Abstract
Objective
Test the hypothesis that active Eustachian tube opening efficiency as measured by sonotubometry is higher in adults with no extant middle-ear disease and no history of previous otitis media (Group-1) when compared to adults with no middle-ear disease but a positive history for otitis media (Group-2).
Methods
Eustachian tube function for 1 ear of 33 otherwise healthy adult subjects, 16 assigned to Group-1 and 17 to Group-2, was tested by sonotubometry using a standard protocol. For each test, the sound envelopes for 3 swallows were abstracted independently by 2 observers from the data stream and 7 descriptive parameters related to sound envelope “shape” were calculated. Interrelatedness among the values for the parameters was explored using correlation analysis. The contributions of swallow, observer and group to the variance in each parameter were evaluated for significance using a General Linear Model.
Results
The shape parameters reflecting envelope height, area and rise and fall rates were highly inter-correlated, but those reflecting envelope widths were not. There was no effect of “swallow” on any of the parameters; but there was a significant “observer” effect on all measures of envelope width, greater for observer-2, and a significant “group” effect for 5 of the 7 shape parameters, all greater in Group-1.
Conclusions
Quantifiable measures of the sound signal “shape” recorded by sonotubometry during swallowing were significantly different between the 2 groups of subjects. This is interpretable as evidencing a more efficient Eustachian tube opening-function in adults with healthy middle ears who do not have a previous history of otitis media when compared to similar adults with a history of prior otitis media. Inefficient Eustachian tube function as children may not be completely resolved by adulthood increasing adult otitis media risk when Eustachian tube function is down-graded by extant upper respiratory diseases that provoke nasopharyngeal inflammation.
Keywords: Otitis media, Eustachian tube, adults, sonotubometry, methodology, otitis media risk
INTRODUCTION
The Eustachian tube (ET) represents a potential, gas-phase communication between the middle ear (ME) and nasopharynx. While usually closed, the ET lumen is opened periodically for short times by contraction of the Tensor Veli Palatini muscle (mTVP) with perhaps the assistance of the Levator Veli Palatini muscle (mLVP). These transient, muscle-assisted ET openings allow for the gradient driven, bolus gas exchange between the ME and nasopharynx1. Such gas transfers decrease the pre-opening ME-ambient pressure gradient that is constantly being perturbed by changes in atmospheric pressure and by changes in ME pressure secondary to diffusive gas transfer from the ME to mucosal blood2. Experiments in monkeys (and other animal species) show that inefficient ET opening causes the successive development of ME under-pressures (ref. ambient), ME mucosal inflammation and effusion accumulation in the usually gas-filled ME cavity3. This presentation is similar, if not identical, to that for otitis media (OM) with effusion (OME), a common disease in infants and children that also occurs in adults4.
As used here, “Eustachian tube function” (ETF) refers to the relative efficiency of ET openings to maintain a near ambient ME pressure5. It is believed that for individual ears, there is a constitutive ETF imposed by the physical composition (e.g. distribution of elastic fibers in the periluminal tissues), mechanical properties (e.g. compliance and hysteresis of the periluminal tissues) and geometry (e.g. paratubal muscle-ET vector relationships) of the ET system6. Using a variety of different ETF test protocols, past research reported that constitutive ETF is: poorer in patients with OME and other ME diseases when compared to age-matched subjects without disease (by manometric testing)7-11; poorer in young children when compared to adults (by pressure chamber testing)12; and, at all ages, downgraded during periods with extant co-morbid disease conditions that provoke inflammation in or around the ET (by 9 Step testing)13,14.
An analysis of the data for one recent study of adults with no extant ME disease that measured ETF using a relatively invasive protocol, the Forced-Response test, showed that constitutive ETF, as reflected in the efficiency of ET opening, was poorer in those subjects with a childhood history of ME disease when compared to those without a past disease history15. This observation suggests that, while improved during growth and development, the poor constitutive ETF in children with ME disease continues to be expressed as a more subtle defect when they achieve adulthood. If valid, adults with a childhood history of OM would be at increased risk for ME disease when extant constitutive ETF is situationally compromised.
Testing that hypothesis using similar procedures in a large, mixed population of adults with healthy MEs is not feasible because the Forced-Response test, which provides quantitative, scaled measures for both the passive and active properties of the ET, needs direct access to the ME via a tympanic membrane perforation or a functional tympanostomy tube, conditions that are uncommon in disease-free ears9. In contrast, sonotubometry is a non-invasive test of ET opening that only needs an effusion-free ME and patent nasal passages16,17. To perform the test, sound is introduced into the nasopharynx and sound-pressure levels are continuously monitored in the ear-canals. ET opening during swallowing (or other maneuvers) will cause an increase over baseline in the ear-canal sound pressure as the sound is transmitted from the nasopharynx to the ME via an open ET lumen16,17. A variety of sonotubometric configurations (introduced sound frequencies, specified sound pressures, filters etc.) have been described in the literature16,18-24, but common to all is the need to reliably abstract specific characteristics of the sound signal that delineate an ET opening from the noise background associated with swallowing with or without ET opening17. Usually, the envelope of the sound signal during a swallow is analyzed and the results are expressed as a dichotomous, yes/no assignment of ET opening based on a pre-defined threshold change in a signal amplitude and/or width, but no study has established the parameter(s) and their cutoffs that yield the highest specificity and sensitivity for assigning a tubal opening20,25. Alternatively, a few studies treated parametric measures of the shape of the sound envelope recorded during swallowing as continuous variables that capture quantitative information on ET opening efficiency22-24,26.
In this pilot study, we tested ETF by sonotubometry in adult subjects with no extant ME disease but with and without a history of significant OM. The envelopes of the ear-canal sound signal associated with swallowing were extracted from the data stream and 7 sound envelope “shape” parameters believed to reflect ET opening efficiency were measured for each swallow. Using these data, we tested the directional hypothesis that the values for sound-envelope “shape” parameters are greater for ears of adult subjects with no extant ME disease and no prior history of OM when compared to those for adult subjects with no extant ME disease but a history of OM.
METHODS
A total of 33 otherwise healthy adult subjects with no extant ME disease, 16 without (Group-1) and 17 (Group-2) with a significant history of OM defined by past ventilation tube insertion(s) were enrolled. After recruitment, the subjects presented to our ME physiology laboratory and a general history was taken, an ENT exam was done, and the ears were examined bilaterally using pneumatic otoscopy and tympanometry. Subjects were excluded from participation if they reported or had signs/symptoms of active allergic rhinitis, a cold (viral upper respiratory tract infection) or non-specific nasal inflammation; if their nasal passages were not bilaterally patent; if the tympanic membrane was judged to have low mobility or present other abnormalities, or if abnormal ME pressures or extant ME disease was diagnosed. Then, one ear from each person was randomly chosen (8 right ears in Groups 1 and 2) and the ET opening function was evaluated using the standardized sonotubometry protocol described below. The study was reviewed and approved by the IRB at the University of Pittsburgh and written informed consent was obtained from all subjects prior to performing any procedure.
The sonotubometer was a customized instrument developed in our laboratory and previously described20. For testing, the subject was seated in an upright position, and a microphone (Knowles Electronic BT-21834-000-Itasca, Illinois, USA) fitted within an appropriate-sized plastic plug was inserted into the ear-canal on the test side to monitor ear-canal sound pressure. The external ears were covered with a standard ear-protector (EARMUFF 1000-Aearo Technologies, Indianapolis, IN) to reduce ear-canal ambient noise level. A plastic nasal probe (2 cm in length, 8 mm in diameter) fitted to a hand-held plastic box containing a speaker (Piezo Electric Tweeter SS-990-Herald Electronics, Lincolnwood, IL) was inserted part-way into the nostril on the most patent side of the nasal cavity for sound delivery. A submental surface electrode (Noraxon Dual Electrodes, Noraxon USA Inc) placed over the anterior belly of the digastric muscle and referenced to a surface electrode (Meditrace 100, ECG Conductive Adhesive Electrodes, Tyco Healthcare Group LP) placed over the tip of the mastoid was used to monitor swallowing activity. The speaker’s output in terms of the frequency spectrum (white noise) and sound-pressure level (110 dB) at the probe tip was controlled by a custom-built circuitry box. The speaker was activated to deliver sound to the nose and nasopharynx, and the subject performed three saliva swallows at intervals of approximately 5 seconds. During the test period, the signals from the ear-canal microphone and the submental surface electrode were continuously routed to a PL3504 PowerLab 4/35 data acquisition system for conditioning and then to a PC running Lab Chart software 7.3.6 (AD Instruments, Bella Vista, NSW Australia) for storage, display, filtering and analysis.
For the analysis, the microphone signal was high pass filtered at 1000 Hz and then smoothed using an arithmetic function. Two observers, blinded to subject group assignment, independently reviewed the synchronously displayed raw and processed microphone signals and the raw submental EMG signal for each test period. The time interval for the three swallows was defined based on an observed increase in the EMG signal and then the processed microphone signals for each of those periods was bracketed by each observer using a detector. The data for the bracketed region were analyzed using the Peak Analysis Module, a subroutine that automatically extracted and stored measures of 7 “user-defined” parametric “shape” features of the signal amplitude-time profile for each swallow demarcated by each observer (See Figure 1). Note that in presenting the data, we use mV as the dimension for signal amplitude since voltage is an untransformed measure of microphone output. Others use the transformed dimension of dB for this measure, where Signal Height in dB = 20 log10 (Vp/Vb), Vp is peak voltage and Vb is the baseline/background voltage.
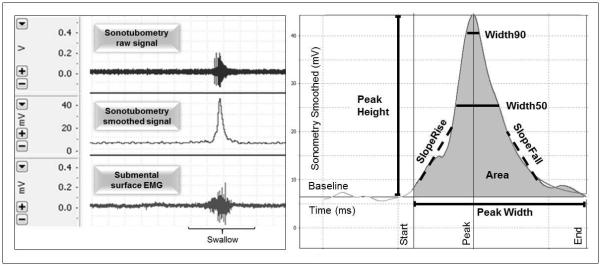
Left Panel: An example of the raw data streams (mV) recorded from the ear-canal microphone (upper row) and the submental electrode (bottom row), and the processed data stream (filtered and smoothed) for the ear-canal microphone (middle row). Right Panel: Idealized ear-canal signal envelope recorded as microphone voltage (mV) as a function of time (ms) and bracketed for the period of a swallow. The seven analyzed “shape” parameters are labeled as: Width, the time between onset and termination of an increase in signal amplitude; Height, the maximum signal amplitude; Area, the integrated area under the amplitude-time curve; SlopeRise, the maximum rate of amplitude increase; SlopeFall, the maximum rate of amplitude decrease, and Width50 and Width90, the lengths of time within a defined change in amplitude at 50% and 90% of Height, respectively.
For each of these 7 parameters, standard summary statistics (average+standard deviation) were calculated across all groups and swallows for each observer, across all observers and groups for each swallow and across all swallows and observers for each group. The inter-relatedness among the 7 parametric measures was explored using pair-wise, least-square, linear correlation operating on the individual data sets for each observer. The contributions of swallow number, observer and group (and their interactions) to the variance in the values for each parameter were evaluated for statistical significance using a 3×2×2 analysis of variance for balanced data. Using these data, we also performed a preliminary analysis focused on a peripheral issue; i.e. the need to use sophisticated pattern analyses algorithms to abstract information on ET opening from the data stream. There, we defined a simple triangle based on the observer’s direct measurement of signal width and height and then calculated an envelope area as 0.5*maximum signal height times signal width. We then regressed the program-calculated signal area based on an algorithm that integrated signal amplitude over time on this simple “triangle” area and determined the percent variance in the former explained by the latter (100% × r2). All data analyses were performed using the NCSS 2007 statistical software package (Kaysville, Utah). In the text, the format average+standard deviation is used consistently.
RESULTS
The average age for Group-1 subjects was 35±12 (range=18 to 54) years; 7 of the individuals were male, and 8 reported their race as being “white”. Three subjects reported a history of allergy and none of gastroesophageal reflux disease; and 3 had had a tonsillectomy and 1 an adenoidectomy at some time in their past. None reported a history of ME disease and none had tympanostomy tubes inserted at any time. The average age for Group 2-subjects was 30±9 (range= 20 to 49) years; 8 of the individuals were male, and 16 reported their race as being “white”. Six subjects reported a history of allergy and 3 of gastroesophageal reflux disease; and 7 had had a tonsillectomy and 7 an adenoidectomy at some time in the past. By self-report, ventilation tubes were inserted into the ears of Group-2 subjects during infancy in 4, childhood in 10, adolescence in 2 and early adulthood in 1.
Table I lists the Pearson Correlation Coefficients between all measured values of paired signal “shape” parameters, separately for the two observers. The pattern of relatedness among parameters was similar for the two observers. There, “Height”, “Area”, “SlopeRise” and “SlopeFall” were highly inter-correlated, but the three “Width” measures were poorly correlated among themselves and with the values for the other parameters. Table II lists the average and standard deviations of the measured values of the signal parameters for subsets defined by swallow, observer and group. There were no obvious differences among swallows in these summary measures. However, the average values for most, if not all, parameters were greater for Observer 2 when compared to Observer 1, with the fold-difference ranging from 1.0 for “SlopeFall” to 1.5 for “Width”. With the exception of “Width”, the average values for all parameters were greater in Group-1 when compared to Group-2, and were more than two-fold greater for “Height”, “SlopeRise” and “SlopeFall”. The coefficient of variation (Standard deviation/average) was calculated for each parameter separately for each observer and group and then averaged between the two observers. For Group-1, the average coefficient of variation measured for the 3 width parameters (range: 0.48 to 0.60) was less than that for the other parameters (range: 1.27 to 1.35). A similar pattern held for the average coefficients of variation for the 3 width (range: 0.59 to 0.87) and the 5 other parameters (range: 1.14 to 2.30) in Group-2 subjects. In general, these coefficients were greater for Group-2 when compared to the paired values for Group-1.
Table 1.
Pairwise Pearson Product Moment Correlation Coefficients (r) among the paired values for envelope “shape” parameters measured by Observer 1 (above diagonal) and Observer 2 (below diagonal).
| Width (ms) |
Width50 (ms) |
Width90 (ms) |
Height (mV) |
Area (mV.ms) |
SlopeRise (mV/ms) |
SlopeFall (mV/ms) |
|
|---|---|---|---|---|---|---|---|
| Width (ms) | 1.00 | 0.65 | 0.27 | 0.02 | 0.20 | −0.06 | 0.07 |
| Width50 (ms) | 0.65 | 1.00 | 0.45 | 0.20 | 0.39 | 0.12 | −0.09 |
| Width90 (ms) | 0.18 | 0.44 | 1.00 | 0.47 | 0.51 | 0.43 | −0.34 |
| Height (mV) | 0.02 | 0.04 | 0.47 | 1.00 | 0.94 | 0.94 | −0.92 |
| Area (mV.ms) | 0.34 | 0.30 | 0.46 | 0.87 | 1.00 | 0.83 | −0.77 |
| SlopeRise (mV/ms) | −0.04 | −0.02 | 0.47 | 0.95 | 0.79 | 1.00 | −0.94 |
| SlopeFall (mV/ms) | 0.00 | 0.03 | −0.43 | −0.94 | −0.78 | −0.93 | 1.00 |
r ≥±0.3, significant at p≤0.5
Table 2.
Average (avg) and standard deviation (std) of the 7 “shape” parameters for data subsets organized by swallow, observer and group.
| Width (ms) |
Width50 (ms) |
Width90 (ms) |
Height (mV) |
PeakArea (mV.ms) |
SlopeRise (mV/ms) |
SlopeFall (mV/ms) |
||
|---|---|---|---|---|---|---|---|---|
| Swallow 1 | Avg | 521 | 203 | 48 | 18.4 | 4994 | 0.24 | −0.21 |
| Std | 299 | 155 | 29 | 30.1 | 9015 | 0.33 | 0.32 | |
| Swallow 2 | Avg | 612 | 214 | 52 | 16.1 | 4610 | 0.24 | −0.21 |
| Std | 473 | 164 | 32 | 24.2 | 7330 | 0.35 | 0.27 | |
| Swallow 3 | Avg | 584 | 205 | 45 | 16.0 | 4508 | 0.23 | −0.19 |
| Std | 395 | 148 | 24 | 27.7 | 7373 | 0.36 | 0.24 | |
|
| ||||||||
| Observer 1 | Avg | 452 | 183 | 45 | 15.9 | 3984 | 0.23 | −0.19 |
| Std | 258 | 135 | 27 | 27.0 | 7108 | 0.35 | 0.27 | |
| Observer 2 | Avg | 693 | 232 | 52 | 17.7 | 5424 | 0.24 | −0.21 |
| Std | 467 | 170 | 30 | 27.8 | 8609 | 0.35 | 0.28 | |
|
| ||||||||
| Group 1 | Avg | 566 | 224 | 57 | 24.2 | 6108 | 0.32 | −0.28 |
| Std | 292 | 138 | 31 | 32.7 | 7965 | 0.43 | 0.35 | |
| Group 2 | Avg | 578 | 192 | 40 | 9.7 | 3356 | 0.15 | −0.13 |
| Std | 475 | 169 | 24 | 18.4 | 7649 | 0.21 | 0.15 | |
The data is presented in mV as the dimension for signal amplitude and the transformed measure of Signal Height in dB = 20 log10(Vp/Vb), where Vp is peak voltage and Vb is the baseline/background voltage.
For each signal “shape” parameter, Table III lists the f and p values derived from the Analysis of Variance for the three primary factors (the interaction effects were omitted since none was statistically significant). There was no significant effect of “swallow” on the values for any of the 7 parameters. There was a significant effect of “observer” on the measured values for “Width” and “Width50”, but not for the other 5 parameters. There was a highly significant effect of “Group” on the measures for “Width90”, “Height”, “Area”, “SlopeRise” and “SlopeFall”, but not for the other 2 width parameters. Post-hoc testing for directional, pair-wise differences revealed that the absolute values for “Width90”, “Height”, “Area”, “SlopeRise” and “SlopeFall” were significantly greater for Group-1 when compared to Group-2.
Table 3.
The values of the “f” statistic and associated significance levels (p) for the contribution of the three primary factors to the variance in each of the “shape” parameters as determined using a saturated Analysis of Variance Model for balanced data.
| Swallow | df=2* | Observer | df=1 | Group | df=1 | |
|---|---|---|---|---|---|---|
| f | p | f | p | f | p | |
| Width (ms) | 0.9 | 0.39 | 19.5 | <0.01 | 0.1 | 0.80 |
| Width50 (ms) | 0.1 | 0.91 | 5.0 | 0.03 | 2.0 | 0.16 |
| Width90 (ms) | 0.8 | 0.44 | 2.9 | 0.09 | 17.4 | <0.01 |
| Height (mV) | 0.2 | 0.85 | 0.2 | 0.65 | 14.6 | <0.01 |
| Area (mV.ms) | 0.1 | 0.93 | 1.6 | 0.20 | 6.2 | 0.01 |
| SlopeRise (mv/ms) | 0.0 | 0.97 | 0.0 | 0.84 | 13.1 | <0.01 |
| SlopeFall (mv/ms) | 0.2 | 0.85 | 0.1 | 0.76 | 15.4 | <0.01 |
There were no significant interactions for the model
df=degrees of freedom
There was a strong linear relationship between the area of a triangle calculated from the simple measures of “Height” and “Width” and the “Area” parameter calculated by the program using integration of signal amplitude over time. There, the regression equation was Area = 0.83 × Triangle Area + 454, and the percent variance in “Area” explained by the regression (r2×100%) was 90%. For this analysis with a forced “0” intercept, the regression equation was Area = 0.96 × Triangle Area, and the percent variance in “Area” explained by the regression (r2×100%) was 93%.
DISCUSSION
The purpose of this study was to explore the validity of an interesting observation made in an earlier study that tested a small group of adults with healthy MEs using a semi-invasive, quantitative ETF test, the Forced-Response test, and reported that ET opening efficiency was higher in the 15 healthy adults with no extant ME disease and a negative OM history when compared to the 5 healthy adults with no extant ME disease and a positive OM history15. While a number of past studies have shown that ETF is much poorer in adults with concurrent ME-disease when compared to adults without disease7,9,11,15, a possible difference in constitutive ETF between adults with healthy MEs, but with and without childhood OM, has not been formally tested.
In this study, we tested the hypothesis that ETF, assessed by sonotubometry, is better in adult subjects with no extant ME disease and no previous OM history when compared to that for adult subjects with no extant ME disease but an OM history. Sonotubometry was used to test ETF because, unlike the Forced-Response test used in the previous study which requires a preparatory myringotomy in otherwise healthy MEs15, sonotubometry is non-invasive and can assess, at a minimum, the presence/absence of ET openings with swallowing16. For each sonotubometric test, sound was introduced into the nasopharynx via the nose and ear-canal sound pressure was continuously monitored over a period sufficient to capture 3 sequential saliva swallows, a maneuver associated with mTVP activity and ET opening. Rather than comparing the between-group frequency in a dichotomous, yes/no ET opening response defined at a threshold-change in the sound signal, we characterized the “shape” of the ear-canal sound envelope for each swallow by measuring 7 parametric descriptors that can be related to specific tubal opening characteristics and, then, performed between group comparisons using the more powerful statistics applicable to continuous data. Of those parameters, it is expected that signal “Width” is a measure of the length of time that the tubal lumen is open during a swallow, signal “Height” is proportional to the minimum cross-sectional area of the open lumen during a swallow, signal “Area” scales to the potential volume gas-flow transferred during a swallow at specified pressure gradients and, less intuitively, that “SlopeRise” is a rate measure of mTVP induced displacement of the lateral ET luminal wall, and “SlopeFall” is a rate measure of the passive return of the ET lateral wall to a closed position. The analysis showed that the values for 5 of these 7 parameters were greater in the group of adults without a history of OM. Because the absolute value for each of these parameters is expected to reflect directly the efficiency of ET opening, these results support our stated hypothesis.
To explain the apparent conditioning of adult ETF by childhood OM experience, we propose a model whereby childhood OM burden is an inverse measure of the extant, constitutive ETF (i.e. poor ETF drives OM risk) and constitutive ETF improves at a similar relative rate across all individuals as the ET system matures during growth and development. There, ETF will improve and, by consequence, OM burden lessen between childhood and adulthood in all individuals, but relative differences in constitutive ETF between groups defined by childhood OM experience will be retained into adulthood. If valid, adults and older children who experienced a high OM burden in childhood are at increased risk for the development of OM under conditions where constitutive ETF is down-graded as, for example, by concurrent diseases that provoke nasopharyngeal inflammation, or stressed, as for example, during air-flights and diving. Moreover, relatively simple assessments of ETF by sonotubometry may have prognostic value in assigning an individual’s risk for these “conditional” disease expressions. These possibilities should be explored in future studies.
For this analysis, the “shape” of the detected sound envelope was represented by 7 characteristic parameters, some requiring integration of sound amplitude over time. Cross-correlations among the values for the 7 parameters showed high percent shared variances (100% × r2) for the 4 parameters geometrically related to envelope amplitude; “Height”, “Area”, “SlopeRise” and “SlopeFall”. Consequently, these parameters capture highly redundant information which is reflected in the fact that all were significantly different between groups defined by the presence/absence of an OM history. In contrast, the extent of the independent information captured by the width parameters is less clear. For example, “Width” shares no variance (information) with the 4 amplitude related parameters, but “Width90” shares between 9% and 25% of its variance with the 4 amplitude related parameters. We also showed that fitting a simple triangle to sound signal envelope shape based on simple measures of maximum signal width and peak height captured more than 90% of the variance in the “Area” parameter when calculated as the integrated amplitude over time. This again suggests redundant information available from most of the shape parameters. However, it should be noted that the sonotubometry tests performed in this study were done on adults with no extant ME disease and, presumably, a sufficient constitutional ETF to maintain a disease-free ME under most conditions. The redundancy in information provided by the various parameters observed for those tests may not characterize tests in younger children or in individuals with more recent OM where constitutive ETF is expected to be much more variable. Because only the minimal parameter set that captures the maximum “shape” information is needed for group discriminations based on sound envelopes, identifying membership in that set is an important goal for future studies.
A caveat to the interpretation of the sonotubometry data for this or any previous study is the absence of empiric data relating an ear-canal sound envelope parameter during a swallow to the presence and extent of an ET luminal opening during the maneuver. While the principal underlying the test methodology for sonotubometry is firmly grounded in theory17, in vivo mechanisms for the production of ear-canal sound envelopes during swallowing that may be similar to those predicted for an ET opening when a true opening does not occur have been suggested17,22,24,27. For example, some researchers have argued that variations in the nasopharyngeal “noise” level caused by the act of swallowing could be transmitted to the ear-canal by bone conduction and recommended the use of test sounds at frequencies higher than those typical for swallowing sounds16,18,19,22. However, this mechanism for the production of “false positive” test results presumes that the amplitude and duration of a bone-conducted swallowing sound signal are of similar magnitude and duration at the ear-canal as an ET conducted sound signal which is unlikely and unproven. Moreover, the suggested remedy fails to recognize that the physical dimensions of the individual ET lumen for each swallow selectively “filters” the sound frequencies transmitted through the tube and thereby distorts nasopharyngeal sound frequencies when represented at the ear-canal. Others recognized that the sound pressure introduced into the nasopharynx by the test system will, if the nasal passages are blocked (e.g. by the sound source), increase and then decrease with swallowing as the nasopharyngeal volume is changed with the rise and fall of the soft palate17,27. As above, these “sound” variations could be transmitted to the ear-canal by bone-conduction whether or not the ET opens. Suggested remedies include the use of a large volume speaker for sound presentation to the nose and ensuring that the contra-lateral nasal cavity is open to the environment (both employed by us). However, the magnitude of the change in nasopharyngeal sound pressure caused by changes in nasopharyngeal volume has not been measured under any conditions. For these reasons, there are a large number of proposed sonotubometry test configurations with different source sounds and different methods for sound signal processing in current use. In this study, as in previous studies by us14,20,26,28-33, we used the configuration of presented sound (110 dB at speaker source, white noise) and signal filters recommended by Murti and colleagues17,34 based on their spectral analysis of sound transmission through the adult ET, but we expect that our results will hold over a wide range of test configurations. We cannot argue for the superiority or inferiority of this or of any of the sonotubometry configurations in current use because the results for none have been cross-correlated with those for a “gold standard” test of ET opening such as the detection of transET gas flow or of a decrease in the ME-nasopharyngeal pressure gradient during a maneuver (e.g. swallow). Validating sonotubometry is an urgent need and is a focus of ongoing experiments in our laboratory.
In summary, the results for the present study support the hypothesized poorer ETF in health adults with a past OM history when compared to those without such a history. If confirmed, the poorer ETF in adults with a past OM history may translate to an increased, situational risk for OM and other ME diseases that is assignable based on the results for sonotubometry. Future studies are needed to define the sonotubometry test methods and configurations that yield the highest accuracy for diagnosing the presence/absence of a ET opening during a specified maneuver and to validate and extend these interesting observations.
ACKNOWLEDGEMENTS
This study was supported in part by a grant from the National Institutes of Health (P50 DC007667), and by the Hamburg and Eberly Endowments to the Division of Pediatric Otolaryngology, University of Pittsburgh. These sources provided funding for the study, but did not have input into the design, analyses or interpretation of the data.
Footnotes
CONFLICT OF INTEREST STATEMENT None of the listed authors has any real or apparent conflicts of interest with respect to the material presented in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Bluestone CD, Doyle WJ. Anatomy and physiology of eustachian tube and middle ear related to otitis media. J Allergy Clin Immunol. 1988;81(5 Pt 2):997–1003. doi: 10.1016/0091-6749(88)90168-6. [DOI] [PubMed] [Google Scholar]
- 2.Doyle W. Middle ear pressure regulation. In: Rosowski JJ, Merchant SN, editors. The Function and Mechanics of Normal, Diseased and Reconstructed Middle Ears. Kugler Publications; The Netherlands: 2000. [Google Scholar]
- 3.Doyle WJ. Functional eustachian tube obstruction and otitis media in a primate model. A review. Acta Otolaryngol Suppl. 1984;414:52–57. doi: 10.3109/00016488409122882. [DOI] [PubMed] [Google Scholar]
- 4.Bluestone CD, Klein JO. Otitis Media in Infant and Children. 5th ed BC Decker Inc; Hamilton, Ontario: 2007. [Google Scholar]
- 5.Bluestone CD. Eustachian Tube Structure, Function, Role in Otitis Media. BC Decker Inc; Hamilton, Ontario: 2005. [Google Scholar]
- 6.Doyle WJ, Mandel EM, Seroky JT, Swarts JD, Casselbrant ML. Reproducibility of the forced response test in children with chronic otitis media with effusion. Otol Neurotol. 2013;34(1):16–21. doi: 10.1097/MAO.0b013e31827853f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swarts JD, Bluestone CD. Eustachian tube function in older children and adults with persistent otitis media. Int J Pediatr Otorhinolaryngol. 2003;67(8):853–859. doi: 10.1016/s0165-5876(03)00127-7. [DOI] [PubMed] [Google Scholar]
- 8.Falk B, Magnuson B. Eustachian tube closing failure in children with persistent middle ear effusion. Int J Pediatr Otorhinolaryngol. 1984;7(2):97–106. doi: 10.1016/s0165-5876(84)80034-8. [DOI] [PubMed] [Google Scholar]
- 9.Cantekin EI, Saez CA, Bluestone CD, Bern SA. Airflow through the eustachian tube. Ann Otol Rhinol Laryngol. 1979;88(5 Pt 1):603–612. doi: 10.1177/000348947908800504. [DOI] [PubMed] [Google Scholar]
- 10.Bhat VK, Kumar PR, Nag M, Hegde J. Comparison of a eustachian barotubometer with a tympanometer to evaluate eustachian tube function in chronic suppurative otitis media. J Otolaryngol Head Neck Surg. 2009;38(4):456–461. [PubMed] [Google Scholar]
- 11.Doyle WJ, Swarts JD, Banks J, Casselbrant ML, Mandel EM, Alper CM. Sensitivity and specificity of eustachian tube function tests in adults. JAMA Otolaryngol Head Neck Surg. 2013;139(7):719–727. doi: 10.1001/jamaoto.2013.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bylander A. Comparison of eustachian tube function in children and adults with normal ears. Ann Otol Rhinol Laryngol Suppl. 1980;89(3 Pt 2):20–24. doi: 10.1177/00034894800890s308. [DOI] [PubMed] [Google Scholar]
- 13.Friedman RA, Doyle WJ, Casselbrant ML, Bluestone C, Fireman P. Immunologic-mediated eustachian tube obstruction: a double-blind crossover study. J Allergy Clin Immunol. 1983;71(5):442–447. doi: 10.1016/0091-6749(83)90459-1. [DOI] [PubMed] [Google Scholar]
- 14.McBride TP, Doyle WJ, Hayden FG, Gwaltney JMJr. Alterations of the eustachian tube, middle ear, and nose in rhinovirus infection. Arch Otolaryngol Head Neck Surg. 1989;115(9):1054–1059. doi: 10.1001/archotol.1989.01860330044014. [DOI] [PubMed] [Google Scholar]
- 15.Swarts JD, Alper CM, Mandel EM, Villardo R, Doyle WJ. Eustachian tube function in adults without middle ear disease. Ann Otol Rhinol Laryngol. 2011;120(4):220–225. doi: 10.1177/000348941112000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Virtanen H. Sonotubometry. An acoustical method for objective measurement of auditory tubal opening. Acta Otolaryngol. 1978;86(1-2):93–103. doi: 10.3109/00016487809124724. [DOI] [PubMed] [Google Scholar]
- 17.Murti KG, Stern RM, Cantekin EI, Bluestone CD. Classification of spectral patterns obtained from eustachian tube sonometry. IEEE Trans Biomed Eng. 1982;29(6):472–477. doi: 10.1109/TBME.1982.324978. [DOI] [PubMed] [Google Scholar]
- 18.Jonathan DA, Chalmers P, Wong K. Comparison of sonotubometry with tympanometry to assess eustachian tube function in adults. Br J Audiol. 1986;20(3):231–235. doi: 10.3109/03005368609079020. [DOI] [PubMed] [Google Scholar]
- 19.Palva T, Marttila T, Jauhiainen T. Comparison of pure tones and noise stimuli in sonotubometry. Acta Otolaryngol. 1987;103(3-4):212–216. [PubMed] [Google Scholar]
- 20.McBride TP, Derkay CS, Cunningham MJ, Doyle WJ. Evaluation of noninvasive eustachian tube function tests in normal adults. Laryngoscope. 1988;98(6 Pt 1):655–658. doi: 10.1288/00005537-198806000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Morita M, Matsunaga T. Sonotubometry with a tubal catheter as an index for the use of a ventilation tube in otitis media with effusion. Acta Otolaryngol Suppl. 1993;501:59–62. doi: 10.3109/00016489309126216. [DOI] [PubMed] [Google Scholar]
- 22.Di Martino EF, Thaden R, Antweiler C, Reineke T, Westhofen M, Beckschebe J, et al. Evaluation of Eustachian tube function by sonotubometry: results and reliability of 8 kHz signals in normal subjects. Eur Arch Otorhinolaryngol. 2007;264(3):231–236. doi: 10.1007/s00405-006-0172-1. [DOI] [PubMed] [Google Scholar]
- 23.Asenov DR, Nath V, Telle A, Antweiler C, Walther LE, Vary P, et al. Sonotubometry with perfect sequences: First results in pathological ears. Acta Otolaryngol. 2010;130(11):1242–1248. doi: 10.3109/00016489.2010.492481. [DOI] [PubMed] [Google Scholar]
- 24.Di Martino EF, Nath V, Telle A, Antweiler C, Walther LE, Vary P. Evaluation of Eustachian tube function with perfect sequences: technical realization and first clinical results. Eur Arch Otorhinolaryngol. 2010;267(3):367–374. doi: 10.1007/s00405-009-1074-9. [DOI] [PubMed] [Google Scholar]
- 25.Handzel O, Poe D, Marchbanks RJ. Synchronous endoscopy and sonotubometry of the eustachian tube: a pilot study. Otol Neurotol. 2012;33(2):184–191. doi: 10.1097/MAO.0b013e3182423242. [DOI] [PubMed] [Google Scholar]
- 26.Leclerc JE, Doyle WJ, Karnavas W. Physiological modulation of eustachian tube function. Acta Otolaryngol. 1987;104(5-6):500–510. doi: 10.3109/00016488709128281. [DOI] [PubMed] [Google Scholar]
- 27.Lildholdt T, Brask T, Hvidegaard T. Interpretation of sonotubometry. A critical view of the acoustical measurement of the opening of the eustachian tube. Acta Otolaryngol. 1984;98(3-4):250–254. doi: 10.3109/00016488409107561. [DOI] [PubMed] [Google Scholar]
- 28.Stillwagon PK, Doyle WJ, Fireman P. Effect of an antihistamine/decongestant on nasal and eustachian tube function following intranasal pollen challenge. Ann Allergy. 1987;58(6):442–446. [PubMed] [Google Scholar]
- 29.Doyle WJ, McBride TP, Skoner DP, Maddern BR, Gwaltney JM, Jr, Uhrin M. A double-blind, placebo-controlled clinical trial of the effect of chlorpheniramine on the response of the nasal airway, middle ear and eustachian tube to provocative rhinovirus challenge. Pediatr Infect Dis J. 1988;7(3):229–238. doi: 10.1097/00006454-198803000-00033. [DOI] [PubMed] [Google Scholar]
- 30.Doyle WJ, Skoner DP, Fireman P, Seroky JT, Green I, Ruben F, et al. Rhinovirus 39 infection in allergic and nonallergic subjects. J Allergy Clin Immunol. 1992;89(5):968–978. doi: 10.1016/0091-6749(92)90219-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doyle WJ, Seroky JT, Angelini BL, Gulhan M, Skoner DP, Fireman P. Abnormal middle ear pressures during experimental influenza A virus infection--role of Eustachian tube function. Auris Nasus Larynx. 2000;27(4):323–326. doi: 10.1016/s0385-8146(00)00075-4. [DOI] [PubMed] [Google Scholar]
- 32.Buchman CA, Doyle WJ, Pilcher O, Gentile DA, Skoner DP. Nasal and otologic effects of experimental respiratory syncytial virus infection in adults. Am J Otolaryngol. 2002;23(2):70–75. doi: 10.1053/ajot.2002.30634. [DOI] [PubMed] [Google Scholar]
- 33.Alper CM, Swarts JD, Singla A, Banks J, Doyle WJ. Relationship between the electromyographic activity of the paratubal muscles and eustachian tube opening assessed by sonotubometry and videoendoscopy. Arch Otolaryngol Head Neck Surg. 2012;138(8):741–746. doi: 10.1001/archoto.2012.1293. [DOI] [PubMed] [Google Scholar]
- 34.Murti KG, Stern RM, Cantekin EI, Bluestone CD. Sonometric evaluation of eustachian tube function using broadband stimuli. Ann Otol Rhinol Laryngol Suppl. 1980;89(3 Pt 2):178–184. doi: 10.1177/00034894800890s342. [DOI] [PubMed] [Google Scholar]


