH 7.4 at 4°C for 24 hours then to 30% sucrose in 0.1 M phosphate buffer pH 7.4 at 4°C until the brain was submerged. The cryoprotected rat brain was frozen and used for cryostat sectioning. Coronal sections were cut in 35 μm thickness in a cryostat (Jung CM 1800; Brodersen Instrument, Valencia, PA) and collected in 24-well culture plates contained with 0.1 M PBS.
Immunohistochemistry for TH were conducted in 24-well culture plates using the free-floating technique. Sections were pre-blocked with 10% normal rabbit serum (NRS) and 0.1% Triton X-100 in 0.1 M PBS. Sections were incubated with primary antibody, mouse anti-tyrosine hydroxylase monoclonal antibody (1:3,000; Chemicon International, Temecula, CA), with 5% NRS and 0.1% Triton X-100 in 0.1 M PBS at 4°C for 16-24 hours. Affinity-purified rabbit anti-mouse IgG (1:50; Jackson ImmunoResearch Laboratories, West Grove, PA) was incubated as secondary antibody with 5% NRS and 0.1% Triton X-100 in 0.1 M PBS at 4°C for 2 hours on the shaker. Mouse peroxidase-anti-peroxidase (PAP) soluble immune complexes (50 μg/ml; Jackson ImmunoResearch Laboratories, West Grove, PA) was used to visualize immunoreactivity with 2% NRS and 0.1% Triton X-100 in 0.1 M PBS at 4° C for 3 hours on the shaker. Tissue was rinsed between all steps with 0.1% Triton X-100 in 0.1 M PBS three times for at least 10 min each time. The peroxidase reaction was developed with DAB Substrate Kit (Vector, Burlingame, CA) until a dark brown reaction product was evident. Sections were rinsed several times in 0.1 M Tris-buffered saline (TBS), mounted on subbed slides, dehydrated in alcohols, defatted in xylenes, and coverslipped for light microscopic analysis. A subset of sections were counterstained with hematoxylin (Vector, Burlingame, CA). At least five sections of brain tissue through the striatum and the substantia nigra for each animal were processed for TH immunohistochemistry.
Control experiments were run in parallel to confirm specificity. Primary antibody was either omitted or replaced with normal mouse serum (Sigma, St. Louis, MO) at 4°C for 16 hours. Purified TH protein (Cell 2 Cell, San Clemente, CA) was used for adsorption of the TH antibody as the antibody specific control.
4.5. Western blot
Thirty-six deeply anesthetized animals were sacrificed for Western blot at 1 day, 7 days, and 28 days after CCI injury and sham surgery (n = 6 in each group for each time point). Briefly, after deeply anesthetized with pentobarbital (Nembutal, 80-100 mg/kg; Abbott Laboratories, North Chicago, IL), animals were decapitated, and the brains were quickly removed and chilled on ice. Tissue from the striatum and substantia nigra were dissected out. Tissues were washed with ice cold 0.1 M PBS, then homogenized and sonicated in the lysis buffer (suspension buffer) which contains 0.1M NaCl, 0.01M Tris-Cl (pH 7.6), 0.001 M EDTA (pH 8.0), 1 μg/ml aprotinin, 100 μg/ml phenylmethylsulfonyl fluoride (PMSF), and centrifuged at 15,000 g for 30 minutes. Only the supernatant (soluble protein) was used for the Western blot study. The protein concentrations were determined by a 96-well micro-plate reader (Spectra Max 340; Molecular Devices, Sunnyvale, CA) using a BCA protein Assay Kit (PIERCE, Rockford, IL). Samples containing 20-100 μg of protein were electrophoresed on the 8% SDS-polyacrylamide gel and transferred to nitrocellulose membranes. The membrane was blocked in 5% non-fat dry milk in 0.1 M PB S with 0.05% Tween-20 (PBST) at room temperature for one hour, and immunolabeled with mouse anti-tyrosine hydroxylase monoclonal antiserum (1:20,000, Chemicon International, Inc., Temecula, CA) at room temperature for one hour followed by goat anti-mouse immunoglobulin G conjugated to peroxidase (1:20,000; PIERCE, Rockford, IL) at room temperature for one hour. Proteins were visualized with a chemiluminescence detection system (SuperSignal, PIERCE, Rockford, IL). To assure equal loading and/or normalize to protein content, the membrane blots were co-incubated with mouse anti-β-actin monoclonal antibody (1:10,000, Sigma, St. Louis, MO) or re-striped and re-blotted with rabbit anti-actin antibody (1:15,000, Sigma, St. Louis, MO). The TH optical densities were normalized to β-actin. There is no statistical difference of TH optical densities with and without normalizing to β-actin. The injured tissue and the sham control were loaded together in the same gel for comparison. Purified TH protein (Cell 2 Cell, San Clemente, CA) was used as standard and pre-adsorbed control of the TH antibody specificity. Blots were exposed to autoradiographic X-ray film for 10 sec to 1 min and bands were semi-quantitated using SCION ImagePC (Frederick, MD) software. Values are given as a ratio (percentage change) of optical density of injured samples versus sham control within individual blots for each hemisphere.
Data are expressed as the group means ± standard error (SE) of the mean. Statistical evaluations were performed according to one-way analysis of variance (ANOVA) followed by Scheffé test as a post hoc comparison test. When appropriate, Pearson correlation test, followed by Bonferroni correction to control Type 1 error rate, was applied. A significance level of P < 0.05 was used for all tests.
Acknowledgments
This work was supported by grants NIH-NS 33150, NIH-NS 40125 and NIH-NS 30318. We thank Dr. Elizabeth L. Hooghe-Peters for critically reviewing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anden NE, Carlsson A, Dahlstroem A, Fuxe K, Hillarp NA, Larsson K. Demonstration and mapping out of nigro-neostriatal dopamine neurons. Life Sci. 1964;3:523–530. doi: 10.1016/0024-3205(64)90161-4. [DOI] [PubMed] [Google Scholar]
- Backman L, Robins-Wahlin TB, Lundin A, Ginovart N, Farde L. Cognitive deficits in Huntington's disease are predicted by dopaminergic PET markers and brain volumes. Brain. 1997;120:2207–2217. doi: 10.1093/brain/120.12.2207. [DOI] [PubMed] [Google Scholar]
- Bankiewicz KS, Plunkett RJ, Jacobowitz DM, Porrino L, di Porzio U, London WT, Kopin IJ, Oldfield EH. The effect of fetal mesencephalon implants on primate MPTP-induced parkinsonism. Histochemical and behavioral studies. J Neurosurg. 1990;72:231–244. doi: 10.3171/jns.1990.72.2.0231. [DOI] [PubMed] [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Bertler A, Rosengren E. Occurrence and distribution of catecholamines in brain. Acta Physiol Scand. 1959;47:350–361. [PubMed] [Google Scholar]
- Blanchard V, Chritin M, Vyas S, Savasta M, Feuerstein C, Agid Y, Javoy-Agid F, Raisman-Vozari R. Long-term induction of tyrosine hydroxylase expression: compensatory response to partial degeneration of the dopaminergic nigrostriatal system in the rat brain. J Neurochem. 1995;64:1669–1679. doi: 10.1046/j.1471-4159.1995.64041669.x. [DOI] [PubMed] [Google Scholar]
- Blanchard V, Anglade P, Dziewczapolski G, Savasta M, Agid Y, Raisman-Vozari R. Dopaminergic sprouting in the rat striatum after partial lesion of the substantia nigra. Brain Res. 1996;709:319–325. doi: 10.1016/0006-8993(95)01391-1. [DOI] [PubMed] [Google Scholar]
- Carlsson A. The occurrence, distribution and physiological role of catecholamines in the nervous system. Pharmacol Rev. 1959;11:490–493. [PubMed] [Google Scholar]
- Dietrich WD, Alonso O, Busto R, Prado R, Zhao W, Dewanjee MK, Ginsberg MD. Posttraumatic cerebral ischemia after fluid percussion brain injury: an autoradiographic and histopathological study in rats. Neurosurgery. 1998;43:585–593. doi: 10.1097/00006123-199809000-00105. [DOI] [PubMed] [Google Scholar]
- Ding Y, Yao B, Lai Q, McAllister JP. Impaired motor learning and diffuse axonal damage in motor and visual systems of the rat following traumatic brain injury. Neurol Res. 2001;23:193–202. doi: 10.1179/016164101101198334. [DOI] [PubMed] [Google Scholar]
- Divac I, Rosvold HE, Szwarcbart MK. Behavioral effects of selective ablation of the caudate nucleus. J Comp Physiol Psychol. 1967;63:184–190. doi: 10.1037/h0024348. [DOI] [PubMed] [Google Scholar]
- Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Doder M, Jahanshahi M, Turjanski N, Moseley IF, Lees AJ. Parkinson's syndrome after closed head injury: a single case report. J Neurol Neurosurg Psychiatry. 1999;66:380–385. doi: 10.1136/jnnp.66.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnemiller E, Brenneis C, Wissel J, Scherfler C, Poewe W, Riccabona G, Wenning GK. Impaired dopaminergic neurotransmission in patients with traumatic brain injury: a SPECT study using 123I-beta-CIT and 123I-IBZM. Eur J Nucl Med. 2000;27:1410–1414. doi: 10.1007/s002590000308. [DOI] [PubMed] [Google Scholar]
- Dravid A, Jaton AL, Enz A, Frei P. Spontaneous recovery from motor asymmetry in adult rats with 6-hydroxydopamine-induced partial lesions of the substantia nigra. Brain Res. 1984;311:361–365. doi: 10.1016/0006-8993(84)90101-x. [DOI] [PubMed] [Google Scholar]
- Dunn-Meynell B, Pan S, Levin BE. Focal traumatic brain injury causes widespread reductions in rat brain norepinephrine turnover from 6 to 24 h. Brain Res. 1994;660:88–95. doi: 10.1016/0006-8993(94)90842-7. [DOI] [PubMed] [Google Scholar]
- Dunn-Meynell AA, Levin BE. Histological markers of neuronal, axonal and astrocytic changes after lateral rigid impact traumatic brain injury. Brain Res. 1997;761:25–41. doi: 10.1016/s0006-8993(97)00210-2. [DOI] [PubMed] [Google Scholar]
- Fiandaca MS, Kordower JH, Hansen JT, Jiao SS, Gash DM. Adrenal medullary autografts into the basal ganglia of Cebus monkeys: injury-induced regeneration. Exp Neurol. 1988;102:76–91. doi: 10.1016/0014-4886(88)90080-5. [DOI] [PubMed] [Google Scholar]
- Friedman JH. Progressive parkinsonism in boxers. South Med J. 1989;82:543–546. doi: 10.1097/00007611-198905000-00002. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Grzanna R. Restoration of ascending noradrenergic projections by residual locus coeruleus neurons: compensatory response to neurotoxin-induced cell death in the adult rat brain. J Comp Neurol. 1992;321:421–441. doi: 10.1002/cne.903210309. [DOI] [PubMed] [Google Scholar]
- Gilad GM, Reis DJ. Collateral sprouting in central mesolimbic dopamine neurons: biochemical and immunocytochemical evidence of changes in the activity and distribution of tyrosine hydroxylase in terminal fields and in cell bodies of A10 neurons. Brain Res. 1979;160:17–26. doi: 10.1016/0006-8993(79)90597-3. [DOI] [PubMed] [Google Scholar]
- Goldstein LB. Neuropharmacology of TBI-induced plasticity. Brain Inj. 2003;17:685–694. doi: 10.1080/0269905031000107179. [DOI] [PubMed] [Google Scholar]
- Guterman A, Smith RW. Neurological sequelae of boxing. Sports Med. 1987;4:194–210. doi: 10.2165/00007256-198704030-00004. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Duyckaerts C, Javoy-Agid F, Hauw JJ, Agid Y. Does adrenal graft enhance recovery of dopaminergic neurons in Parkinson's disease? Ann Neurol. 1990;27:676–682. doi: 10.1002/ana.410270615. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Ungerstedt U. Electron and fluorescence microscopical studies on the nucleus caudatus putamen of the rat after unilateral lesions of ascending nigro-neostriatal dopamine neurons. Acta Physiol Scand. 1969;76:415–426. doi: 10.1111/j.1748-1716.1969.tb04489.x. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Johansson O, Fuxe K, Goldstein M, Park D. Immunohistochemical studies on the localization and distribution of monoamine neuron systems in the rat brain. I Tyrosine hydroxylase in the mes- and diencephalon. Med Biol. 1976;54:427–453. [PubMed] [Google Scholar]
- Hokfelt T, Johansson O, Fuxe K, Goldstein M, Park D. Immunohistochemical studies on the localization and distribution of monoamine neuron systems in the rat brain II. Tyrosine hydroxylase in the telencephalon. Med Biol. 1977;55:21–40. [PubMed] [Google Scholar]
- Hornykiewicz O. Parkinson's disease and the adaptive capacity of the nigrostriatal dopamine system: possible neurochemical mechanisms. Adv Neurol. 1993;60:140–147. [PubMed] [Google Scholar]
- Howells DW, Donnan GA, Wong JY, Kaczmarczyk SJ, Chilcho PJ, Fabinyi GC, Mendelsohn FA. Surgical damage stimulates proliferation of dopamine uptake sites in normal mouse brain. Brain Res. 1993;622:285–288. doi: 10.1016/0006-8993(93)90830-g. [DOI] [PubMed] [Google Scholar]
- Jaeger CB, Joh TH, Reis DJ. The effect of forebrain lesions in the neonatal rat: survival of midbrain dopaminergic neurons and the crossed nigrostriatal projection. J Comp Neurol. 1983;218:74–90. doi: 10.1002/cne.902180105. [DOI] [PubMed] [Google Scholar]
- Joh TH, Reis DJ. Different forms of tyrosine hydroxylase in central dopaminergic and noradrenergic neurons and sympathetic ganglia. Brain Res. 1975;85:146–151. doi: 10.1016/0006-8993(75)91021-5. [DOI] [PubMed] [Google Scholar]
- Joyce JN, Smutzer G, Whitty CJ, Myers A, Bannon MJ. Differential modification of dopamine transporter and tyrosine hydroxylase mRNAs in midbrain of subjects with Parkinson's, Alzheimer's with parkinsonism, and Alzheimer's disease. Mov Disord. 1997;12:885–897. doi: 10.1002/mds.870120609. [DOI] [PubMed] [Google Scholar]
- Kampfl A, Zhao X, Whitson JS, Posmantur R, Dixon CE, Yang K, Clifton GL, Hayes RL. Calpain inhibitors protect against depolarization-induced neurofilament protein loss of septo-hippocampal neurons in culture. Eur J Neurosci. 1996;8:344–352. doi: 10.1111/j.1460-9568.1996.tb01218.x. [DOI] [PubMed] [Google Scholar]
- Katzman R, Bjorklund A, Owman C, Stenevi U, West KA. Evidence for regenerative axon sprouting of central catecholamine neurons in the rat mesencephalon following electrolytic lesions. Brain Res. 1971;25:579–596. doi: 10.1016/0006-8993(71)90462-8. [DOI] [PubMed] [Google Scholar]
- Kline AE, Massucci JL, Marion DW, Dixon CE. Attenuation of working memory and spatial acquisition deficits after a delayed and chronic bromocriptine treatment regimen in rats subjected to traumatic brain injury by controlled cortical impact. J Neurotrauma. 2002;19:415–425. doi: 10.1089/08977150252932370. [DOI] [PubMed] [Google Scholar]
- Kobori N, Dash PK. Reversal of brain injury-induced prefrontal glutamic acid decarboxylase expression and working memory deficits by D1 receptor antagonism. J Neurosci. 2006;26:4236–4246. doi: 10.1523/JNEUROSCI.4687-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopin IJ, Bankiewicz KS, Plunkett RJ, Jacobowitz DM, Oldfield EH. Tissue implants in treatment of parkinsonian syndromes in animals and implications for use of tissue implants in humans. Adv Neurol. 1993;60:707–714. [PubMed] [Google Scholar]
- Kordower JH, Cochran E, Penn RD, Goetz CG. Putative chromaffin cell survival and enhanced host-derived TH-fiber innervation following a functional adrenal medulla autograft for Parkinson's disease. Ann Neurol. 1991;29:405–412. doi: 10.1002/ana.410290411. [DOI] [PubMed] [Google Scholar]
- Levin HS. Cognitive function outcomes after traumatic brain injury. Curr Opin Neurol. 1998;11:643–646. doi: 10.1097/00019052-199812000-00006. [DOI] [PubMed] [Google Scholar]
- Lotocki G, Alonso OF, Frydel B, Dietrich WD, Keane RW. Monoubiquitination and cellular distribution of XIAP in neurons after traumatic brain injury. J Cereb Blood Flow Metab. 2003;23:1129–1136. doi: 10.1097/01.WCB.0000086938.68719.E0. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Flashman LA, Sparling MB, Saykin AJ. Working memory deficits after traumatic brain injury: catecholaminergic mechanisms and prospects for treatment -- a review. Brain Inj. 2004;18:331–350. doi: 10.1080/02699050310001617370. [DOI] [PubMed] [Google Scholar]
- McIntosh TK, Yu T, Gennarelli TA. Alterations in regional brain catecholamine concentrations after experimental brain injury in the rat. J Neurochem. 1994;63:1426–1433. doi: 10.1046/j.1471-4159.1994.63041426.x. [DOI] [PubMed] [Google Scholar]
- Miyoshi E, Wietzikoski S, Camplessei M, Silveira R, Takahashi RN, Da Cunha C. Impaired learning in a spatial working memory version and in a cued version of the water maze in rats with MPTP-induced mesencephalic dopaminergic lesions. Brain Res Bull. 2002;58:41–47. doi: 10.1016/s0361-9230(02)00754-2. [DOI] [PubMed] [Google Scholar]
- Muller U, Wachter T, Barthel H, Reuter M, von Cramon DY. Striatal [123I]beta-CIT SPECT and prefrontal cognitive functions in Parkinson's disease. J Neural Transm. 2000;107:303–319. doi: 10.1007/s007020050025. [DOI] [PubMed] [Google Scholar]
- National Institute of Health. Rehabilitation of persons with traumatic brain injury. NIH Consensus Statement. 1998;16:1–41. [PubMed] [Google Scholar]
- Newcomb JK, Kampfl A, Posmantur RM, Zhao X, Pike BR, Liu SJ, Clifton GL, Hayes RL. Immunohistochemical study of calpain-mediated breakdown products to alpha-spectrin following controlled cortical impact injury in the rat. J Neurotrauma. 1997;14:369–383. doi: 10.1089/neu.1997.14.369. [DOI] [PubMed] [Google Scholar]
- Onn SP, Berger TW, Stricker EM, Zigmond MJ. Effects of intraventricular 6-hydroxydopamine on the dopaminergic innervation of striatum: histochemical and neurochemical analysis. Brain Res. 1986;376:8–19. doi: 10.1016/0006-8993(86)90894-2. [DOI] [PubMed] [Google Scholar]
- Otto D, Unsicker K. Basic FGF reverses chemical and morphological deficits in the nigrostriatal system of MPTP-treated mice. J Neurosci. 1990;10:1912–1921. doi: 10.1523/JNEUROSCI.10-06-01912.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasinetti GM, Osterburg HH, Kelly AB, Kohama S, Morgan DG, Reinhard JF, Jr, Stellwagen RH, Finch CE. Slow changes of tyrosine hydroxylase gene expression in dopaminergic brain neurons after neurotoxin lesioning: a model for neuron aging. Brain Res Mol Brain Res. 1992;13:63–73. doi: 10.1016/0169-328x(92)90045-d. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Joh TH, Field PM, Becker CG, Reis DJ. Cellular localization of tyrosine hydroxylase by immunohistochemistry. J Histochem Cytochem. 1975;23:1–12. doi: 10.1177/23.1.234988. [DOI] [PubMed] [Google Scholar]
- Pifl C, Hornykiewicz O. Dopamine turnover is upregulated in the caudate/putamen of asymptomatic MPTP-treated rhesus monkeys. Neurochem Int. 2006;49:519–524. doi: 10.1016/j.neuint.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Plunkett RJ, Bankiewicz KS, Cummins AC, Miletich RS, Schwartz JP, Oldfield EH. Long-term evaluation of hemiparkinsonian monkeys after adrenal autografting or cavitation alone. J Neurosurg. 1990;73:918–926. doi: 10.3171/jns.1990.73.6.0918. [DOI] [PubMed] [Google Scholar]
- Posmantur R, Hayes RL, Dixon CE, Taft WC. Neurofilament 68 and neurofilament 200 protein levels decrease after traumatic brain injury. J Neurotrauma. 1994;11:533–545. doi: 10.1089/neu.1994.11.533. [DOI] [PubMed] [Google Scholar]
- Posmantur RM, Kampfl A, Liu SJ, Heck K, Taft WC, Clifton GL, Hayes RL. Cytoskeletal derangements of cortical neuronal processes three hours after traumatic brain injury in rats: an immunofluorescence study. J Neuropathol Exp Neurol. 1996;55:68–80. doi: 10.1097/00005072-199601000-00007. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Levivier M, Kostic V, Jackson-Lewis V, Dollison A, Gash DM, Fahn S, Cadet JL. Sham transplantation protects against 6-hydroxydopamine-induced dopaminergic toxicity in rats: behavioral and morphological evidence. Brain Res. 1991;550:231–238. doi: 10.1016/0006-8993(91)91323-s. [DOI] [PubMed] [Google Scholar]
- Ragnarsson KT. Results of the NIH consensus conference on “rehabilitation of persons with traumatic brain injury. Restor Neurol Neurosci. 2002;20:103–8. [PubMed] [Google Scholar]
- Rinne JO, Portin R, Ruottinen H, Nurmi E, Bergman J, Haaparanta M, Solin O. Cognitive impairment and the brain dopaminergic system in Parkinson's disease: [18F]fluorodopa positron emission tomographic study. Arch Neurol. 2000;57:470–475. doi: 10.1001/archneur.57.4.470. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Clark WA, Goldstein M, Roth RH, Deutch AY. Effects of 6-hydroxydopamine lesions of the prefrontal cortex on tyrosine hydroxylase activity in mesolimbic and nigrostriatal dopamine systems. Neuroscience. 1992;48:831–839. doi: 10.1016/0306-4522(92)90271-3. [DOI] [PubMed] [Google Scholar]
- Sasajima H, Nakagawa K, Yokosawa H. Antiproliferative proteins of the BTG/Tob family are degraded by the ubiquitin-proteasome system. Eur J Biochem. 2002;269:3596–3604. doi: 10.1046/j.1432-1033.2002.03052.x. [DOI] [PubMed] [Google Scholar]
- Soriano MA, Justicia C, Ferrer I, Rodriguez-Farre E, Planas AM. Striatal infarction in the rat causes a transient reduction of tyrosine hydroxylase immunoreactivity in the ipsilateral substantia nigra. Neurobiol Dis. 1997;4:376–385. doi: 10.1006/nbdi.1997.0166. [DOI] [PubMed] [Google Scholar]
- Tomac A, Lindqvist E, Lin LF, Ogren SO, Young D, Hoffer BJ, Olson L. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995;373:335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- Von Voigtlander PF, Moore KE. In vivo electrically evoked release of [3H]noradrenaline from cat brain. J Pharm Pharmacol. 1971;23:381–382. doi: 10.1111/j.2042-7158.1971.tb09933.x. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Sokoloski JE, Ren D, Chen X, Khan AS, Zafonte RD, Michael AC, Dixon CE. Controlled cortical impact injury affects dopaminergic transmission in the rat striatum. J Neurochem. 2005;95:457–465. doi: 10.1111/j.1471-4159.2005.03382.x. [DOI] [PubMed] [Google Scholar]
- White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem. 2002;77:125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- Williams AJ, Dave JR, Tortella FC. Neuroprotection with the proteasome inhibitor MLN519 in focal ischemic brain injury: relation to nuclear factor kappaB (NF-kappaB), inflammatory gene expression, and leukocyte infiltration. Neurochem Int. 2006;49:106–112. doi: 10.1016/j.neuint.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Wilson MS, Chen X, Ma X, Ren D, Wagner AK, Reynolds IJ, Dixon CE. Synaptosomal dopamine uptake in rat striatum following controlled cortical impact. J Neurosci Res. 2005;80:85–91. doi: 10.1002/jnr.20419. [DOI] [PubMed] [Google Scholar]
- Yan HQ, Yu J, Kline AE, Letart P, Jenkins LW, Marion DW, Dixon CE. Evaluation of combined fibroblast growth factor-2 and moderate hypothermia therapy in traumatically brain injured rats. Brain Res. 2000;887:134–143. doi: 10.1016/s0006-8993(00)03002-x. [DOI] [PubMed] [Google Scholar]
- Yan HQ, Kline AE, Ma X, Hooghe-Peters EL, Marion DW, Dixon CE. Tyrosine hydroxylase, but not dopamine beta-hydroxylase, is increased in rat frontal cortex after traumatic brain injury. Neuroreport. 2001;12:2323–2327. doi: 10.1097/00001756-200108080-00009. [DOI] [PubMed] [Google Scholar]
- Yan HQ, Kline AE, Ma X, Li Y, Dixon CE. Traumatic brain injury reduces dopamine transporter protein expression in rat frontal cortex. Neuroreport. 2002;13:1899–1901. doi: 10.1097/00001756-200210280-00013. [DOI] [PubMed] [Google Scholar]
- Yao XL, Liu J, Lee E, Ling GS, McCabe JT. Cullin 5 gene expression in the rat cerebral cortex and hippocampus following traumatic brain injury (TBI) Neurosci Lett. 2006;409:65–69. doi: 10.1016/j.neulet.2006.09.015. [DOI] [PubMed] [Google Scholar]