Abstract
Background
Single nucleotide polymorphisms (SNPs) are the most common source of genetic variation. Although microvascular pathology is associated with cardiovascular events, genetic phenotypes causing microvascular disease remain largely unknown. This study identifies gender specific SNPs associated with coronary microvascular dysfunction.
Methods and Results
Six-hundred and forty-three patients without significant obstructive coronary heart disease (CHD) were enrolled, referred for cardiac catheterization, and underwent invasive coronary microcirculatory assessment. Patient data was collected from 1529 autosomal SNPs and 7 X chromosome SNPs which were selected to represent the variability from 76 candidate genes having published associations with coronary vasoreactivity, angiogenesis, inflammation, vascular calcification, atherosclerosis risk factors, female hormones, blood coagulation, or CHD. Coronary flow reserve (CFR) was assessed by intracoronary injection of adenosine. Patients were categorized according to a CFR above or below 2.5 and were stratified by sex.
After adjusting for age, sex, and BMI, this study demonstrates that SNPs within VEGFA and CDKN2B-AS1 are associated with abnormal CFR (P<0.005). SNPs within MYH15, VEGFA and NT5E are associated with abnormal CFR in men. No SNPs were associated with abnormal CFR in women.
Conclusions
Genetic variation within defined regions of VEGFA and CDKN2B-AS1 genes are associated with coronary microvascular dysfunction. Furthermore, sex-specific allelic variants within MYH15, VEGFA and NT5E are associated with an increased risk of coronary microvascular dysfunction in men.
Keywords: Single nucleotide polymorphisms, coronary microvascular dysfunction, coronary flow reserve
INTRODUCTION
Many genomic variants underlie variation causing cardiovascular disease. The large number of new loci associated with cardiovascular risk factors, subclinical indexes, and disease end points have provided insights into the biologic pathways that underlie disease (1). Although conventional risk factors are important, both rare and common genetic variants account for more than 50% of a person’s susceptibility to coronary heart disease (CHD)(2). Single nucleotide polymorphisms (SNPs) are the most common sources of genetic variations (2).
The development of coronary heart disease depends on a person’s genetic predisposition and accumulation of risk factors that lead to a wide array of molecular and cellular abnormalities within vessel wall, such as those related to sustenance and regeneration of microvessels(3). These abnormalities can contribute to the development of coronary microvascular dysfunction and atherosclerosis(3).
Coronary microvascular dysfunction, defined as abnormal coronary flow reserve in response to adenosine, is associated with an increased annual major adverse cardiovascular event rate which includes death, nonfatal myocardial infarction, nonfatal stroke, and congestive heart failure(4). Previous studies on the genetic predictors of cardiovascular disease have primarily focused on macrovascular disease traits and genetic analyses of microvascular disease phenotypes which are unavailable, although both macrovascular and microvascular pathology are associated with cardiovascular disease(5).
Several studies demonstrate gender-specific variations in coronary microcirculation (6–8) however; there is a lack of understanding as to how gender-related pathobiologic and genetic differences influence the development of vascular abnormalities that underlie CHD. Knowledge of such genotypic predictors may enhance our understanding of the molecular mechanisms causing coronary microvascular dysfunction. Thus, the current study was designed to assess the association between SNPs and invasive assessment of coronary microvascular dysfunction in humans and to investigate the sex-specific SNPs related to coronary microvascular dysfunction.
METHODS
Study population
This study includes 643 subjects (426 women and 217 men) who underwent invasive coronary microcirculatory assessment from 1993 to 2010. The referral for cardiac catheterization and coronary microvascular function testing was made at the discretion of the referring cardiologist and operating interventionalist. Patients did not have significant fixed coronary artery stenosis, meaning that they had less than 30% obstruction in their coronary arteries. The majority of subjects were of European ancestry (93% white + 5.6% unknown and presumed white). The median age was 51 for women (range 20 to 75) and 46 for men (range 18 to 78).
Pre-defined exclusion criteria were unstable angina pectoris, uncontrolled systemic hypertension, valvular heart disease, left ventricular ejection fraction<40%, and/or significant endocrine, hepatic, renal, or inflammatory disease. Systemic hypertension was defined as a history of elevated blood pressure requiring long-term therapy. Hypercholesterolemia was defined as either a total cholesterol serum concentration of ≥240 mg/dL or intake of lipid-lowering therapy. Diabetes mellitus diagnosis was based on the patient’s clinical record.
The study was approved by the Mayo Clinic Institutional Review Board, and informed consent was obtained from every patient. This study complies with the Helsinki Declaration of 1975, as revised in 2008.
Study protocol
As previously described(9–14), all patients refrained from taking any vasoactive medication for at least 36 hours prior to catheterization. Patients refrained from any food, drinks, and tobacco for at least 12 hour prior to catheterization. Diagnostic coronary angiography was performed using a 6F or 7F guiding catheter with a standard femoral percutaneous approach. Unfractionated intravenous heparin was administered to achieve an activated clotting time of approximately 250 seconds. Non-ionic contrast material was used for all patients. No nitroglycerin was given prior to the diagnostic procedure.
Coronary vascular reactivity responses were studied, as previously reported (9–12). In brief, a 0.014-inch Doppler tipped guidewire (FloWire: Volcano Corp, CA, USA) was introduced into the left anterior descending coronary artery (LAD). Coronary flow reserve (CFR) was assessed by escalating doses of intracoronary bolus injection of adenosine (36–60 µg) until maximal CFR was obtained. Doppler flow velocity spectra were analyzed on-line to determine averaged peak velocity (APV). CFR was calculated as the ratio of maximal CBF induced by adenosine to basal CBF.
Patients were divided into two groups according a CFR greater than, less than or equal to 2.5. These cut-off points were derived from previous reports (9–12). Impaired coronary microvascular function was defined as CFR less than 2.5, and favorable coronary microvascular function was defined as CFR more than or equal to 2.5. Furthermore, the patients were sub-classified into two groups stratified by gender.
Genomic data and blood collection
DNA was extracted from all samples at the same time, after storing them all at −80 degrees C, by the Biospecimens Accessioning and Processing (BAP) facility. Picogreen analysis was run on all the samples to assess quality. The data was genotyped at the Mayo Genotyping Core facility using an Illumina custom GoldenGate panel (15). Per 96 well plate, there were 85 unique samples, 5 duplicate DNA samples, and 6 quality control samples. A total of 1529 autosomal tag SNPs and 7 SNPs on the X chromosome were originally chosen to represent 76 genes with known associations to coronary vasoreactivity, angiogenesis, inflammation, artery calcification, atherosclerosis risk factors, female hormone, blood coagulation system, or prevalence of CHD. Of these, 351 of these were eliminated from the analysis due to minor allele frequencies less than 5%, Hardy Weinberg Equilibrium (HWE) p-values less than 0.001, or SNP call rates less than 95% (i.e. missing values for at least 5% of the subjects). The majority of SNPs failed because they were monomorphic (had the same value for all subjects) or had a very low minor allele frequency (an alternate SNP value was seen in only a few subjects). Genetic positions were listed in Build 36. Expanded methods are included in the supplementary materials.
Ethical Considerations
This study was approved by the Mayo Clinic Institutional Review Board. This study also complies with principles stated in the Declaration of Helsinki. All patients enrolled in the study signed consent forms after reviewing the protocol for the study which included the risks, burdens and benefits of the study. Precautions were taken to maintain confidentiality of all identifying patient information used in this study using secure firewalled, pass-word-secured databases and special consideration was taken to prevent patients undergoing cardiac catheterization from enduring any unnecessary punctures.
Statistical analysis
Categorical data were analyzed using the chi-square test; continuous variables were analyzed using the two sample t-test and summarized using mean ±SD. Logistic regression was run using the endpoint of CFR<2.5 to determine if genetic differences existed after adjusting for age, sex, and body mass index (BMI), assuming a log-additive genetic model. Models were also run testing for a sex-SNP interaction. A p-value of less than 0.005 was considered statistically significant (Table 1). Statistical analysis was performed using Plink 1.07 (16).
Table 1.
Patient characteristics
| All (n=643) |
Women (n=426) |
Men (n=217) |
P value |
|
|---|---|---|---|---|
| Age, years | 49.7 (11.4) | 51.3 (10.9) | 46.5 (11.8) | <0.001 |
| BMI, kg/m2 | 29.0 (6.2) | 29.1 (6.8) | 28.9 (4.7) | 0.57 |
| Postmenopausal | 247 (58%) | |||
| CFR | 3.0 (0.7) | 2.8 (0.6) | 3.2 (0.8) | <0.001 |
| Risk factor | ||||
| Diabetes Mellitus | 52 (8%) | 28 (7%) | 24 (11%) | 0.049 |
| Hypertension | 264 (41%) | 165 (39%) | 99 (46%) | 0.09 |
| Dyslipidemia | 352 (55%) | 217 (51%) | 135 (62%) | 0.009 |
| Family history | 409 (65%) | 272 (65%) | 137 (66%) | 0.91 |
| Current smoking | <0.001 | |||
| Never | 326 (51%) | 241 (57%) | 85 (39%) | |
| Former | 233 (36%) | 147 (35%) | 86 (40%) | |
| Current | 82 (13%) | 37 (9%) | 45 (21%) | |
| Drugs | ||||
| Aspirin | 325 (51%) | 205 (48%) | 120 (55%) | 0.09 |
| CCB | 240 (37%) | 150 (35%) | 90 (42%) | 0.12 |
| ACE-I /ARB | 104 (16%) | 62 (15%) | 42 (19%) | 0.12 |
| Beta-blocker | 185 (29%) | 127 (30%) | 58 (27%) | 0.41 |
| Diuretics | 105 (16%) | 84 (20%) | 21 (10%) | 0.001 |
| Lipid-lowering drugs | 249 (39%) | 151 (35%) | 98 (45%) | 0.015 |
| ERT | 119 (28%) |
Values are given as n (%) or mean (standard deviation). P value shows women vs. men. BMI, body mass index; CFR, Coronary flow reserve; CCB, Calcium channel blocker; ACE-I, Angiotensin-converting enzyme - inhibitor; ARB, Angiotensin II receptor blocker; ERT, estrogen replacement therapy
RESULTS
Patient characteristics
There were 643 subjects who had physiologic coronary testing in the cardiac catheterization laboratory (426 women and 217 [34%] men). Patient characteristics are summarized in Table 1. The median age was 51 for women (range 20 to 75) and 46 for men (range 18 to 78). Women had a significantly lower CFR than men (2.8±0.6, 3.2±0.8, p<0.001).
Comparisons between abnormal and normal CFR group are shown in Table 2. Age was significantly higher in the abnormal CFR group than for those in the normal group (53.4±11.0 yrs vs. 48.2±11.2 yrs, p<0.001). BMI and proportion of men was significantly lower in the abnormal CFR group than that in the normal CFR group (27.8±5.5 vs. 29.5±6.3, p<0.001; 2.2 vs. 3.3, p<0.001, respectively). There were no differences in prevalence of diabetes, hypertension, dyslipidemia, family history, and smoking between the two groups. Based on this analysis, age, sex and BMI were used as adjustors prior to investigation of the SNPs.
Table 2.
Comparison of coronary risk factor between the 2 groups divided by CFR
| Parameters | CFR<2.5 (n=184) |
CFR≥2.5 (n=459) |
P value |
|---|---|---|---|
| Age, years | 53.4 (11.0) | 48.2 (11.2) | <0.001 |
| BMI | 27.8 (5.5) | 29.5 (6.3) | 0.001 |
| Postmenopausal | 117 (64%) | 248 (54%) | 0.06 |
| CFR | 2.2 (0.3) | 3.3 (0.6) | |
| Risk Factors | |||
| Men, n (%) | 36 (20%) | 181 (39%) | <0.001 |
| Diabetes, n (%) | 18 (10%) | 34 (7%) | 0.32 |
| Hypertension, n (%) | 77 (42%) | 187 (41%) | 0.73 |
| Dyslipidemia, n (%) | 105 (57%) | 247 (54%) | 0.44 |
| Family history, n (%) | 118 (66%) | 291 (65%) | 0.73 |
| Smoking, n (%) | 0.63 | ||
| Never | 96 (52%) | 230 (50%) | |
| Former | 66 (36%) | 169 (37%) | |
| Current | 22 (12%) | 60 (13%) | |
| Drugs | |||
| Aspirin | 99 (54%) | 226 (49%) | 0.30 |
| Calcium channel blocker | 70 (38%) | 170 (37%) | 0.79 |
| ACE inhibitor/ARB | 36 (20%) | 68 (15%) | 0.14 |
| Beta-blocker | 62 (34%) | 123 (27%) | 0.08 |
| Diuretics | 36 (20%) | 69 (15%) | 0.16 |
| Lipid-lowering drugs | 84 (46%) | 165 (36%) | 0.024 |
| ERT | 53 (29%) | 124 (27%) | 0.65 |
Values are given as n (%) or mean (standard deviation). BMI, body mass index; CFR, coronary flow reserve; ACE, Angiotensin-converting enzyme; ARB, Angiotensin II receptor blocker; ERT, estrogen replacement therapy
Relationship of SNPs with abnormal CFR
Figure 1 displays Manhattan plot showing the minus log-transformed p-values for the individual 1529 SNPs against their genomic position. The solid horizontal line marks the threshold for significance (P=0.005). The top SNPs which are associated with increased risk of abnormal CFR are shown in Table 3. This includes one signal within the gene VEGFA (vascular endothelial growth factor A) represented by one SNP (odd’s ratio = 1.68, p=0.004) and one signal within the gene CDKN2B-AS1 (CDKN2B antisense RNA1) represented by five correlated SNPs (top SNP’s odd’s ratio = 1.5, p=0.003).
Figure 1.
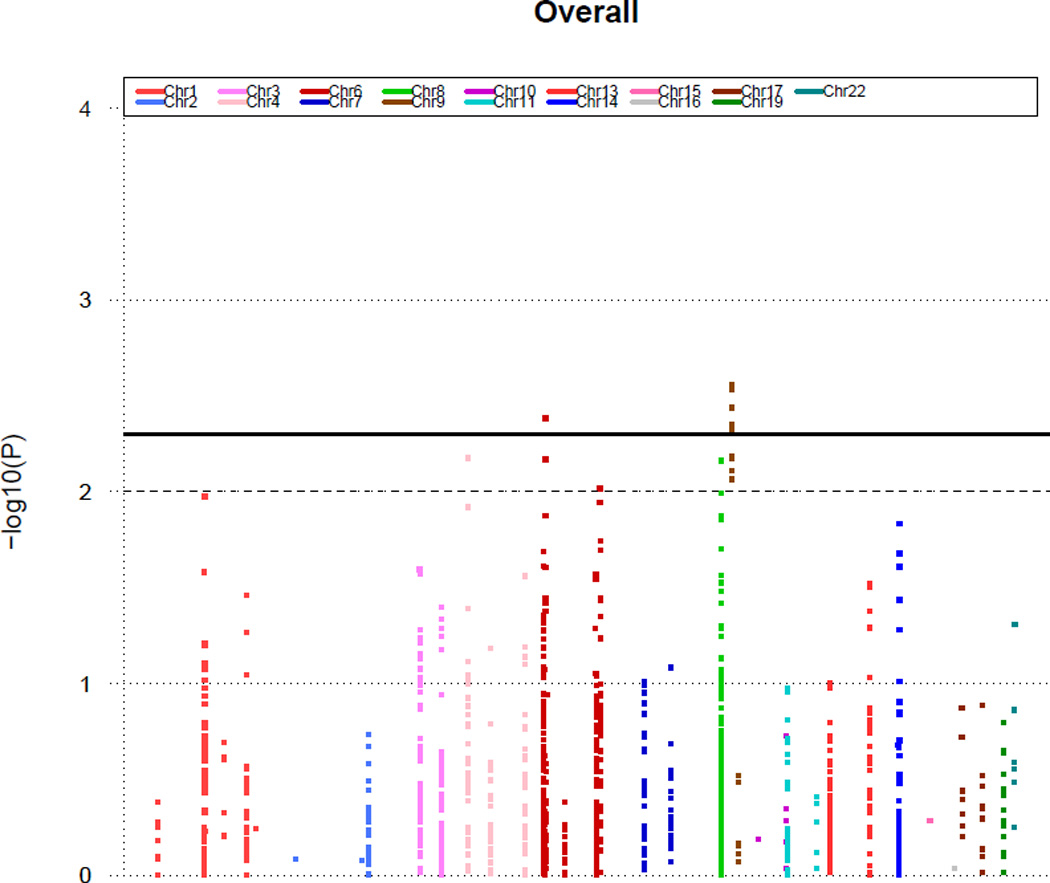
P values (minus log-transformed) of whole population are shown in a signal intensity (Manhattan) plot relative to their genomic position. Each SNP is plotted with respect to its chromosomal location (x axis) and its P value (y axis on the left). The solid horizontal line marks the threshold for significance in our study (P=0.005). The dashed line represents a standard P value (P=0.01).
Table 3.
SNP analysis for CFR <2.5 after adjusting for age, sex, and BMI
| Chr. | SNP | Position | Gene region |
Risk Allele X/Y |
Affected genotype XX/XY/YY |
Unaffected genotype XX/XY/YY |
SNP annotation | OR | 95% CI | P -value |
|---|---|---|---|---|---|---|---|---|---|---|
| 6 | rs3025039 | 43860514 | VEGFA | A/G | 5/57/122 | 7/105/347 | 3'UTR | 1.68 | 1.18–2.39 | 0.0041 |
| 9 | rs10757274 | 22086055 | CDKN2B-AS1 | G/A | 48/94/35 | 95/209/138 | intron | 1.49 | 1.15–1.92 | 0.0028 |
| 9 | rs2383206 | 22105026 | CDKN2B-AS1 | G/A | 59/93/32 | 122/206/131 | intron | 1.43 | 1.12–1.83 | 0.0048 |
| 9 | rs1004638 | 22105589 | CDKN2B-AS1 | T/A | 61/91/32 | 125/205/129 | intron | 1.45 | 1.13–1.85 | 0.0036 |
| 9 | rs2383207 | 22105959 | CDKN2B-AS1 | T/A | 61/91/32 | 124/206/129 | intron | 1.46 | 1.14–1.87 | 0.0029 |
| 9 | rs1333049 | 22115503 | CDKN2B-AS1 | G/C | 52/94/38 | 102/219/138 | 3'downstream | 1.44 | 1.12–1.86 | 0.0045 |
VEGFA, vascular endothelial growth factor A; CDKN2B-AS1, CDKN2B antisense RNA1, 3’UTR, 3’ untranslated region
Sex-specific differences seen in SNPs related to abnormal CFR
We tested for differential effects of SNPs concerning abnormal CFR for males and females by testing for SNP-sex interactions (Table 4). Figures 2 and 3 display Manhattan plots showing minus log-transformed p-values for the individual SNPs against their genomic position in women and men, respectively. The solid horizontal line marks the threshold for significance (P=0.005). Genes with at least one SNP that had different effects between males and females include MYH15, VEGFA, and NT5E. Among these four SNPs (Table 4) associated with those genes, the odd’s ratio for the SNPs ranged from 2.27 to 2.85 for males (p-values from 0.0006–0.0029) and 1.06–1.25 for females (no p-values < 0.005).
Table 4.
Sex-specific estimates for the association of SNPs with CFR < 2.5
| Chr. | SNP | Position | Gene region |
Risk Allele X/Y |
Affected Male genotype XX/XY/YY |
Unaffected Male genotype XX/XY/YY |
SNP annotation |
OR Men |
P value Men |
OR Women |
P value Women |
P value SNP × SEX |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | rs4855559 | 109597726 | MYH15 | A/C | 7/20/2009 | 16/70/95 | intron | 2.27 | 0.0029 | 1.22 | 0.2215 | 0.0008 |
| 3 | rs7630352 | 109605691 | MYH15 | A/G | 11/20/2005 | 24/83/74 | intron | 2.60 | 0.0006 | 1.25 | 0.1454 | 0.0002 |
| 6 | rs3025028 | 43858529 | VEGFA | C/G | 21/13/2 | 53/97/30 | intron | 2.74 | 0.0015 | 1.06 | 0.6909 | 0.0017 |
| 6 | rs6922 | 86262042 | NT5E | C/A | 24/10/2 | 60/96/25 | 3'UTR | 2.85 | 0.0018 | 1.11 | 0.4794 | 0.0027 |
MYH15; myosin, heavy chain 15; VEGFA, vascular endothelial growth factor A; NT5E, 5'-nucleotidase, ecto (CD73)
Figure 2.
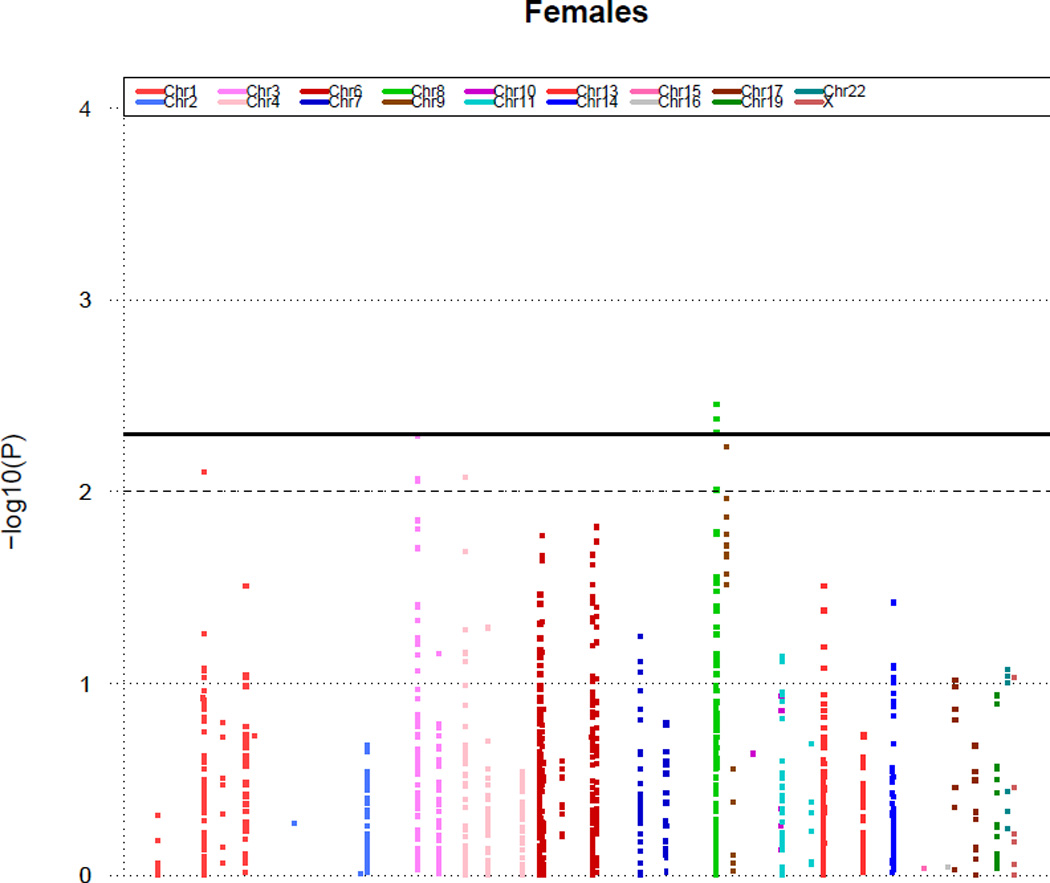
P values (minus log-transformed) of women adjusting for age are shown in a signal intensity (Manhattan) plot relative to their genomic position. Each SNP is plotted with respect to its chromosomal location (x axis) and its P value (y axis on the left). The solid horizontal line marks the threshold for significance in our study (P=0.005). The dashed line represents a standard P value (P=0.01).
Figure 3.
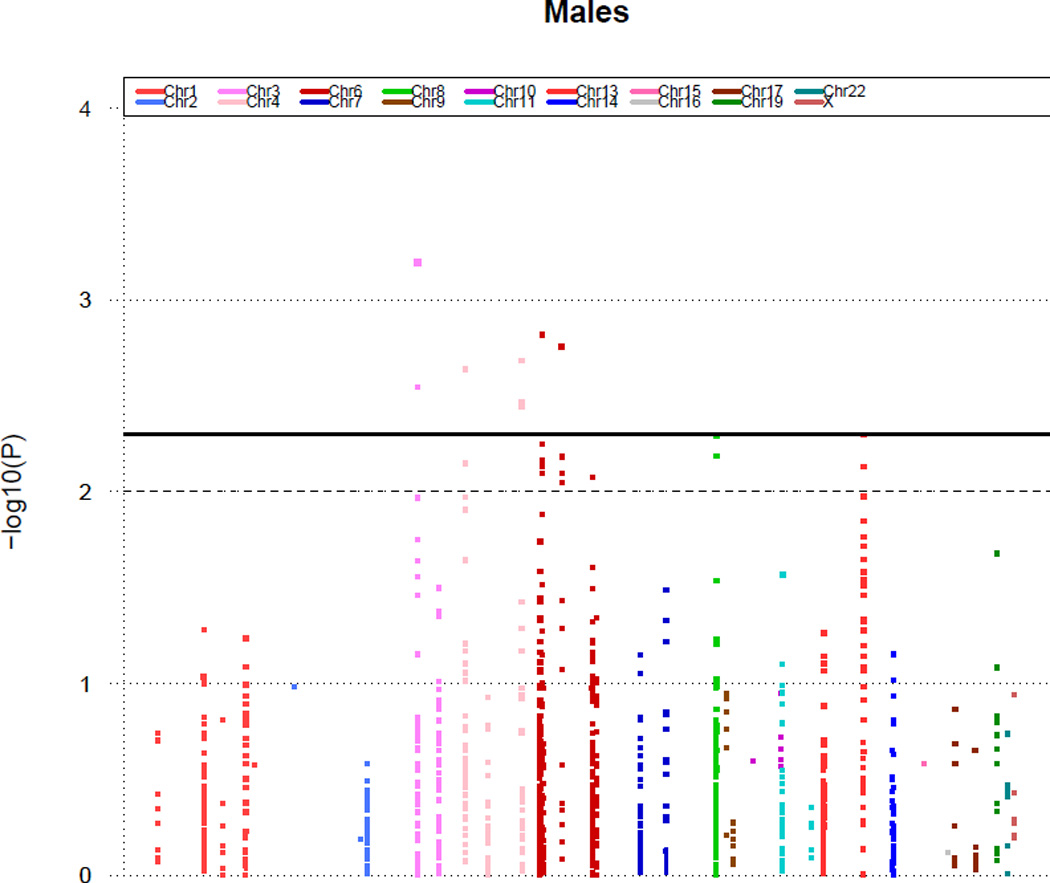
P values (minus log-transformed) of men adjusting for age are shown in a signal intensity (Manhattan) plot relative to their genomic position. Each SNP is plotted with respect to its chromosomal location (x axis) and its P value (y axis on the left). The solid horizontal line marks the threshold for significance in our study (P=0.005). The dashed line represents a standard P value (P=0.01).
DISCUSSION
In this current study, we have indicated novel regions of genetic variation within VEGFA and CDKN2B-AS1 genes that are associated with coronary microvascular dysfunction. Furthermore, there were sex-specific differences in SNPs which are associated with microvascular dysfunction. This study may support a role for genetic variation in the heterogeneity of coronary blood flow reserve that can lead to myocardial ischemia.
SNPs related to the increased risk of abnormal CFR
Our study demonstrated that the risk allele of VEGFA and CDKN2B-AS1 gene SNPs reside at introns which are associated with the increased risk of abnormal CFR.
Coronary morphogenesis constitutes the proliferation and migration of angioblasts, tube formation, and further assembly of the vascular wall (17). Regulators of this process are multiple growth factors including vascular endothelial growth factors (VEGF). During the early stage in myocardial vascularization, VEGF expression is closely related to the sites of vascular tube formation and microvascular permeability (17). The role of VEGF and its receptors is not only to generate new vessels but has a control the function of the vascular system in its on-going process. Recent evidences provided the potential effects of polymorphism in VEGFA genes upon the development of CHD (3, 17, 18). The decrease in VEGFA function induced by gene variants is correlated with vascular dysfunction, including microvascular cell damage, impaired microvascular cell survival, decreased anti-apoptotic effect of VEGF, and abnormal vascular repair (19). All of these VEGF functions can lead to the development of coronary atherosclerosis. The current study suggests that polymorphisms in the VEGF gene are candidate contributors to the pathogenesis of coronary microvascular dysfunction.
Recently, a major genetic susceptibility locus for CHD was identified. This locus is located within 9p21.3, mapping to the large non-coding antisense RNA transcript CDKN2BAS, formerly called ANRIL (20). ANRIL is expressed in vascular endothelial cells and coronary smooth muscle cells (21). Genetic variants of CDKNBAS are associated with angiogenesis and atherosclerosis pathogenesis in vascular cells by mediating the response to inflammatory signaling (22). Modulations of the expression levels of CDKN2BAS may affect vascular cell proliferation and senescence. Its deficiency may cause vascular injury in patients with microvascular dysfunction.
Sex differences of SNPs related to abnormal CFR
Cardiovascular structural and functional adaptation to aging and disease differs substantially between women and men(23). Women with symptomatic ischemic heart disease undergoing coronary angiography have less extensive or obstructive coronary lesions than men (23, 24). Despite this, women have a poorer prognosis compared with men, suggesting the existence of female-specific pattern of coronary artery disease with a high frequency of coronary microvascular dysfunction (23, 24).
In the current study, we observed sex-specific differences in SNPs, and some duplication in the gene regions of vasculature and MYH15 without overlap of SNP between women and men. Sex-specific differences in microvascular blood flow and vasodilatory capacity are observed very early in development. In a study on skin microcirculation in newborn preterm (24–28 weeks) infants, female infants had a lower baseline flow than males (25) This suggests that the mechanism of myocardial ischemia in women may be localized to the microvascular coronary arteries, and that abnormal microvascular function may have prognostic implications (26).
Polymorphisms in MYH15 are associated with MI and an increased risk of CHD (27). MYH15 encodes myosin heavy polypeptide 15 (27). Further studies are needed to clarify how MYH15 might be involved in vascular biology or how this polymorphism in the downstream region of MYH15 affects the risk of vascular disease between women and men.
In men, the gene variant of NT5E is associated with abnormal CFR. Mutations in NT5E are associated with arterial calcification (28). This gene encodes CD73, which converts adenosine monophosphate (AMP) to adenosine, supporting a role for this metabolic pathway in inhibiting vascular calcification (28, 29). CD73 deficiency leads to reduction in extracellular adenosine levels, causing vascular calcification. Double knockout mice for the NT5E gene have reduced but not absent levels of adenosine (30) indicating NT5E SNPs role in microvascular dysfunction.
Han et al (31) described sex differences in atheroma burden and CFR in patients with early coronary atherosclerosis, demonstrating that men have greater atheroma burden and more eccentric atheroma than women and that CFR was significantly lower in women than in men. Gene variant in NT5E may play a role in vascular calcification and function of adenosine, and its role may depend on sex-specific differences. NT5E SNP rs6922 effect on risk is independent of all known risk factors, including dyslipidemia, hypertension, diabetes, obesity, and markers of inflammation; this implies a new biologic pathway that is relevant to CHD in that its effects on adenosine working normally as a vasodilator might possibly be lacking and subsequently lead to earlier development of coronary microvascular dysfunction in patients with this SNP.
The aspects that may account for differences in outcomes between women and men are related to vascular genetic and biological factors such as a smaller atheroma burden and slower progression in women, lower CFR, more vascular stiffness, differences in remodeling, and functional differences of smooth muscle cells in the wall.
Clinical implications
The vast majority of cardiovascular diseases are polygenic, with both heritable and environmental contributions. Familial segregation of cardiovascular disease suggests that these diseases share a common genetic predisposition that interact with the environment and may predispose individuals to vascular disorders, which manifest at different time points throughout life. This study suggests that the mechanism of the development of microvascular dysfunction seen in men may be different than that in women and may suggest that treatment options will need to be tailored in specific ways.
While most SNPs that we identified as significantly associated with abnormal CFR lie within currently presumed non-coding intronic sequences, there are several potential mechanisms that could explain their association. These SNPs may be in linkage disequilibrium with promoter SNPs that have not yet been identified or that were not genotyped in this study. Furthermore, these intronic SNPs may have promoter functions that have not yet been identified, and intronic variants may potentially affect receptor function through alternative splicing mechanisms. SNPs in 5’ upstream region could play a significant role in affecting gene transcription, and those in 3’ untranslated region (3’ UTR) can change mRNA stability and participate in the development of human disease.
Limitations
This study has several limitations. First, this is a cross-sectional study and we have not studied follow-up of cardiovascular events in these patients. Second, the sample size is relatively limited and therefore significance level used (0.005) does not fully account for the multiple testing issues with the SNPs. Given 1185 SNPs and the correlation between the SNPs, the significance level should theoretically be set at 7.1e-5 in order to retain a type I level of 0.05. However, in order to detect odd’s ratios that are clinically reasonable in the size of 1.5–2, this study would need to be triple the sample size. Therefore, we have chosen a lower significance cut-off, recognizing that there are false positive results that need to be validated n additional cohorts. Third, we limited analysis to Caucasian subjects, as we had insufficient number of non-Caucasian subjects to date to allow for statistical accommodation of gene admixture. Our findings were not definitive and should be viewed as exploratory until further validation studies are conducted in other Caucasian populations with microvascular dysfunction. While we have indicated regions that contain genetic variants independently associated with coronary microvascular dysfunction, there is considerable linkage disequilibrium within these regions and the SNPs identified are only indicators that there may be associations in these regions. We do not have a validation set. Because of the challenge of obtaining such a physiologically quantified group and because the assessment of CFR with adenosine in patients without CAD is performed in very few centers, we do not have a validation set. To our knowledge, we are the only center performing CFR assessment with adenosine as well as collecting blood samples on patients without CAD. Finally, our findings do not identify mechanistic pathways that link identified SNP associations to development of coronary microvascular dysfunction. Further investigation is required to determine how these SNPs associations identified in this study relate to development of coronary microvascular dysfunction.
CONCLUSIONS
This clinical study suggests that genetic variation within defined regions of VEGFA and CDKN2B-AS1 genes are associated with coronary microvascular dysfunction. Furthermore, there are sex-specific differences in genetic variation in alleles MYH15, VEGFA, and NT5E which are associated with an increased risk of coronary microvascular dysfunction in men. Finally, there is no overlap of SNPs related to microvascular dysfunction when the analysis is run separately for women and men. Our findings may help focus research on novel genes and pathways involving the microvasculature and its role in the pathogenesis and development of CHD, and lead to potential future directed therapy.
Supplementary Material
AKNOWLEDGEMENTS
none
Sources of Support: This work was supported by the National Institute of Health (NIH Grant HL-92954 and AG-31750 to Amir Lerman and NIH grant HL-77131 to Lilach Lerman) and the Mayo Foundation; CTSA (Grant Number KL2 RR024151 to Patricia Best); Satoshi Yoshino was supported by Fukuda Foundation for Medical Technology, JAPAN.
PB: NIH grant: KL2 RR024151; JMC: NIH grant: CA 15083; LL: Consultancy: AstraZeneca Pharmaceutical; Ongoing Grants: NIH, Stealth Peptides, Inc – research grant support and AHA – Post Doc fellowship support to fellow; Patents pending – Bendavia, humanin (peptides not related to this study); Travel/ accommodations/ meeting expenses unrelated to activities listed – accommodation at scientific meetings in past for international meetings with other academic institutions. AL: Consultancy: Itamar medical (ongoing).
Footnotes
Conflict of Interest: No other authors have anything to disclose.
REFERENCES
- 1.O'Donnell CJ, Nabel EG. Genomics of cardiovascular disease. N Engl J Med. 2011 Dec 1;365(22):2098–2109. doi: 10.1056/NEJMra1105239. [Historical Article Review] [DOI] [PubMed] [Google Scholar]
- 2.McPherson R. Chromosome 9p21 and coronary artery disease. N Engl J Med. 2010 May 6;362(18):1736–1737. doi: 10.1056/NEJMcibr1002359. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Zheng Y, Zhang W, Yu H, Lou K, Zhang Y, Qin Q, Zhao B, Yang Y, Hui R. Polymorphisms of KDR gene are associated with coronary heart disease. Journal of the American College of Cardiology. 2007 Aug 21;50(8):760–767. doi: 10.1016/j.jacc.2007.04.074. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 4.Kothawade K, Bairey Merz CN. Microvascular coronary dysfunction in women: pathophysiology, diagnosis, and management. Curr Probl Cardiol. 2011 Aug;36(8):291–318. doi: 10.1016/j.cpcardiol.2011.05.002. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikram MK, Sim X, Jensen RA, Cotch MF, Hewitt AW, Ikram MA, Wang JJ, Klein R, Klein BE, Breteler MM, Cheung N, Liew G, Mitchell P, Uitterlinden AG, Rivadeneira F, Hofman A, de Jong PT, van Duijn CM, Kao L, Cheng CY, Smith AV, Glazer NL, Lumley T, McKnight B, Psaty BM, Jonasson F, Eiriksdottir G, Aspelund T, Harris TB, Launer LJ, Taylor KD, Li X, Iyengar SK, Xi Q, Sivakumaran TA, Mackey DA, Macgregor S, Martin NG, Young TL, Bis JC, Wiggins KL, Heckbert SR, Hammond CJ, Andrew T, Fahy S, Attia J, Holliday EG, Scott RJ, Islam FM, Rotter JI, McAuley AK, Boerwinkle E, Tai ES, Gudnason V, Siscovick DS, Vingerling JR, Wong TY. Four novel Loci (19q13, 6q24, 12q24, and 5q14) influence the microcirculation in vivo. PLoS Genet. 2010 Oct;6(10):e1001184. doi: 10.1371/journal.pgen.1001184. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pepine CJ, Theodore E. Woodward Award. Ischemic heart disease in women: the role of coronary microvascular dysfunction. Trans Am Clin Climatol Assoc. 1999;110:107–116. [Comparative Study Research Support, U.S. Gov't, P.H.S.]. discussion 17–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Sieber FE, Hurn P, Alkayed NJ, Traystman RJ. Gender-based differences in Na+ -K+ adenosine triphosphatase activity occur in the microcirculation of the diabetic rat brain. Anesthesiology. 2001 Feb;94(2):372–375. doi: 10.1097/00000542-200102000-00037. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 8.Duvernoy CS, Meyer C, Seifert-Klauss V, Dayanikli F, Matsunari I, Rattenhuber J, Hoss C, Graeff H, Schwaiger M. Gender differences in myocardial blood flow dynamics: lipid profile and hemodynamic effects. J Am Coll Cardiol. 1999 Feb;33(2):463–470. doi: 10.1016/s0735-1097(98)00575-0. [Comparative Study Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 9.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000 Mar 7;101(9):948–954. doi: 10.1161/01.cir.101.9.948. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 10.Hasdai D, Gibbons RJ, Holmes DR, Jr, Higano ST, Lerman A. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation. 1997 Nov 18;96(10):3390–3395. doi: 10.1161/01.cir.96.10.3390. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 11.Hamasaki S, Al Suwaidi J, Higano ST, Miyauchi K, Holmes DR, Jr, Lerman A. Attenuated coronary flow reserve and vascular remodeling in patients with hypertension and left ventricular hypertrophy. J Am Coll Cardiol. 2000 May;35(6):1654–1660. doi: 10.1016/s0735-1097(00)00594-5. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 12.Al Suwaidi J, Higano ST, Holmes DR, Jr, Lennon R, Lerman A. Obesity is independently associated with coronary endothelial dysfunction in patients with normal or mildly diseased coronary arteries. J Am Coll Cardiol. 2001 May;37(6):1523–1528. doi: 10.1016/s0735-1097(01)01212-8. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 13.Nishimura RA, Lerman A, Chesebro JH, Ilstrup DM, Hodge DO, Higano ST, Holmes DR, Jr, Tajik AJ. Epicardial vasomotor responses to acetylcholine are not predicted by coronary atherosclerosis as assessed by intracoronary ultrasound. J Am Coll Cardiol. 1995 Jul;26(1):41–49. doi: 10.1016/0735-1097(95)00142-m. [DOI] [PubMed] [Google Scholar]
- 14.Lerman A, Holmes DR, Jr, Bell MR, Garratt KN, Nishimura RA, Burnett JC., Jr Endothelin in coronary endothelial dysfunction and early atherosclerosis in humans. Circulation. 1995 Nov 1;92(9):2426–2431. doi: 10.1161/01.cir.92.9.2426. [Clinical Trial Research Support, Non-U.S. Gov't Research Support, U.S.Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 15.Oliphant A, Barker DL, Stuelpnagel JR, Chee MS. BeadArray technology: enabling an accurate, cost-effective approach to high-throughput genotyping. Biotechniques. 2002 Jun;(Suppl):56–58. 60–61. [Research Support, U.S.Gov't, P.H.S.] [PubMed] [Google Scholar]
- 16.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007 Sep;81(3):559–575. doi: 10.1086/519795. [Research Support,. N.I.H., Extramural Research Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abhary S, Burdon KP, Gupta A, Lake S, Selva D, Petrovsky N, Craig JE. Common sequence variation in the VEGFA gene predicts risk of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009 Dec;50(12):5552–5558. doi: 10.1167/iovs.09-3694. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 18.Hlatky MA, Quertermous T, Boothroyd DB, Priest JR, Glassford AJ, Myers RM, Fortmann SP, Iribarren C, Tabor HK, Assimes TL, Tibshirani RJ, Go AS. Polymorphisms in hypoxia inducible factor 1 and the initial clinical presentation of coronary disease. Am Heart J. 2007 Dec;154(6):1035–1042. doi: 10.1016/j.ahj.2007.07.042. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 19.Mu H, Ohashi R, Lin P, Yao Q, Chen C. Cellular and molecular mechanisms of coronary vessel development. Vasc Med. 2005 Feb;10(1):37–44. doi: 10.1191/1358863x05vm584ra. [Research Support, N.I.H., Extramural Research Support, U.S. Gov't, P.H.S. Review] [DOI] [PubMed] [Google Scholar]
- 20.Schaefer AS, Richter GM, Dommisch H, Reinartz M, Nothnagel M, Noack B, Laine ML, Folwaczny M, Groessner-Schreiber B, Loos BG, Jepsen S, Schreiber S. CDKN2BAS is associated with periodontitis in different European populations and is activated by bacterial infection. J Med Genet. 2011 Jan;48(1):38–47. doi: 10.1136/jmg.2010.078998. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 21.Broadbent HM, Peden JF, Lorkowski S, Goel A, Ongen H, Green F, Clarke R, Collins R, Franzosi MG, Tognoni G, Seedorf U, Rust S, Eriksson P, Hamsten A, Farrall M, Watkins H. Susceptibility to coronary artery disease and diabetes is encoded by distinct, tightly linked SNPs in the ANRIL locus on chromosome 9p. Hum Mol Genet. 2008 Mar 15;17(6):806–814. doi: 10.1093/hmg/ddm352. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 22.Harismendy O, Notani D, Song X, Rahim NG, Tanasa B, Heintzman N, Ren B, Fu XD, Topol EJ, Rosenfeld MG, Frazer KA. 9p21 DNA variants associated with coronary artery disease impair interferon-gamma signalling response. Nature. 2011 Feb 10;470(7333):264–268. doi: 10.1038/nature09753. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olivetti G, Giordano G, Corradi D, Melissari M, Lagrasta C, Gambert SR, Anversa P. Gender differences and aging: effects on the human heart. J Am Coll Cardiol. 1995 Oct;26(4):1068–1079. doi: 10.1016/0735-1097(95)00282-8. [Comparative Study Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 24.Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Pepine CJ, Mankad S, Sharaf BL, Rogers WJ, Pohost GM, Lerman A, Quyyumi AA, Sopko G. Insights from the NHLBI-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Study: Part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006 Feb 7;47(3 Suppl):S21–S29. doi: 10.1016/j.jacc.2004.12.084. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Review] [DOI] [PubMed] [Google Scholar]
- 25.McMurray J, McDonagh T, Morrison CE, Dargie HJ. Trends in hospitalization for heart failure in Scotland 1980–1990. Eur Heart J. 1993 Sep;14(9):1158–1162. doi: 10.1093/eurheartj/14.9.1158. [DOI] [PubMed] [Google Scholar]
- 26.Vaccarino V, Badimon L, Corti R, de Wit C, Dorobantu M, Hall A, Koller A, Marzilli M, Pries A, Bugiardini R. Ischaemic heart disease in women: are there sex differences in pathophysiology and risk factors? Position paper from the working group on coronary pathophysiology and microcirculation of the European Society of Cardiology. Cardiovasc Res. 2011 Apr 1;90(1):9–17. doi: 10.1093/cvr/cvq394. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luke MM, O'Meara ES, Rowland CM, Shiffman D, Bare LA, Arellano AR, Longstreth WT, Jr, Lumley T, Rice K, Tracy RP, Devlin JJ, Psaty BM. Gene variants associated with ischemic stroke: the cardiovascular health study. Stroke. 2009 Feb;40(2):363–368. doi: 10.1161/STROKEAHA.108.521328. [Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.St Hilaire C, Ziegler SG, Markello TC, Brusco A, Groden C, Gill F, Carlson-Donohoe H, Lederman RJ, Chen MY, Yang D, Siegenthaler MP, Arduino C, Mancini C, Freudenthal B, Stanescu HC, Zdebik AA, Chaganti RK, Nussbaum RL, Kleta R, Gahl WA, Boehm M. NT5E mutations and arterial calcifications. N Engl J Med. 2011 Feb 3;364(5):432–442. doi: 10.1056/NEJMoa0912923. [Research Support, N.I.H., Intramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Markello TC, Pak LK, St Hilaire C, Dorward H, Ziegler SG, Chen MY, Chaganti K, Nussbaum RL, Boehm M, Gahl WA. Vascular pathology of medial arterial calcifications in NT5E deficiency: implications for the role of adenosine in pseudoxanthoma elasticum. Mol Genet Metab. 2011 May;103(1):44–50. doi: 10.1016/j.ymgme.2011.01.018. [Case Reports Research Support, N.I.H., Intramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Street SE, Kramer NJ, Walsh PL, Taylor-Blake B, Yadav MC, King IF, Vihko P, Wightman RM, Millan JL, Zylka MJ. Tissue-Nonspecific Alkaline Phosphatase Acts Redundantly with PAP and NT5E to Generate Adenosine in the Dorsal Spinal Cord. J Neurosci. 2013 Jul 3;33(27):11314–11322. doi: 10.1523/JNEUROSCI.0133-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han SH, Bae JH, Holmes DR, Jr, Lennon RJ, Eeckhout E, Barsness GW, Rihal CS, Lerman A. Sex differences in atheroma burden and endothelial function in patients with early coronary atherosclerosis. Eur Heart J. 2008 Jun;29(11):1359–1369. doi: 10.1093/eurheartj/ehn142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


