Abstract
Mac-1 dependent crawling is a new step in the leukocyte recruitment cascade which follows LFA-1 dependent adhesion and precedes emigration. Neutrophil adhesion via LFA-1 has been shown to induce cytoskeletal reorganization through Vav1-dependent signaling, and the current study investigates the role of Vav1 in the leukocyte recruitment process in vivo with particular attention to the events immediately downstream of LFA-1 dependent adhesion. Intravital and spinning-disk-confocal microscopy was used to investigate intravascular crawling in relation to endothelial junctions in vivo in wild-type (WT) and Vav1−/− mice. Adherent WT neutrophils almost immediately began crawling perpendicular to or against blood flow via Mac-1 until they reached an endothelial junction where they often changed direction. This pattern of perpendicular, mechanotactic crawling was recapitulated in vitro when shear was applied. In sharp contrast, the movement of Vav1−/− neutrophils was always in the direction of flow, and appeared more passive as if the cells were dragged in the direction of flow in vivo and in vitro. More than 80% of Vav1−/− neutrophils moved independent of Mac-1 and could be detached with LFA-1 antibodies. An inability to release the uropod was frequently noted for Vav1−/− neutrophils, leading to greatly elongated tails. The Vav1−/− neutrophils failed to stop or follow junctions, and ultimately detached leading to fewer emigrated neutrophils. The Vav1−/− phenotype resulted in fewer neutrophils recruited in a relevant model of infectious peritonitis. Clearly, Vav1 is critical for the complex interplay between LFA-1 and Mac-1 that underlies the programmed intravascular crawling of neutrophils.
Keywords: Neutrophils, chemotaxis, inflammation, rodent
Introduction
Recruitment of circulating leukocytes to the site of inflammation occurs via complex interactions with endothelium; leukocytes tether to, roll along and firmly adhere to the endothelium before transmigrating out of the vasculature (1, 2). In the case of neutrophils, rolling is dependent on selectins, adhesion occurs mainly through β2-integrins and emigration is mediated by integrins as well as PECAM-1, CD99 and JAMs (3, 4, 5, 6, 7). Schenkel and colleagues recently documented another step in the leukocyte recruitment cascade, namely crawling to junctions prior to transendothelial migration (8). Using an in vitro system to visualize monocytes, these investigators reported that cells firmly adhered, flattened and then rapidly sent out pseudopods, and crawled via integrins to junctions where they subsequently transmigrated. The crawling appeared to be random but was ultimately necessary for emigration. More recently, a number of laboratories reported that intraluminal crawling also occurred in vivo (9, 10, 11) and this allowed cells to reach optimal emigration sites at endothelial junctions (10). The crawling of neutrophils did not appear to be biased in the direction of flow suggesting this was not simply a dragging motion due to hydrodynamic force displacement. However, whether the neutrophil crawling was truly a random process or followed an inherent pattern remained unclear, as was the question of whether the crawling was chemotactic, chemokinetic, haptotactic (following chemokine attached to extra cellular matrix) or mechanotactic (driven by mechanical forces).
Neutrophil adhesion and crawling are mediated by two separate molecular mechanisms. Firm adhesion and stabilization (spreading) occurred via αLβ2 integrin (LFA-1), followed immediately by crawling via αMβ2 integrin (Mac-1). Inhibition of LFA-1 prevented adhesion while inhibition of Mac-1 had no effect on adhesion but prevented all subsequent crawling (10). These distinct, molecular steps for LFA-1-induced adhesion followed by Mac-1-induced crawling in vivo, would require exquisite communication (e.g., outside in signaling from LFA-1) for these molecules to function in a sequential, coordinated fashion under flow conditions. Moreover, these in vivo observations bestow added physiologic importance to various in vitro studies that demonstrated that cross linking of LFA-1 leads to outside in signaling and cytoskeletal rearrangements (12, 13) as well as Mac-1 activation (14) presumably leading to crawling. However, intracellular signaling molecules that might be important downstream of adhesion to impact on crawling and resultant emigration have not been identified.
Vav1, a guanine exchange factor for the Rho family GTPases Rac and Cdc42, is a major regulator of the organization of the actin cytoskeleton during leukocyte polarization and migration (15). Importantly, Vav1 regulates activation downstream of LFA-1 in all leukocytes tested including neutrophils, lymphocytes and NK cells (13, 16, 17, 18). Absence of Vav1 under static conditions in vitro leads to impaired neutrophil spreading, membrane ruffling, cytoskeletal rearrangement and polarization. However, the role for Vav1 in crawling becomes equivocal under static conditions in vitro. One group has reported no role for Vav1 in crawling on protein coated coverslips (19), another group observed reduced crawling speed for Vav1−/− cells on plastic (20) whereas a third group reported impaired crawling to some but not other chemoattractants (21). To date no one has examined the role of Vav1 for crawling under the very dynamic shear-dependent in vivo leukocyte recruitment paradigm. In fact, to our knowledge, only one group has visualized leukocyte behavior in vivo in Vav1 deficient mice by adding a brief chemotactic stimulus and examined neutrophil rolling and adhesion for the subsequent 10 min in real time (19). The data revealed no obvious impairment in rolling or adhesion in this very acute setting in Vav1−/− neutrophils; however events downstream of adhesion were not examined.
Using intravital light and multi-channel fluorescence spinning disk confocal microscopy in real time and time-lapse, we systematically examined the transition from adhesion to crawling to emigration in wild-type and Vav1−/− mice. A very striking and consistent Mac-1-dependent crawling behaviour was noted in wild-type mice, which was often perpendicular to blood flow but sometimes changed when an endothelial junction was encountered. In vitro, similar perpendicular crawling was observed in the presence of shear and absence of chemokine gradients on protein coated coverslips suggesting mechanotaxis rather than chemotaxis for the inherent perpendicular crawling behavior. In striking contrast, Vav1−/− neutrophils were unable to crawl perpendicular to the direction of blood flow in vitro and in vivo under physiological shear rates. In fact, adherent Vav1−/− neutrophils appeared to be stretched and dragged in the direction of blood flow. This movement in Vav1−/− mice was Mac-1-independent. Vav1−/− cells also failed to realign and follow any junctions that were not aligned in the direction of flow. This resulted in a greater interval of time for adhesion and crawling in Vav1−/− mice, leading to more detachment and reduced emigration. Finally, these neutrophil recruitment defects translated into significant impairment in recruitment of neutrophils in an infectious disease relevant model of peritonitis.
Materials and methods
All procedures were approved by the University of Calgary Animal Care Committee and conformed to Canadian Council for Animal Care guidelines. Male Vav1+/+ mice (C57Bl/6) and Vav1 deficient mice (C57Bl/6 background, generously provided by Dr. Josef M. Penninger, Institute of Molecular Biotechnology, Vienna, Austria), were anesthetized with 10 mg kg−1 xylazine (Bayer, Inc.) and 200 mg kg−1 ketamin hydrochloride (Biomeda-MTC) i.p. Additional anesthetic was administered through a jugular vein.
Intravital video-microscopy
The behavior of leukocytes in the microcirculation and adjacent tissue was studied in the cremaster muscle preparation through an intravital microscope (Optiphot-2; Nikon, Inc., with a 25/0.35 (E. Leitz, Inc.) or 40/0.75W (Carl Zeiss MicroImaging, Inc. objective) connected to a video camera (5100 HS; Panasonic) (22, 23).
Rolling, adhesion and emigration
The number of rolling, adherent, and emigrated leukocytes in single unbranched venules (25–40 μm in diameter) before and after addition of the chemokine MIP-2 (5nM) to the superfusate was determined using video playback analysis (22, 23). By using MIP-2, more than 90% of the emigrated cells were neutrophils (24).
Crawling
Crawling cells within inflamed vessels (0.5nM MIP-2 superfusion or 0.5μg TNFα in 200μl saline injected intrascraotally 4h prior experiments (23)) were tracked using time-lapse video microscopy. For the best resolution, postcapillary venules ranging from 18–30 μm were imaged in the cremaster, and the 40x water immersion objective was used. The distance of cells crawling within the vessel was measured from when they first adhered until they emigrated or detached and crawling velocity was calculated. Centerline red blood cell velocity (Vrbc) was measured online using an optical Doppler velocimeter (Microcirculation Research Institute, Texas A&M University). Mean red blood cell velocity (Vmean= Vrbc/1.6) assuming cylindrical geometry was used to calculate venular wall shear rate based on the Newtonian definition γ= 8(Vmean/diameter). For blocking experiments, monoclonal antibodies against Mac-1 (30 μg per mouse, eBioscience) or LFA-1 (30 μg per mouse, eBioscience) were given intravenously during experiments. Isotype controls have previously been shown not to affect the measured parameters (10).
Spinning disk confocal microscopy
Endothelial junctions were labeled with monoclonal anti–PECAM-1 (50 ug per mouse; Fitzgerald Industries) conjugated to Alexa Fluor 555 (Molecular Probes) and neutrophils were stained with FITC-labeled GR-1 (40 μg per mouse; eBioscience), a dose previously shown not to affect neutrophil recruitment (10). Images were acquired with an upright microscope (BX51, Olympus) using a 20x/0.95W NA water dipping XLUM Plan F1 objective. The microscope was equipped with a confocal light path (WaveFx, Quorum) based on a modified Yokogawa CSU-10 head (Yokogawa Electric Corporation), and 488 or 561 nm laser excitation (Cobalt, Stockholm, Sweden) was used with the appropriate long pass filters (Semrock). An EMCCD camera (C9100-13, Hamamatsu) was used for fluorescence detection. Volocity Acquisition software (Improvision) was used to drive the confocal microscope.
Circulating leukocyte counts and experimental peritonitis
Peptidoglycan (PGN) from Staphylococcus aureus (Fluka) was sonicated for 1h and injected i.p. After 4 h, mice were anesthetized with Isoflurane (inhaled anesthetic, Bimeda-MTC) and blood was collected via cardiac puncture with a heparinized syringe for circulating leukocyte counts. Next, the peritoneum was lavaged with 3 ml of PBS. Exudate cells were recovered following a 60-second manual massage, and counted with a hemacytometer. Leukocyte differentials were determined from cytospins and Wright-Giemsa staining (25).
Murine neutrophil isolation
Blood was collected as described above, and centrifuged (500 g, 4°C, 15 min). The resulting plasma was diluted to 10% in HBSS and stored at 4°C until needed. Mice were then euthanized and the ends of the femurs and tibias were resected. The bone marrow was removed by perfusion of 5 ml ice-cold PBS and then suspended through a 20-gauge needle. Marrow cells were pelleted in a centrifuge (250 g, 4°C, 12 min) and resuspended in 2 ml PBS. The neutrophils (1.0 × 106 cells ml−1) were isolated over a discontinuous Percoll gradient (stock: 90 ml Percoll, 10 ml 10× HBSS, diluted to 72, 64, and 52% in PBS) by centrifugation (1100 g, 4°C, 30 min) resulting in a band of neutrophils between the 72 and 64% layers.
In vitro Flow Chamber
Glass coverslips were coated with murine plasma by first marking an area in the center of the slide 15 mm × 25 mm in size, with a hydrophobic pen. 200 μl of the 10% murine plasma in HBSS was pipetted into the marked area and then the slides were incubated for 4 hours at room temperature. The slides were then washed 2 times with room temperature HBSS where after 100 μl of isolated murine neutrophils plus 100 μl of fresh HBSS were placed onto the plasma-coated region. The slides were incubated (15 min in a 5% CO2 37 °C), followed by 5 min stimulation either by 10 nM MIP-2, or with vehicle (PBS).
To assay adhesive strength, the slides were mounted in a parallel-plate flow chamber (interior dimensions: 11×22×0.17 mm) connected to a pump, and placed on a microscope (Axiovert, Zeiss) with a heated stage, and imaged using a 20x lens (Zeiss 20/0.30). 37°C HBSS was perfused through the chamber at 10 dynes cm−2, and the results recorded by a DVD recorder for 5 min. Still images of the video were captured every 30 sec using PowerDVD software, imported into ImageJ (U. S. National Institutes of Health, http://rsb.info.nih.gov/ij/), and the number of neutrophils in each frame quantified using the “Analyze Particles” feature. To quantify the rate of neutrophil detachment, the number of cells in each frame was standardized to the number of cells present in the same field of view before flow was initiated.
To assay the role shear plays in mediating intravascular crawling, the preparation was recorded for 5 min without flow. Flow (2 dynes cm−2) was then initiated, and the cells in the chamber were recorded for 5 min. The flow was then stopped and the cells were recorded for an additional 5 min. The videos were time lapsed 600 × (1 frame = 20 sec) and data were split into three parts, corresponding to the pre-flow, flow and post-flow periods.
Average speed was calculated by measuring the total distance traveled by the cell and then dividing that by the length of each time period (5 min). The displacement was calculated by determining the location of each cell at the beginning and end of each time period. The total distance moved along the x-axis (parallel to flow, Δx) and along the y-axis (perpendicular to flow, Δy) was then calculated for each cell. The displacement from flow (DFF) was calculated by dividing Δy by Δx, resulting in a DFF of 1.0 for randomly moving cells; DFF <1.0 for cells moving with flow, and DFF >1.0 for cells moving perpendicular to flow.
Statistics
All data are presented as mean ± standard error of the mean (SEM). ANOVA, single-factor, non-repeated measures followed by Fisher protected least significant difference test was performed for multiple comparisons. For the in vitro data, single factor ANOVA was used with Tukeys test. p<0.05 was deemed statistically significant.
Results
Crawling differs greatly in WT and Vav1−/− mice
Table 1 shows that no neutrophil parameter upstream of adhesion was affected by Vav1 deficiency. In wild-type mice, approximately 80 cells min−1 rolled in unstimulated blood vessels at a rolling velocity of about 35–40 μm sec−1 (Table 1). Vav1−/− mice had similar basal values for rolling flux and rolling velocity, and rolling flux decreased in both groups to approximately 40 cells min−1 following exposure to MIP-2, a ligand for CXCR2 (Table 1). Under basal conditions, very few neutrophils adhered in blood vessels in either strain of mice. However upon exposure to MIP-2, wild-type neutrophils adhered rapidly and within the first minute post-adhesion, began to crawl (Figure 1A). Although it would be reasonable to expect the shear forces to induce neutrophil crawling in the direction of flow, it became apparent that in most instances neutrophils crawled perpendicular to blood flow (Figure 1A, supplemental video 1). In fact, only about 20% of neutrophils initially crawled in the direction of blood flow (Figure 1C). By contrast Vav1−/− neutrophils almost always moved in the direction of blood flow, rarely deviating from this path (Figure 1B and C). The Vav1−/− neutrophils but not WT neutrophils were greatly elongated, with a uropod often longer than the rest of the body of the neutrophil (Figure 1D and E). The Vav1−/− neutrophils were stretched in the direction of flow. Rather than the amoeboid crawling seen in wild-type mice, in Vav1−/− mice a motion more reminiscent of dragging was noted (see supplemental video 2).
Table 1.
Leukocyte-endothelial cell interactions before and after addition of MIP-2 (5nM) to the superfusate. All values are means± SEM of n=7 (C57Bl/6) and n=5 (Vav1−/−).
| Rolling flux (cells/min) | Rolling velocity (μm/s) | |||
|---|---|---|---|---|
|
|
||||
| C57Bl/6 | Vav1−/− | C57Bl/6 | Vav1−/− | |
|
|
||||
| Pre-MIP-2 | 79±4 | 60±9 | 37±5 | 45±2 |
|
|
||||
| 30min Post MIP-2 | 44±7 | 49±11 | 37±3 | 36±3 |
|
|
||||
| 60min post MIP-2 | 44±11 | 42±14 | 32±4 | 30±6 |
|
|
||||
Figure 1. Vav1 deficient but not WT neutrophils migrate intravascularly in the direction of blood flow.
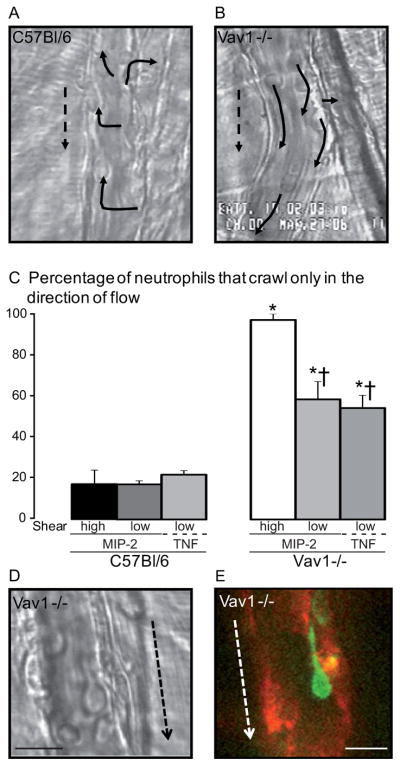
A, B and D are micrographs of MIP-2 (0.5 nM) stimulated venules with adherent neutrophils; dotted arrows indicate the direction of the blood flow. Panels A and B show C57Bl/6 or Vav1−/− venules respectively and solid arrows mark where the adherent cells migrated within the vessel (crawled). The percentages of wild-type (C57Bl/6) or Vav1−/− cells crawling only in the direction of blood flow in MIP-2 or TNF activated vessels with high shear or low shear vessels are shown in panel C, at least 60 cells were analyzed for each strain. The high and low shear was 787±106 s−1 or 860±218 s−1and 279±16 s−1 or 271±86 s−1 for MIP-2 treated C57Bl/6 and Vav1 −/− respectively. Panels D and E show Vav1 −/− neutrophils elongated in the direction of blood flow (arrow). In panel E the neutrophil is marked with GR-1 (green) and endothelial junctions with PECAM-1 (red). All values are means ± SEM and * p<0.05 compared to C57Bl/6 and † p<0.05 compared to Vav1−/− high shear.
To determine whether the shear was mediating the direction of movement of neutrophils, we examined neutrophil crawling in low and high shear. Physiologic shear in postcapillary venules of the cremaster ranges between 200 s−1 and 1000 s−1. The mean value of the observed high shear rate venules (WT 787±106 s−1 and Vav1−/− 860±218 s−1, calculated from the mean red blood cell velocity) and the low shear rate venules (WT 279±16 s−1 and Vav1−/− 271±86 s−1) were not significantly different among the two groups. The percentage of WT neutrophils that crawled only in the direction of blood flow was approximately 20% in both high and low shear vessels (Figure 1C). By contrast, nearly 100% of Vav1−/− neutrophils oriented with blood flow in high shear vessels. In low shear vessels some Vav1−/− neutrophils did appear to crawl in directions other than that of blood flow, however the majority of cells still oriented in the same direction as flow. To investigate if neutrophil crawling behavior would be influenced by endothelial activation, WT and Vav1−/− mice were pretreated with TNFα, which has previously been shown to reduce velocity of red blood cells and venular shear (23). No difference in crawling behavior compared to MIP-2 superfusion in low shear venules could be detected, as only 22±2% of WT neutrophils oriented with flow, but still the majority of Vav1−/− cells (54±6 %) crawled only in the direction of blood flow. These data suggest that at sufficiently low shear, some Vav1−/− neutrophils can crawl perpendicularly but at physiologic shear these cells mostly oriented in the direction of flow.
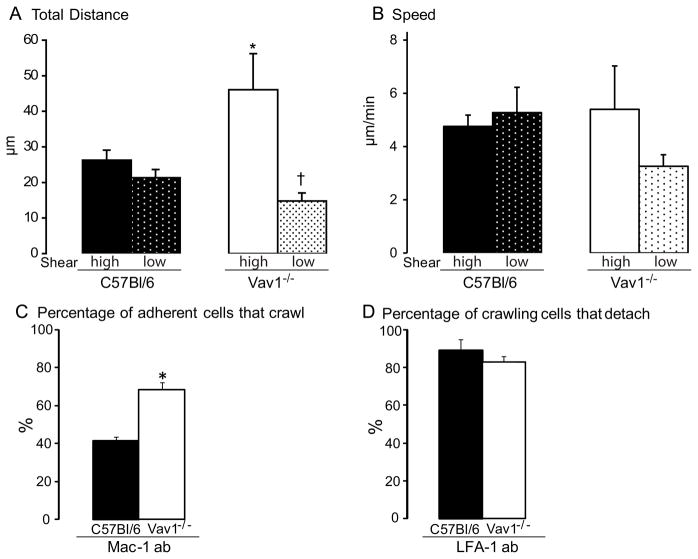
The total displacement was also different among the two experimental mouse strains. WT neutrophils crawled approximately 25 μm, whereas Vav1−/− neutrophils were displaced almost 50 μm in high shear venules (Figure 2A). The total distance wild-type neutrophils crawled was unaffected by shear (Figure 2A). By contrast, the distance that Vav1−/− neutrophils moved in the low shear vessels was greatly reduced, and comparable to wild-type cells (Figure 2A). The speed of the displacement did not differ between the WT and Vav1−/− neutrophils (Figure 2B). Because of the substantial phenotypical difference in motility between wild-type and Vav1−/− mice, with a more passive less amoeboid type crawling in the latter, we examined whether the Mac-1-dependent molecular mechanism was being used in Vav1−/− neutrophils. Surprisingly, addition of Mac-1 antibody to Vav1−/− neutrophils had little effect upon crawling (Figure 2C), while in WT neutrophils, more than 50% of already crawling cells were induced to stop. This strongly supports the view that unlike WT neutrophils, Vav1−/− neutrophils do not engage Mac-1 to crawl. Addition of anti-LFA-1 antibody caused an ~80% decrease in the number of adherent WT and Vav1−/− neutrophils. This was entirely due to prevention of new adhesion in both groups of mice (Figure 2D). The few cells that still adhered continued to crawl (WT) or moved in the direction of blood flow (Vav1−/−), suggesting a very limited role for LFA-1 in crawling in these cells.
Figure 2. Time-lapse microscopy of inflamed (MIP-2, 0.5 nM) postcapillary venules with high (787±106 s−1) or low shear (A and B) and number of crawling cells after Mac-1 or LFA-1 blocking antibody (C and D).
Panel A shows the total distance neutrophils crawled and the speed of the crawling (B) was calculated by dividing distance crawled with the time the cells were followed. The high and low shear was 787±106 s−1 or 860±218 s−1and 279±16 s−1 or 271±86 s−1 for C57Bl/6 and Vav1 −/− respectively. C shows the percentage of adherent cells that crawled after Mac-1 blocking antibody. Panel D show the percentage of the crawling cells that detached 20 minutes after administration of LFA-1 blocking antibody. All values are means ± SEM and * p<0.05 compared to C57Bl/6 high and low shear and † p<0.05 compared to C57Bl/6 and Vav1−/− high shear.
Mechanotaxis is impaired in Vav1−/− neutrophils
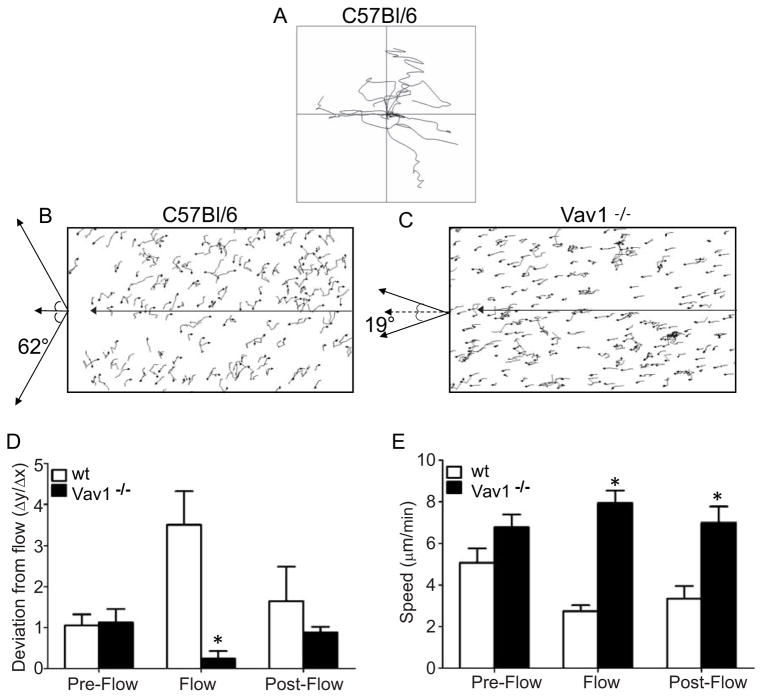
To further examine the mechanism(s) underlying crawling behavior, we examined Vav1−/− and WT neutrophil displacement in vitro in parallel-plate flow chambers in which the shear stress could be reproducibly applied to adherent neutrophils in the absence of endothelium to eliminate any potential chemotactic gradient on the surface of this substratum. Wild-type neutrophils were first placed on a plasma protein coated coverslip which we previously demonstrated has ligands for LFA-1, Mac-1 and α4-integrin (26). Under static (non-flow) conditions, WT (Figure 3A) and Vav1−/− (data not shown) neutrophils migrated randomly in all directions. When shear was applied all WT neutrophils, almost in unison, began to crawl perpendicularly to flow (Figure 3B). On average the neutrophils crawled at 62° relative to flow direction. Since no chemotactic or haptotactic gradient was present in this assay, the perpendicular movement was clearly a mechanotactic response. Perpendicular crawling in response to flow was not seen for the Vav1−/− neutrophils, which instead were oriented in the direction of flow when shear was applied (Figure 3C), in accordance with our in vivo observation (Figure 1B and C). On average, Vav1−/− neutrophils deviated from flow by only 19°.
Figure 3. Effect of flow on the direction of IL-8 stimulated neutrophil crawling in vitro.
Panels A shows random migration with frequent turns of wild-type neutrophils before flow is initiated. When flow is initiated (2 dynes cm−2) the majority of WT neutrophils migrate perpendicular to the direction of flow (B). Arrow indicates direction of flow. Vav1−/− neutrophils are unable to migrate in opposition to flow, and are observed to move strictly downstream of flow (C). Panel D shows the deviation from flow for WT and Vav1−/− neutrophils (y/x), and flow induces directional migration. Prior to flow neutrophils migrate randomly (Pre-Flow), but when flow is initiated cells move in a preferential manner which for the wt neutrophils is perpendicular to flow (Flow), while the Vav1−/− neutrophils preferentially follow the direction of flow. When flow is stopped this directional migration ceases and the cells resume random migration (Post-Flow). Panel E shows the speed of crawling for WT and Vav1−/− neutrophils (μm min−1). Flow induces a decrease in the migratory speed of wt neutrophils, while Vav1−/− neutrophils have a significantly higher speed. All values are means ± SEM and n=3 in E and F * p<0.05 compared to wt.
These data are quantitatively summarized in Figure 3D and 3E as deviation from the direction of flow and the crawling speed respectively. We approximate the displacement in the flow chambers as occurring in two dimensions within the plane of the flow, where the displacement in x and y, Δx and Δy, are defined as the displacement parallel and perpendicular to the direction of flow, respectively. Figure 3D demonstrates that prior to flow, neutrophils crawled equally in the y and x direction (Δy/Δx = 1). Upon introduction of flow in the x direction the cells re-oriented perpendicular to flow such that the majority of crawling occurred in the y direction (Δy/Δx > 1). In fact, WT neutrophils were 4 times more prone to crawl in the y direction than the x direction. Once flow was terminated, the cells returned to random migration in all directions. Although, the Vav1−/− neutrophils also migrated randomly under static conditions (Figure 3D), application of shear caused the exact opposite effect; the cells now moved with much greater propensity in the x direction (with flow) than in the y direction (perpendicular to flow). Once flow was terminated, the cells returned to a random crawling behavior. Figure 3E demonstrates that WT and Vav1−/− neutrophils crawled with essentially the same speed in static conditions. However, when shear was applied, the speed with which WT neutrophils crawled was altered whereas Vav1−/− neutrophils did not decrease their speed. Interestingly, although random migration was restored upon cessation of shear, the reduced speed was not immediately reversed in WT neutrophils.
Mechanotaxis leads neutrophils to junctions
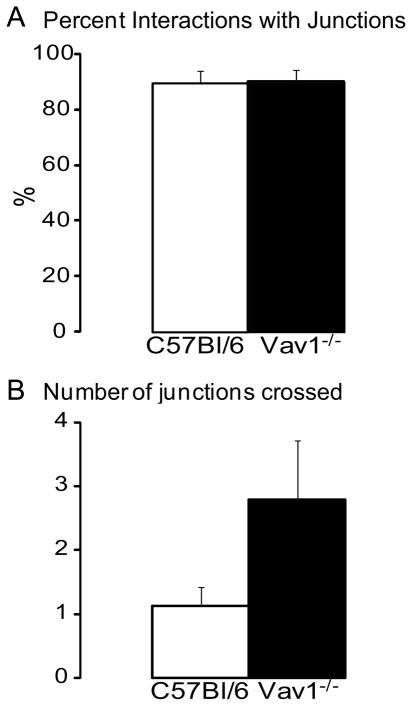
Next we examined the importance of the mechanotaxic program of the crawling cells. Using intravital multichannel spinning disk microscopy, we noted that 90% of crawling neutrophils did follow endothelial junctions at some point during their recruitment (Figure 4A, supplemental video 1) accounting for sudden radical changes in direction that were observed. If neutrophils adhered at junctional regions, they often began following the junction regardless of direction. In WT mice the cells found an emigration site quickly and rarely crossed additional junctions (Figure 4B). By contrast, Vav1−/− neutrophils often crossed junctions (Figure 4B) that would have required radical directional changes for the neutrophils to initiate junctional crawling. Vav1−/− neutrophils eventually followed junctions but always in the direction of flow (Figure 4A).
Figure 4. In vivo data showing where neutrophils crawled in relation to junctions.
Panel A shows the percent interactions with junctions and panel B show how many junctions that were crossed during crawling. All values are means ± SEM.
Sustained Adhesion is Impaired in Vav1−/− cells
Intravital microscopy of the cremaster muscle microcirculation revealed that adhesion increased tenfold in wild-type mice in response to MIP-2 (5nM, Figure 5A). Although Vav1−/− cells also adhered, they were not able to sustain adhesion for 30 sec. Since 30 sec is by definition deemed adhesion, the overall adhesion was significantly impaired for Vav1−/− neutrophils (Figure 5A) as a result of detachment. Figure 5B summarizes that during the adhesion and crawling process leading ultimately to emigration, approximately 20% of WT neutrophils detached whereas close to 60% of Vav1−/− neutrophils detached (Figure 5B). To determine whether detachment was also increased in vitro, we used a parallel-plate flow chamber, coated with a multi-integrin ligand substratum (plasma proteins). WT and Vav1−/− neutrophils adhered equally well under static conditions. When high shear (10 dynes/cm2) was applied, unstimulated WT and Vav1−/− neutrophils both adhered poorly, with nearly 50% of neutrophils detaching during 5 minutes of flow (Figure 5C). MIP-2 stimulation resulted in an enhanced ability of WT but not Vav1−/− neutrophils to resist high shear (Figure 5C). Vav1−/− neutrophils detached significantly more than WT neutrophils, entirely consistent with the Vav1−/− neutrophils detaching more in vivo (Figure 5B).
Figure 5. Vav1−/− neutrophils adhere poorly both in vivo and in vitro.
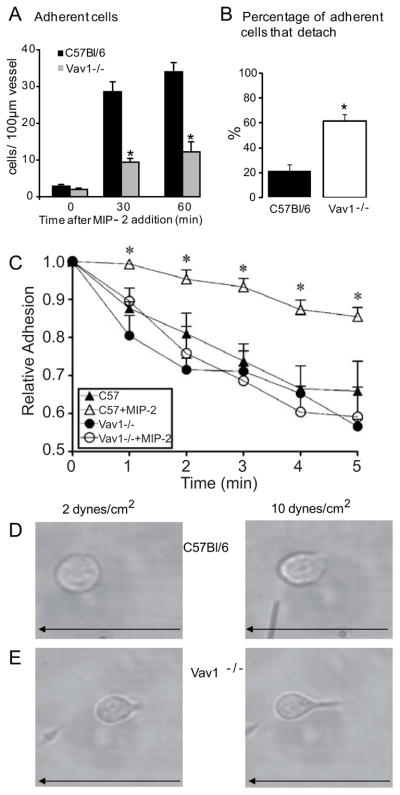
Panel A shows number of adherent cells in vivo (stationary for more than 30 s within a 100 μm length of venule during 5 min) in C57Bl/6 (n=7) and Vav1−/− (n=5) before (time 0) or 30 and 60 min after addition of MIP-2 (5 nM) in the superfusate. Panel B show the percentages of the adherent cells that detached. Panel C show the relative adhesion of wild-type (C57Bl/6) and Vav1−/− neutrophils in vitro under flow, with and without 10 nM MIP-2 pre-stimulation (n = 4). Panels D and E show the phenotype of wild-type and Vav1−/− neutrophils under low (2 dynes/cm2) and high (10 dynes/cm2) flow. Arrow indicates direction of flow. All values are means ± SEM. *p<0.05, compared to C57Bl/6.
Under these flow conditions, neutrophils from C57Bl/6 and Vav1−/− mice displayed strikingly different structural phenotypes. Whereas neutrophils from C57Bl/6 mice maintained a flattened round or slightly polarized morphology at 10dynes/cm2 flow (Figure 5D), Vav1−/− neutrophils took on an unusual phenotype where the cell body was attached to the site of adhesion by a narrow prolonged uropod (Figure 5E). Often these prolonged uropods were two to three cell diameters in length. In addition, frequently the cell body would detach from the substratum and further elongate. As already mentioned the elongated Vav1−/− phenotype was also seen in vivo (Figure 1D and supplemental video 2). Occasionally WT neutrophils would form a similar structure, but these structures were rare, very short lived, not as pronounced as in Vav1−/− neutrophils and resulted very quickly in cell detachment.
Emigration is dramatically impaired in Vav1−/− mice
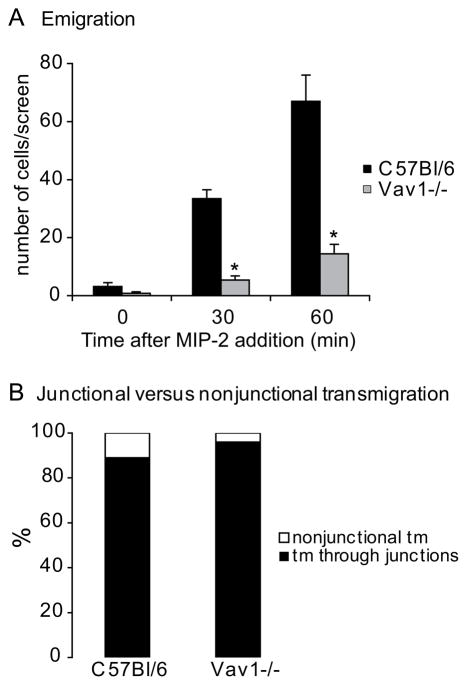
Ultimately, the biggest impact of the impaired mechanotaxis of Vav1−/− neutrophils was on the process of emigration. In wild-type mice, about 70 neutrophils emigrated out of the vessel during 60 min with MIP-2 superfusion (Figure 6A). In the Vav1−/− mice only 10 cells emigrated during the same time-period with MIP-2 superfusion (Figure 6A). Spinning disk confocal microscopy and PECAM-1-labeled junctions revealed that the majority of WT neutrophils emigrated at junctions (Figure 6B). The few Vav1−/− neutrophils also emigrated at junctions, suggesting that when the Vav1−/− cells were able to engage junctions and follow them, they would then emigrate. Interestingly, in the few Vav1−/− neutrophils where the neutrophils began emigrating, the uropod would not detach causing cells to struggle to emigrate (supplemental video 3). Once detachment occurred, these same cells migrated efficiently in the interstitium (supplemental video 3) suggesting not all crawling is impaired in Vav1−/− cells.
Figure 6. In vivo leukocyte emigration in inflamed venules (5 nM MIP-2).
Panel A shows the number of emigrated cells (in the extra vascular space, within an area of 200 × 300 μm area) before (time 0) or 30 and 60 min after addition of MIP-2 in the superfusate. Panel B show where leukocyte transmigration occurred in relation to junctions. All values are means of n=7 (C57Bl/6) and n=5 (Vav1−/−) ± SEM. * p<0.05 compared to C57Bl/6 mice.
Vav1 deficiency leads to an impaired innate immune response
To determine whether the impaired crawling and emigration in Vav1 deficiency could impact on a clinically relevant innate immune response, WT and Vav1−/− mice were challenged with Staphylococcus aureus-derived peptidoglycan (PGN; 5 mg kg−1 i.p.) and leukocyte recruitment into the peritoneal cavity was examined (after 4 and 24 hours). As shown in Figure 7A, treatment of WT or Vav1−/− mice with either PGN or saline did not result in any significant changes in circulating leukocyte counts. WT mice exhibited a significant leukocyte recruitment in peritoneum following challenge with PGN (Figure 7B). Interestingly, peritoneal leukocyte counts were lower (12 × 106) in the Vav1−/− mice than in WT mice (22 × 106) following PGN challenge. Nearly all of the recruited cells in the WT and Vav1−/− mice were neutrophils, as determined by leukocyte differentials, and Vav1−/− mice had less neutrophil recruitment into the peritoneal cavity than WT mice in response to PGN (Figure 7C).
Figure 7.
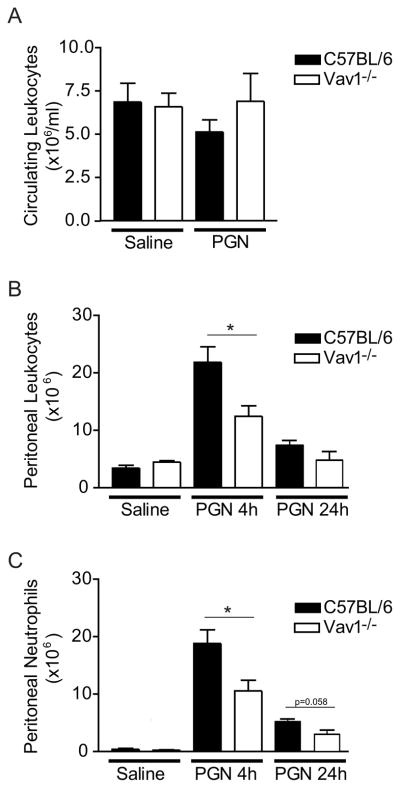
Effect of Vav1 deficiency on circulating leukocyte counts and leukocyte accumulation in the peritoneum. C57Bl/6 or Vav1−/− mice were challenged i.p. with saline or with PGN (5 mg kg−1) for 4 or 24h. Panel A show the blood circulating leukocyte counts (A) at time 4h. Panel B and C show the total leukocyte counts and the number of neutrophils respectively accumulated in the peritoneal cavity, 4 and 24h after PGN challange. All values are mean ± SEM and * p<0.05 compared to C57Bl/6.
Leukocyte recruitment was also studied in WT and Vav1−/− mice for 24 hours after challenge (PGN; 5 mg kg−1 i.p.) to determine if neutrophil emigration was just delayed in Vav1−/− mice. A decreased number of accumulated leukocytes in the peritoneum was detected at 24 hours compared to 4 hours in both WT (7.4±0.8 106) and Vav1−/− (4.8±01.4 106) mice (Figure 7B), as described previously for WT mice (25), and the vast majority of recruited cells were again identified as neutrophils (Figure 7C). Fewer Vav1−/− neutrophils (3±0.8 106) compared to WT (5.2±0.5 106) were recruited also at this delayed time point (Figure 7C, p=0.058). This indicates that the crawling defect observed in the Vav1−/− neutrophils, concomitant with the decrease in emigration reduces the capacity for an innate immune response and not just a delayed response in a model of peritoneal inflammation.
Discussion
There is a growing body of evidence that interposed between leukocyte adhesion and emigration, exists another step in the recruitment cascade, namely intraluminal crawling (8, 9, 10, 11). This step eluded investigators partly because the crawling required time lapse video microscopy, a technique generally not applied to the microvasculature where dynamic events need to be imaged in real time. In this study we report that an inherent characteristic of adherent neutrophils is to crawl perpendicularly to fluid shear. This behavior can be replicated on protein-coated coverslips in vitro but only in the presence of shear and entirely independent of the presence of a chemotactic gradient. In vivo, the neutrophils crawl perpendicularly to blood flow until they overlay a junction. At this stage, the shear induced perpendicular crawling can be superseded by junctional proteins and/or haptotactic gradients and the neutrophils often change direction sometimes radically and begin to follow the path of the junction and ultimately emigrate out of the vasculature. To date, no one has assessed any of the intracellular signaling pathways that lead to the crawling in blood vessels under flow conditions. Herein we report for the first time that the guanine exchange factor Vav1, a major regulator of the actin cytoskeleton, is critical for the shear-induced perpendicular crawling seen in WT neutrophils. The underlying molecular impairment appears to be an inability for Vav1−/− neutrophils to crawl via Mac-1. Indeed, much of the crawling in wild-type neutrophils was Mac-1 dependent, whereas Mac-1 antibody did not impact on Vav1−/− neutrophil behavior in vivo.
Vav1 is found only in hematopoietic cells (27) and is a very important signaling molecule downstream of LFA-1 (13, 16, 17, 18) and cross-linking LFA-1 does indeed induce Vav1 dependent cytoskeletal changes (15) leading to in vitro phenotypes such as neutrophil spreading, membrane ruffling, and polarization (12, 13). It has previously been shown that RhoA and actin-myosin bundles (molecules activated downstream of Vav1) localize to the uropod when neutrophil motility is initiated (28). In this study we extend these observations by identifying severely defective Mac-1-dependent mechanotactic crawling after LFA-1 dependent adhesion in Vav1−/− cells under shear conditions. While WT neutrophils crawled via Mac-1, the downstream movement of Vav1−/− neutrophils was not dependent on Mac-1 under shear conditions suggesting a failure to activate this molecule. The sequential LFA-1 activation followed by Mac-1 activation has been documented in vitro by Simon and colleagues further supporting our in vivo observation (14).
Neutrophils initiate lamellipodia which dictate direction while deadhesion occurs in the uropod allowing for a smooth forward motion. Previous work in vitro under static conditions in lymphocytes revealed that LFA-1 affinity is increased at the leading edge of a migrating cell (29) reaching peak levels in the centre of the cell (30). Although the adhesion at the uropod is also LFA-1 dependent, de-adhesion is necessary at this site to allow for effective movement forward. Accordingly, Tohyama et al. have shown that while affinity changes regulated LFA-1 binding at the front of the cell, avidity changes in LFA-1 mediated adhesion in the uropod perhaps allowing for more effective detachment (31). It should be noted that all the aforementioned work was done under static in vitro conditions. Crawling under flow conditions would require both the molecular complexities of migration observed in tissues, but also the necessary molecular mechanisms resulting in tethering to endothelium that would prevent detachment due to shear. We would hypothesize that an exquisite communication between LFA-1 and Mac-1 must exist where LFA-1 binds first for firm adhesion and would perhaps disengage only after Mac-1 bound its ligand. It would seem intuitive that LFA-1 would remain activated until Mac-1 was engaged. In our in vivo imaging, it was clear that Vav1−/− cells were able to adhere in blood vessels via LFA-1, but the leading edge and body often detached leaving the uropod as the only point of contact for extended periods. This reduced surface area of Vav1−/− neutrophils in contact with endothelium, frequently resulted in detachment both in vivo and in vitro perhaps due to lack of engagement of Mac-1. Indeed Mac-1 is localized to the leading edge and body of the crawling neutrophil (32).
Although the neutrophils were able to emigrate at junctions in both strains of mice, the number of Vav1−/− cells that emigrated was dramatically reduced. This is quite interesting when one considers that Mac-1 deficient neutrophils are incapable of crawling but emigrate at similar levels as wild-type neutrophils albeit with some delay (10). Clearly, Vav1−/− neutrophils cannot emigrate as effectively as Mac-1 deficient cells suggesting a more severe defect in the former. Indeed, Mac-1−/− neutrophils did not detach and were able to extend pseudopods into the endothelium for both paracellular and transcellular migration. By contrast, detachment of the leading edge and cell body would prevent pseudopod extension at the leading edge of Vav1−/− neutrophils reducing transmigration. In addition the Vav1−/− neutrophils detach more than wt neutrophils also likely contributing to the lack of emigration. Interestingly increased detachment of neutrophils has been reported previously but only in Vav1/3 double knockout but not Vav1−/− mice (19). However, these investigators examined detachment only over 90 sec post very brief administration of a chemokine. Indeed, we observed substantial adhesion in Vav1−/− neutrophils over this period of time with detachment only occurring at a delayed time point. To our knowledge neither Gakidis et al. (19), nor anyone else has examined crawling and emigration in Vav1−/− mice.
Endothelial cells are cobblestone when grown under static conditions, but when exposed to shear as is the case in vivo, these cells are elongated such that they can be up to 200 um in length (tapering at the ends) but only 30–50 um in width. When taking into consideration this elongated shape of venular endothelial cells, the closest junction and by default the fastest way for a neutrophil to find a junction would be to move left or right. Therefore, intuitively it makes sense that WT neutrophils immediately begin migrating perpendicularly to blood flow. This process was unlikely to require a chemotactic gradient as even in vitro in the absence of chemokine gradients, all neutrophils began crawling in straight lines perpendicular to applied shear forces, but immediately returned to random migration when flow was terminated suggesting that the neutrophil has the capacity to detect and react to shear. Our data however do not dismiss the possibility that in vivo the neutrophils can detect chemotactic gradients in blood vessels, further modifying their crawling directionality, depending on the source of the chemoattractant (i.e., left or right).
The ability to detect mechanical forces (mechanoception) has been identified in a large variety of cells. In fact, it is well known that a variety of single cell organisms as well as motile cells in multi-cellular organisms can respond to shear. For example, Dicytostelium aligns in the direction of flow (33). Neutrophils behave very similarly to Dicytostelium and in many respects have similar molecular processes to permit crawling. Interestingly, multiple integrins have been identified as mechanoreceptors, capable of detecting shear. Indeed, studies have shown shear-dependent regulation of integrins on other myeloid cells (34). Shear-dependent regulation of CD18 on neutrophils has been reported (35), suggesting that the activity of integrins on neutrophils can be regulated by shear. We submit that the activation of integrin mechanoreceptors induced the activation of signaling molecules such as Vav1, which plays a central role in mediating neutrophil motility (28, 36, 37, 38, 39, 40). Clearly, mechanosensing is an essential cue, leading to effective neutrophil emigration thereby responding in a timely fashion to infection and immunity.
Haptotaxis (following stationary signals) is suggested to be important in leukocyte recruitment during inflammation (41, 42, 43). We found that when the perpendicularly crawling neutrophils reached an endothelial junction, some changed direction and started to follow the junctions, indicating a switch from mechanotaxis to haptotaxis as stationary junctional specific signals were followed. Our data thus demonstrates that crawling involves two distinct stages. In the initial stage, the cell moves perpendicular to flow, using the mechanotactic signal of flow to determine direction. This mechanotactic movement is terminated when the cell detects that it is crossing an endothelial junction. At this point the cell ignores the mechanotactic signal provided by flow, and begins to follow the haptotactic signal, provided by junctional proteins, to the site of emigration.
In conclusion, an inherent behavior of neutrophils exposed to shear was detected, as the neutrophils migrated perpendicular to flow in vitro as well as in vivo. In vivo this led the neutrophils to the closest endothelial junction, which they often followed for periods of time before transmigrating out of the vasculature. This feature is absent in Vav1−/− neutrophils, which suggests Vav1 is down-stream of adhesion, and dependent upon LFA-1 outside-in signaling required for optimal Mac-1 dependent crawling under physiological shear. Finally, in a model of peritonitis, we found that fewer Vav1−/− cells were recruited to the peritoneal cavity, indicating the importance of Vav1-dependent intravascular crawling to optimal sites for emigration in innate immunity.
Supplementary Material
Acknowledgments
This work was supported by the CIHR, CHIR group grant and Swedish Research Council (57P-20680, 57x-20675). PK is an Alberta Heritage Foundation for Medical Research Chair and the Snyder Chair in Critical Care Medicine. MP is a Canadian Association of Gastroenterology Postdoctoral fellow.
The authors would like to thank Krista McRae and Sara Massena Santos for excellent technical assistance, the Microscopy and Imaging Facility and The Live Cell Imaging Facility at the Faculty of Medicine, Calgary University.
References
- 1.Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 2.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 4.Issekutz AC, Issekutz TB. The contribution of LFA-1 (CD11a/CD18) and MAC-1 (CD11b/CD18) to the in vivo migration of polymorphonuclear leucocytes to inflammatory reactions in the rat. Immunology. 1992;76:655–661. [PMC free article] [PubMed] [Google Scholar]
- 5.Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood. 2005;106:584–592. doi: 10.1182/blood-2004-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lou O, Alcaide P, Luscinskas FW, Muller WA. CD99 is a key mediator of the transendothelial migration of neutrophils. J Immunol. 2007;178:1136–1143. doi: 10.4049/jimmunol.178.2.1136. [DOI] [PubMed] [Google Scholar]
- 7.Petri B, Phillipson M, Kubes P. The physiology of leukocyte recruitment: an in vivo perspective. J Immunol. 2008;180:6439–6446. doi: 10.4049/jimmunol.180.10.6439. [DOI] [PubMed] [Google Scholar]
- 8.Schenkel AR, Mamdouh Z, Muller WA. Locomotion of monocytes on endothelium is a critical step during extravasation. Nat Immunol. 2004;5:393–400. doi: 10.1038/ni1051. [DOI] [PubMed] [Google Scholar]
- 9.Wojciechowski JC, I, Sarelius H. Preferential binding of leukocytes to the endothelial junction region in venules in situ. Microcirculation. 2005;12:349–359. doi: 10.1080/10739680590934763. [DOI] [PubMed] [Google Scholar]
- 10.Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal Crawling of Neutrophils to Emigration Sites in Inflamed Blood Vessels: A Molecularly Distinct Process from Adhesion in the Recruitment Cascade. J Ex Med. 2006;203:2569–2575. doi: 10.1084/jem.20060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryschich E, Kerkadze V, Lizdenis P, Paskauskas S, Knaebel HP, Gross W, Gebhard MM, Buchler MW, Schmidt J. Active leukocyte crawling in microvessels assessed by digital time-lapse intravital microscopy. J Surg Res. 2006;135:291–296. doi: 10.1016/j.jss.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Fernandez JL, Gomez M, Luque A, Hogg N, Sanchez-Madrid F, Cabanas C. The interaction of activated integrin Lymphocyte Function-associated Antigen 1 with ligand Intracellular Adhesion Molecule 1 induces activation and redistribution of focal adhesion kinase and proline-rich tyrosine kinase 2 in T lymphocytes. Mol Biol Cell. 1999;10:1891–1907. doi: 10.1091/mbc.10.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez-Martin L, Sanchez-Sanchez N, Gutierrez-Lopez MD, Rojo AI, Vicente-Manzanares M, Perez-Alvarez MJ, Sanchez-Mateos P, Bustelo XR, Cuadrado A, Sanchez-Madrid F, Rodriguez-Fernandez JL, Cabanas C. Signaling through the leukocyte integrin LFA-1 in T cells induces a transient activation of Rac-1 that is regulated by Vav and PI3K/Akt-1. J Biol Chem. 2004;279:16194–16205. doi: 10.1074/jbc.M400905200. [DOI] [PubMed] [Google Scholar]
- 14.Green CE, Schaff UY, Sarantos MR, Lum AF, Staunton DE, Simon SI. Dynamic shifts in LFA-1 affinity regulate neutrophil rolling, arrest, and transmigration on inflamed endothelium. Blood. 2006;107:2101–2111. doi: 10.1182/blood-2005-06-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mor A, Dustin ML, Philips MR. Small GTPases and LFA-1 reciprocally modulate adhesion and signaling. Immunol Rev. 2007;218:114–125. doi: 10.1111/j.1600-065X.2007.00538.x. [DOI] [PubMed] [Google Scholar]
- 16.Riteau B, Barber DF, Long EO. Vav1 phosphorylation is induced by beta2 integrin engagement on natural killer cells upstream of actin cytoskeleton and lipid raft reorganization. J Exp Med. 2003;198:469–474. doi: 10.1084/jem.20021995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schymeinsky J, Then C, Walzog B. The non-receptor tyrosine kinase Syk regulates lamellipodium formation and site-directed migration of human leukocytes. J Cell Physiol. 2005;204:614–622. doi: 10.1002/jcp.20323. [DOI] [PubMed] [Google Scholar]
- 18.Schymeinsky J, Sindrilaru A, Frommhold D, Sperandio M, Gerstl R, Then C, Mócsai A, Scharffetter-Kochanek K, Walzog B. The Vav binding site of the non-receptor tyrosine kinase Syk at Tyr 348 is critical for beta2 integrin (CD11/CD18)-mediated neutrophil migration. Blood. 2006;108:3919–3927. doi: 10.1182/blood-2005-12-030387. [DOI] [PubMed] [Google Scholar]
- 19.Gakidis MA, Cullere X, Olson T, Wilsbacher JL, Zhang B, Moores SL, Ley K, Swat W, Mayadas T, Brugge JS. Vav GEFs are required for beta2 integrin-dependent functions of neutrophils. J Cell Biol. 2004;166:273–282. doi: 10.1083/jcb.200404166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells CM, Bhavsar PJ, Evans IR, Vigorito E, Turner M, Tybulewicz V, Ridley AJ. Vav1 and Vav2 play different roles in macrophage migration and cytoskeleton organization. Exp Cell Res. 2005;310:303–310. doi: 10.1016/j.yexcr.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Kim C, Marchal CC, Penninger J, Dinauer MC. The hemopoietic Rho/Rac guanine nucleotide exchange factor Vav1 regulates N-formyl-methionyl-leucyl-phenylalanine-activated neutrophil functions. J Immunol. 2003;171:4425–4430. doi: 10.4049/jimmunol.171.8.4425. [DOI] [PubMed] [Google Scholar]
- 22.Kanwar S, Bullard DC, Hickey MJ, Smith CW, Beaudet AL, Wolitzky BA, Kubes P. The association between alpha4-integrin, P-selectin, and E-selectin in an allergic model of inflammation. J Exp Med. 1997;185:1077–1087. doi: 10.1084/jem.185.6.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L, Cara DC, Kaur J, Raharjo E, Mullaly SC, Jongstra-Bilen J, Jongstra J, Kubes P. LSP1 is an endothelial gatekeeper of leukocyte transendothelial migration. J Exp Med. 2005;201:409–418. doi: 10.1084/jem.20040830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cara DC, Kaur J, Forster M, McCafferty DM, Kubes P. Role of p38 mitogen-activated protein kinase in chemokine-induced emigration and chemotaxis in vivo. J Immunol. 2001;167:6552–6558. doi: 10.4049/jimmunol.167.11.6552. [DOI] [PubMed] [Google Scholar]
- 25.Mullaly SC, Kubes P. The role of TLR2 in vivo following challenge with Staphylococcus aureus and prototypic ligands. J Immunol. 2006;177:8154–8163. doi: 10.4049/jimmunol.177.11.8154. [DOI] [PubMed] [Google Scholar]
- 26.Heit B, Colarusso P, Kubes P. Fundamentally different roles for LFA-1, Mac-1 and alpha4-integrin in neutrophil chemotaxis. J Cell Sci. 2005;118:5205–5220. doi: 10.1242/jcs.02632. [DOI] [PubMed] [Google Scholar]
- 27.Garrett TA, Van Buul JD, Burridge K. VEGF-induced Rac1 activation of endothelial cells is regulated by the guanine nucleotide exchange factor Vav2. Exp Cell Res. 2007;313:3285–3297. doi: 10.1016/j.yexcr.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pestonjamasp KN, Forster C, Sun C, Gardiner EM, Bohl B, Weiner O, Bokoch GM, Glogauer M. Rac1 links leading edge and uropod events through Rho and myosin activation during chemotaxis. Blood. 2006;108:2814–2820. doi: 10.1182/blood-2006-01-010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katagiri K, Maeda A, Shimonaka M, Kinashi T. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat Immunol. 2003;4:741–748. doi: 10.1038/ni950. [DOI] [PubMed] [Google Scholar]
- 30.Smith A, Stanley P, Jones K, Svensson L, McDowall A, Hogg N. The role of the integrin LFA-1 in T-lymphocyte migration. Immunol Rev. 2007;218:135–146. doi: 10.1111/j.1600-065X.2007.00537.x. [DOI] [PubMed] [Google Scholar]
- 31.Tohyama Y, Katagiri K, Pardi R, Lu C, Springer TA, Kinashi T. The critical cytoplasmic regions of the alphaL/beta2 integrin in Rap1-induced adhesion and migration. Mol Biol Cell. 2003;14:2570–2582. doi: 10.1091/mbc.E02-09-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rochon YP, Kavanagh TJ, Harlan JM. Analysis of integrin (CD11b/CD18) movement during neutrophil adhesion and migration on endothelial cells. J Microsc. 2000;197:15–24. doi: 10.1046/j.1365-2818.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 33.Décave E, Rieu D, Dalous J, Fache S, Brechet Y, Fourcade B, Satre M, Bruckert F. Shear flow-induced motility of Dictyostelium discoideum cells on solid substrate. J Cell Sci. 2003;116:4331–4343. doi: 10.1242/jcs.00726. [DOI] [PubMed] [Google Scholar]
- 34.Zwartz GJ, Chigaev A, Dwyer DC, Foutz TD, Edwards BS, Sklar LA. Real-time analysis of very late antigen-4 affinity modulation by shear. J Biol Chem. 2004;279:38277–38286. doi: 10.1074/jbc.M402944200. [DOI] [PubMed] [Google Scholar]
- 35.Fukuda S, Schmid-Schonbein GW. Regulation of CD18 expression on neutrophils in response to fluid shear stress. Proc Natl Acad Sci. 2003;100:13152–13157. doi: 10.1073/pnas.2336130100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coughlin MF, Schmid-Schonbein GW. Pseudopod projection and cell spreading of passive leukocytes in response to fluid shear stress. Biophys J. 2004;87:2035–2042. doi: 10.1529/biophysj.104.042192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makino A, Glogauer M, Bokoch GM, Chien S, Schmid-Schonbein GW. Control of neutrophil pseudopods by fluid shear: role of Rho family GTPases. Am J Physiol Cell Physiol. 2005;288:C863–C871. doi: 10.1152/ajpcell.00358.2004. [DOI] [PubMed] [Google Scholar]
- 38.Makino A, Prossnitz ER, Bunemann M, Wang JM, Yao W, Schmid-Schonbein GW. G protein-coupled receptors serve as mechanosensors for fluid shear stress in neutrophils. Am J Physiol Cell Physiol. 2006;290:C1633–C1639. doi: 10.1152/ajpcell.00576.2005. [DOI] [PubMed] [Google Scholar]
- 39.Mazaki Y, Hashimoto S, Tsujimura T, Morishige M, Hashimoto A, Aritake K, Yamada A, Nam JM, Kiyonari H, Nakao K, Sabe H. Neutrophil direction sensing and superoxide production linked by the GTPase-activating protein GIT2. Nat Immunol. 2006;7:724–731. doi: 10.1038/ni1349. [DOI] [PubMed] [Google Scholar]
- 40.Szczur K, Xu H, Atkinson S, Zheng Y, Filippi MD. Rho GTPase CDC42 regulates directionality and random movement via distinct MAPK pathways in neutrophils. Blood. 2006;108:4205–4213. doi: 10.1182/blood-2006-03-013789. [DOI] [PubMed] [Google Scholar]
- 41.Duperray A, Mantovani A, Introna M, Dejana E. Endothelial cell regulation of leukocyte infiltration in inflammatory tissues. Mediators Inflamm. 1995;4:322–330. doi: 10.1155/S0962935195000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y, Yan J, De P, Chang HC, Yamauchi A, Christopherson KW, II, Paranavitana NC, Peng X, Kim C, Munugulavadla V, Kapur R, Chen H, Shou W, Stone JC, Kaplan MH, Dinauer MC, Durden DL, Quilliam LA. Rap1a null mice have altered myeloid cell functions suggesting distinct roles for the closely related Rap1a and 1b proteins. J Immunol. 2007;179:8322–8331. doi: 10.4049/jimmunol.179.12.8322. Erratum in: 2008. J Immunol. 180: 3612. V. Munugalavadla. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Toledo KA, Bernardes ES, Baruffi MD, Roque-Barreira MC. Neutrophil haptotaxis induced by mouse MNCF: interactions with extracellular matrix glycoproteins probably contribute to overcoming the anti-inflammatory action of dexamethasone. Inflamm Res. 2007;56:368–376. doi: 10.1007/s00011-007-6159-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.