Abstract
A series of phosphine-ligated palladium precatalysts based on N-methyl- and N-phenyl-2-aminobiphenyl have been developed. Substitution at the nitrogen center prevents the presence of traces of aminobiphenyls that contain a free −NH2 group from contaminating cross-coupling products. These precatalysts produce N-substituted carbazoles upon activation, which cannot consume starting materials. These precatalysts were efficiently generated from 2-aminobiphenyl with minimal purification and found to be highly effective in Suzuki–Miyaura and C–N cross-coupling reactions.
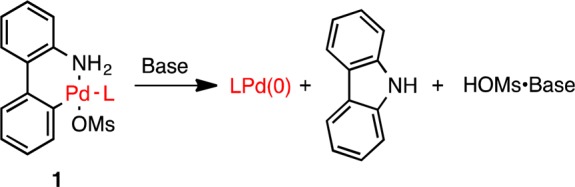
We recently reported palladium precatalysts based on a ligated 2-aminobiphenylpalladium methanesulfonate palladacycle (1, Scheme 1).1 Precatalyst 1 activates through the deprotonation of the palladium-bound amine to give a Pd–amido complex which then reductively eliminates to form carbazole, a methanesulfonate salt, and LPd(0). Precatalysts of type 1 can accommodate a variety of ligands and are applicable to numerous palladium-catalyzed transformations.2
Scheme 1. Palladium Methanesulfonate Precatalysts and Their Generic Activation.
Despite the advantages of 1, a few drawbacks limit their utility in some applications, including (1) the carbazole byproduct generated through the activation of 1 can be N-arylated, consuming valuable starting materials or potentially complicating work-up/purification of the desired product,1b and (2) there is some concern regarding the presence of trace amounts of residual NH2-aminobiphenyls in pharmaceutical samples due to potential health risks.3N-Alkyl and N-aryl analogues of 1 would overcome these concerns and provide a useful alternative to 1.
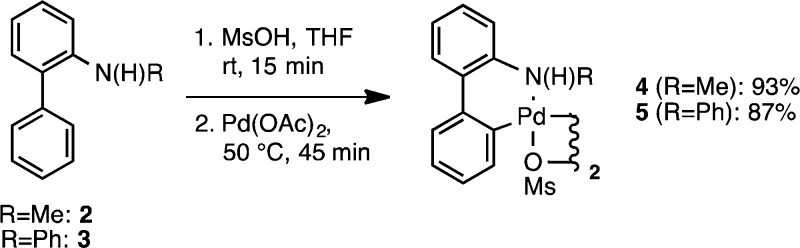
N-Substituted 2-aminobiphenyls could be readily prepared on a 30 mmol scale via N-methylation and N-arylation as shown in Scheme 2. The unpurified products4 from these reactions can be directly used to prepare the corresponding palladacycles. Treatment of N-methyl-2-aminobiphenyl, 2, and N-phenyl-2-aminobiphenyl, 3, with methanesulfonic acid followed by heating the resulting salt solution with Pd(OAc)2 provided the dimeric palladacycles 4 and 5. These procedures were amenable to scale up, providing the desired palladium dimers in excellent yields at a 30 mmol scale (Scheme 3).
Scheme 2. Preparation of N-Methyl- and N-Phenyl-2-aminobiphenyl Derivatives.
(i) nBuLi, THF, 0 °C, 30 min; (ii) MeI, 0 °C to rt, 30 min, quant conversion.
1 mol % 1·L1, PhCl, NaOtBu, dioxane, 100 °C, 30 min, 99%.
Scheme 3. Preparation of N-Substituted μ-OMs Palladium Dimers.
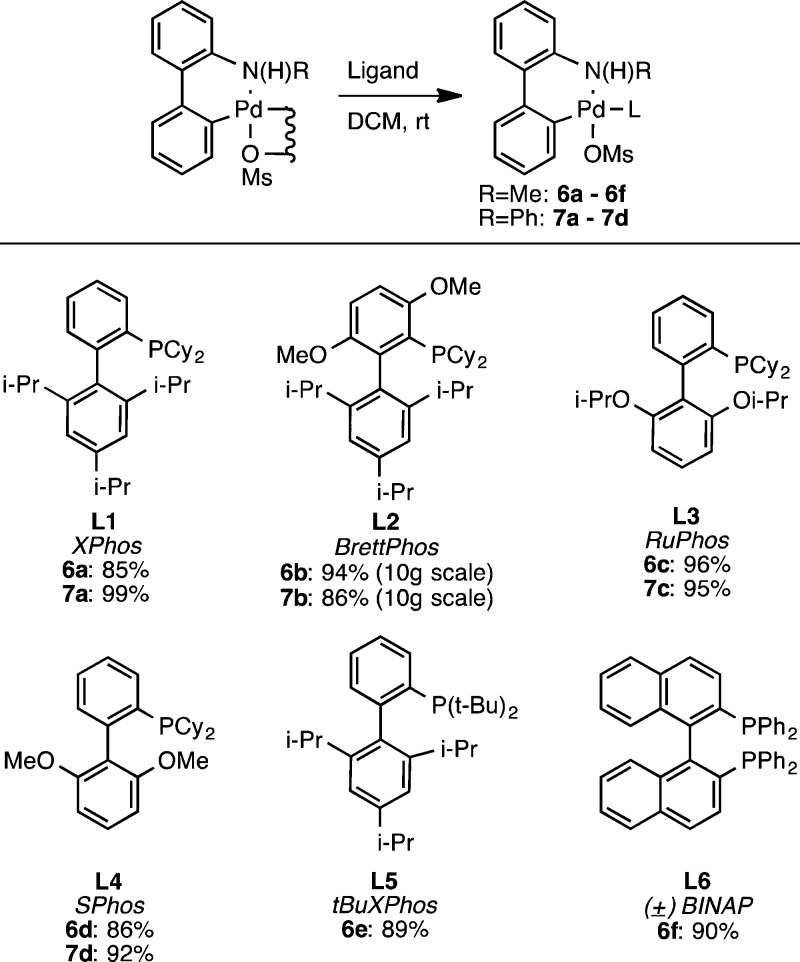
These were subsequently treated with phosphines at room temperature in dichloromethane to provide the N-substituted precatalysts (Scheme 4). Precatalysts that incorporated a variety of ligands could be prepared as shown in Scheme 3. In contrast, however, to what we observed with 1, we were unable to make precatalysts containing the largest of our ligands: attempts to incorporate tBuBrettPhos, RockPhos, and AdBrettPhos were unsuccessful.5 Additionally, while we could prepare 6f from (±)-BINAP, we were unable to obtain the corresponding N-phenyl analogue, 7f.6
Scheme 4. Preparation of N-Substituted Palladium Methanesulfonate Precatalysts.
The solid-state structures of 6a and 7a were determined by single-crystal X-ray crystallography (Figure 1). Both possess a tetracoordinated Pd(II) center with a slightly distorted square planar geometry. The phosphine is bound to the palladium center cis to the Pd–C bond. Additionally the methanesulfonate anion is directly bound to the palladium center. This is similar to what was previously observed for 1 (L = XPhos).
Figure 1.
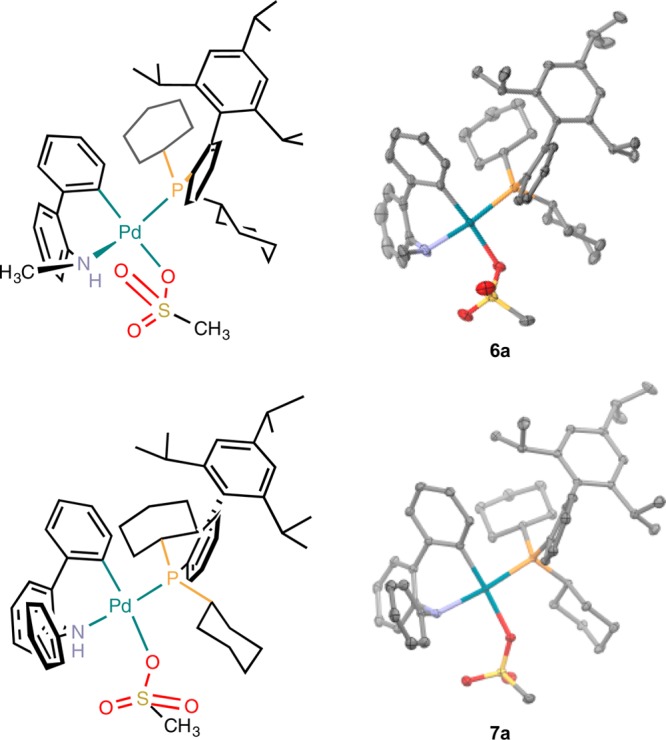
Crystallographically determined X-ray structures of 5a and 6a (thermal ellipsoid plot at 50% probability, hydrogen atoms are omitted for clarity).
To evaluate the reactivity of precatalysts 6 and 7, we first examined their efficacy in promoting the Suzuki–Miyaura coupling of aryl halides with arylboronic acids that are prone to rapid protodeboronation under standard cross-coupling reaction conditions. As previously described, the rapid generation of a highly active LPd(0) is essential for success of these reactions.7 These reactions allowed us to test whether 6 and 7 activate rapidly at room temperature. As shown in Scheme 5, both 6a and 7a were highly effective precatalysts in the coupling of (hetero)aryl halides and unstable boronic acids, providing the arylated products in uniformly good yields.
Scheme 5. Suzuki–Miyaura Coupling of Unstable Boronic Acids with Precatalysts 6a and 7a.
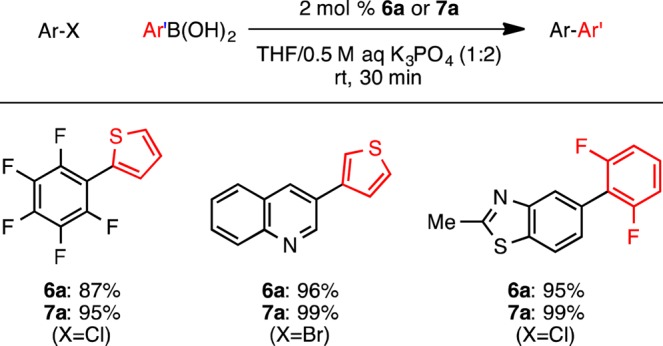
General conditions: ArX (1 mmol), Ar’B(OH)2 (1.5 mmol), 6a or 7a (2 mol %), 0.5 M K3PO4 (aq) (4 mL), THF (2 mL), rt, 30 min, average of two isolated yields.
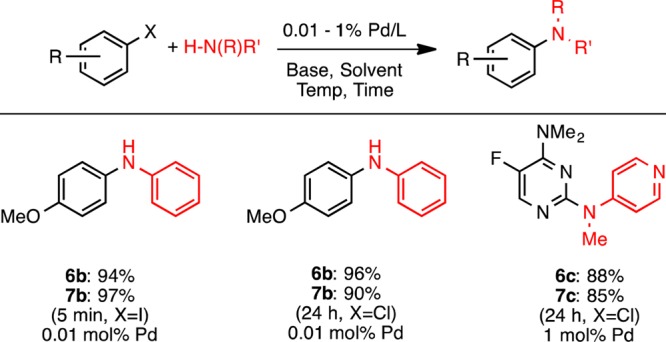
We also evaluated precatalysts of type 6 and 7 in C–N cross-coupling reactions. They were found to be effective in the arylation of primary amines, secondary amines, as well as primary amides (Scheme 6). The precatalyst was able to be employed at low catalyst loadings (0.01 mol %) for the arylation of aniline with 4-chloro- and 4-iodoanisole.
Scheme 6. N-Arylation of Amines with Precatalysts 6 and 7.
In conclusion, we have developed a series of precatalysts based on the N-methyl- and N-phenyl-2-aminobiphenylpalladium methanesulfonate scaffold. By utilizing N-substituted 2-aminobiphenyls, the chance of the trace contamination of reaction products with NH2-aminobiphenyls is eliminated. Additionally, the N-substituted carbazole that results during precatalyst activation cannot be further arylated, preventing the waste of the aryl halide substrate. We believe that these precatalysts, like 1, will find many applications in palladium-catalyzed cross-coupling chemistry in both academia and industry.
Experimental Section
General Information
General Reagent Information
THF and toluene were purchased in solvent-delivery kegs and vigorously purged with argon for 2 h. The solvents were further purified by passing it under argon pressure through two packed columns of neutral alumina (for THF) or through neutral alumina and copper(II) oxide (for toluene). Anhydrous tribasic potassium phosphate and sodium tert-butoxide were purchased from commercial suppliers. These bases were stored in a nitrogen-filled glovebox and removed in small quantities. They were stored on the bench in a desiccator for up to 2 weeks. Pd(OAc)2 and all ligands were acquired from commercial suppliers. All other reagents were purchased from commercial suppliers and used as received. Aqueous 0.5 M K3PO4 solution was prepared by dissolving K3PO4 (1.06 g, 5 mmol) in deionized water (19 mL), and the solution was degassed by performing three sets of evacuation and argon refill cycles under sonication. Flash chromatography was performed with 230–400 mesh silica gel.
General Analytical Information
All compounds (starting materials and products) were characterized by 1H NMR, 13C NMR, 31P NMR (when applicable), 19F NMR (when applicable), and IR spectroscopy, melting point (when applicable), and elemental analysis. The 1H, 13C, 31P, and 19F NMR spectra can be found in the Supporting Information. 1H, 13C, 31P, and 19F NMR were recorded on 300, 400, or 500 MHz spectrometers. The spectra were calibrated according to residual solvent peaks (CDCl3: 7.26 ppm for 1H NMR and 77.0 ppm for 13C NMR; CD2Cl2: 5.32 ppm for 1H NMR and 53.84 ppm for 13C NMR; CD3OD: 3.31 for 1H NMR and 49.00 for 13C NMR), an external reference (H3PO4: 0 ppm for 31P; CFCl3: 0 ppm for 19F), or an internal reference (CF3Ph: −63.7 ppm for 19F). The 13C and 31P NMR spectra were obtained with 1H decoupling, and the 19F NMR spectra were obtained without 1H decoupling. The following abbreviations were used to explain the multiplicities: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br = broad, app = apparent. Reactions were monitored by GC and thin-layer chromatography (TLC) carried out on 0.25 mm glass-backed silica gel plates using UV light as a visualizing agent.
N-Methyl-2-aminobiphenylpalladium Methanesulfonate (4)
Step 1: N-Methyl-2-aminobiphenyl (2)
A flame-dried 300 mL round-bottomed flask equipped with a magnetic stir bar was charged with 2-aminobiphenyl and capped with a rubber septum. The flask was evacuated and backfilled with argon, followed by the addition of THF (100 mL). The mixture was cooled to 0 °C in an ice bath. At 0 °C, nBuLi (2.5 M in hexanes, 12.6 mL, 31.5 mmol, 1.05 equiv) was added slowly. After the addition of nBuLi was complete the bright yellow reaction mixture was stirred for 1 h at 0 °C. Then iodomethane (1.89 mL, 30.3 mmol, 1.01 equiv) was added slowly, at which time the color faded to a dull yellow. The mixture was stirred for an additional 30 min at rt. Saturated NaHCO3 (aq) (25 mL) and water (25 mL) were added, and the aqueous layer was extracted with diethyl ether (3 × 50 mL). The combined organic layers were dried over MgSO4 and concentrated under vacuum to provide the title compound as a yellow oil as a 95:5 mixture of mono-/dimethylated product, as determined by gas chromatography and 1H NMR. The crude mixture was used directly in the next step without further purification. Yield: 5.45 g, 99%. 1H NMR (500 MHz, CDCl3): δ 7.49–7.42 (m, 4H), 7.40–7.34 (m, 1H), 7.30 (ddd, J = 8.1, 7.4, 1.7 Hz, 1H), 7.12 (dd, J = 7.4, 1.6 Hz, 1H), 6.80 (td, J = 7.4, 1.1 Hz, 1H), 6.72 (dd, J = 8.2, 1.0 Hz, 1H), 3.98 (s, 1H), 2.82 (s, 3H) ppm. 13C NMR (126 MHz, CDCl3): δ 146.4, 139.7, 130.2, 129.6, 129.1, 129.0, 127.8, 127.4, 117.0, 110.0, 31.0 ppm. IR (neat, cm–1): 1613, 1603, 1509, 1490, 1435, 1284, 1008, 770, 746, 701, 615.
Step 2: Palladium Methanesulfonate Dimer (4)
A 200 mL round-bottomed flask equipped with a magnetic stir bar was charged with N-methyl-2-aminobiphenyl (5.52 g, 30.0 mmol, 1.00 equiv, 95:5 mono-/dimethylated) and THF (60 mL). With stirring, methanesulfonic acid (1.84 mL, 28.5 mmol, 0.95 equiv (1 equiv relative to monomethylated amine)) was added slowly and the reaction mixture stirred for 15 min at room temperature. Palladium acetate (6.38 g, 28.5 mmol, 0.95 equiv) was added in one portion and rinsed off the walls of the flask with additional THF (15 mL). The flask was capped with a rubber septum, and the deep red slurry was stirred at 50 °C for 45 min. After the mixture was cooled to room temperature, the dark yellow solution was filtered through a plug of cotton to remove traces of palladium black and ∼95% of the solvent was removed with the aid of a rotary evaporator. Diethyl ether (150 mL) was added to the flask, and the mixture was sonicated to precipitate the product. The solid was isolated via vacuum filtration and dried under vacuum overnight to provide the title compound as a tan solid. Yield: 10.2 g, 93%. 1H NMR (500 MHz, CD2Cl2 + 10 μL pyridine-d5): δ 7.70 (d, J = 7.7 Hz, 1H), 7.66–7.56 (m, 1H), 7.52–7.47 (m, 1H), 7.44 (d, J = 7.7 Hz, 1H), 7.36 (t, J = 7.6 Hz, 1H), 7.28 (t, J = 7.7 Hz, 1H), 7.15 (t, J = 7.4 Hz, 1H), 6.94–6.84 (m, 1H), 6.50 (d, J = 7.7 Hz, 1H), 2.70 (d, J = 5.6 Hz, 3H), 2.61 (s, 3H) ppm. 13C NMR (126 MHz, CD3OD): δ 142.0, 140.7, 133.4, 128. 9, 128.5, 127.2, 126.5, 125.2, 39.0 ppm. IR (neat, cm–1): 3205, 1230, 1136, 1118, 1026, 735, 727 714, 589.
N-Phenyl-2-aminobiphenylpalladium Methanesulfonate (5)
Step 1: N-Phenyl-2-aminobiphenyl (3)
A 100 mL oven-dried round-bottomed flask equipped with a magnetic stir bar was charged with 2-aminobiphenyl (5.07 g, 30.0 mmol, 1.00 equiv), sodium tert-butoxide (3.07 g, 32.0 mmol, 1.07 equiv), and XPhos precatalyst 1·L1 (306 mg, 0.30 mmol, 1 mol %). The flask was capped with a rubber septum and subsequently evacuated and backfilled with argon (this procedure was repeated one additional time). Chlorobenzene (3.04 mL, 30.0 mmol, 1.00 equiv) was then added by syringe, followed by dioxane (30 mL). The reaction mixture was stirred at 100 °C for 30 min. It was then cooled to room temperature, diluted with ethyl acetate (50 mL), and filtered through a plug of silica gel layered on top of Celite, eluting the mixture with additional ethyl acetate. The mixture was concentrated with the aid of a rotary evaporator, and the product was obtained as a dark yellow oil, containing traces of N,N-diphenyl-2-aminobiphenyl and 9-phenylcarbazole. It was used for the next step without further purification. 1H NMR (500 MHz, CDCl3): δ 7.53–7.46 (m, 4H), 7.46–7.38 (m, 2H), 7.34–7.27 (m, 4H), 7.12–7.02 (m, 3H), 6.97 (tt, J = 7.4, 1.1 Hz, 1H), 5.66 (bs, 1H) ppm. 13C NMR (126 MHz, CDCl3): δ 144.1, 140.9, 139.7, 132.2, 131.6, 130.1, 130.1, 129.6, 129.0, 128.2, 121.8, 121.8, 118.9, 118.2. IR (neat, cm–1): 1611, 1500, 1479, 1434, 1008, 749, 721, 702.
Step 2: Palladium Methanesulfonate Dimer (5)
A 300 mL round-bottomed flask equipped with a magnetic stir bar was charged with N-phenyl-2-aminobiphenyl (7.35 g, 30.0 mmol, 1.00 equiv) and THF (50 mL). Methanesulfonic acid (1.95 mL, 30.0 mmol, 1.00 equiv) was added slowly with vigorous stirring. After the reaction mixture was stirred for 15 min, palladium acetate (6.72 g, 30.0 mmol, 1.00 equiv) was added to the flask in one portion and rinsed off the walls of the flask with additional THF (25 mL). The flask was capped with a rubber septum, and the deep red slurry was stirred at 50 °C for 45 min. Over the course of the reaction the deep red color dissipated and a tan slurry formed. The reaction mixture was cooled to room temperature and poured into an Erlenmeyer flask containing pentane (75 mL) and diethyl ether (75 mL). The solid was isolated via vacuum filtration and dried under vacuum overnight to afford the title compound as a tan powder. Yield: 12.1 g, 87%. 1H NMR (500 MHz, CD3CN): δ 10.47 (s, 1H), 7.83–7.79 (m, 1H), 7.52 (ddd, J = 7.8, 6.9, 1.9 Hz, 1H), 7.47–7.39 (m, 3H), 7.20–7.13 (m, 2H), 7.13–7.09 (m, 2H), 7.09–7.03 (m, 2H), 7.00 (dd, J = 7.8, 1.2 Hz, 1H), 6.89 (ddd, J = 7.9, 7.2, 1.6 Hz, 1H), 2.75 (s, 3H). 13C NMR (126 MHz, CD3CN): δ 146.4, 142.4, 140.6, 137.5, 136.8, 136.5, 130.0, 129.8, 129.5, 129.3, 127.8, 127.1, 127.1, 126.7, 123.8, 122.1, 40.3 ppm. IR (neat, cm–1): 3115, 1493, 1218, 1130, 1025, 762, 735, 716, 606.
XPhos Precatalyst 6a: Representative Procedure
A 24 mL screw-top test tube equipped with a stir bar was charged with 4 (384 mg, 0.50 mmol, 0.50 equiv) XPhos (476 mg, 1.00 mmol, 1.00 equiv). Dichloromethane (5 mL) was added, and the reaction mixture was stirred at room temperature for 1 h. The solvent was removed with the aid of rotary evaporation. Pentane (25 mL) was added to the residue to precipitate the precatalyst, which was then isolated via vacuum filtration and dried under vacuum overnight to provide the title compound as a tan solid. Yield: 730 mg, 85%. 1H NMR (500 MHz, CD3OD): δ 7.97 (ddd, J = 9.2, 4.9, 2.9 Hz, 1H), 7.65 (dd, J = 7.8, 1.7 Hz, 2H), 7.63–7.57 (m, 3H), 7.41–7.23 (m, 5H), 7.16–7.10 (m, 1H), 7.04–6.94 (m, 2H), 3.39 (h, J = 6.9 Hz, 1H), 2.94 (hept, J = 6.8 Hz, 1H), 2.69 (s, 3H), 2.61–2.49 (m, 1H), 2.37 (qt, J = 12.4, 3.1 Hz, 1H), 2.29 (d, J = 10.9 Hz, 1H), 2.09 (dd, J = 6.0, 2.6 Hz, 4H), 1.98 (ddd, J = 13.4, 9.5, 5.0 Hz, 2H), 1.94–1.75 (m, 5H), 1.56 (dd, J = 6.9, 2.5 Hz, 7H), 1.51–1.23 (m, 7H), 1.22–1.06 (m, 6H), 0.94–0.83 (m, 4H), 0.67 (d, J = 6.8 Hz, 2H), 0.15 (m, 1H) ppm. 13C NMR (126 MHz, CD3OD): δ 157.5, 156.3, 151.5, 145.3, 145.2, 144.4, 135.6, 134.1, 133.1, 132.9, 131.8, 130.5, 130.4, 129.5, 129.1, 129.1, 128.6, 128.5, 128.2, 128.0, 127.8, 127.2, 126.9, 125.9, 124.6, 123.4, 122.1, 41.9, 40.8, 40.7, 39.7, 39.6, 38.5, 37.4, 35.8, 35.6, 34.5, 33.9, 33.2, 32.9, 32.4, 31.4, 30.3, 30.2, 28.4, 28.0, 26. 8, 26.6, 25.6, 25.1, 24.6, 24.1, 23.2, 22.8 ppm (observed complexity due to C–P splitting). 31P NMR (121 MHz, CD3OD) δ 39.49 ppm. IR (neat, cm–1): 2924, 1462, 1420, 1144, 1020, 1003, 876, 766, 738.
XPhos Precatalyst 7a
Tan solid. Yield: 913 mg, 99%. 1H NMR (500 MHz, CD3OD): δ 8.02–7.94 (m, 1H), 7.77–7.71 (m, 2H), 7.69–7.60 (m, 3H), 7.56–7.48 (m, 2H), 7.26–7.19 (m, 1H), 7.13–7.06 (m, 2H), 7.05–6.93 (m, 4H), 6.89 (tt, J = 7.4, 1.3 Hz, 1H), 6.79–6.73 (m, 1H), 6.65 (dd, J = 8.4, 1.4 Hz, 2H), 3.20 (hept, J = 6.9 Hz, 1H), 2.97 (hept, J = 6.8 Hz, 1H), 2.69 (s, 3H), 2.56–2.30 (m, 3H), 2.09 (d, J = 12.8 Hz, 1H), 2.05–1.86 (m, 4H), 1.86–1.68 (m, 2H), 1.64–1.37 (m, 3H), 1.37–1.20 (m, 6H), 1.20–1.05 (m, 6H), 0.97 (ddd, J = 16.6, 8.1, 3.7 Hz, 1H), 0.93–0.84 (m, 5H), 0.70 (d, J = 6.8 Hz, 2H), −0.09 (dh, J = 17.1, 4.7, 3.9 Hz, 1H) ppm. 13C NMR (126 MHz, CD3OD): δ 156.0, 155.0, 152.1, 145.5, 145.3, 143.4, 142.9, 142.0, 139.9, 137.5, 135.3, 135.2, 133.6, 133.4, 133.3, 132.4, 130.1, 129.1, 129.1, 129.0, 128.9, 128.8, 128.8, 128.5, 127.3, 126.6, 125.9, 125.3, 123.2, 38.9, 36.6, 36.4, 34.9, 34.6, 33.4, 32.8, 32.6, 32.1, 30.8, 30.0, 28.4, 28.3, 27.9, 27.8, 26.8, 26.7, 26.5, 26.4, 26.4, 26.3, 26.1, 25.5, 25.5, 24.5, 23.7, 22.9, 22.8, 22.7, 13.8 ppm (observed complexity due to C–P splitting). 31P NMR (121 MHz, CD3OD): δ 40.59 ppm. IR (neat, cm–1): 2923, 1422, 1254, 1145, 1024, 1002, 773, 760, 740, 691.
BrettPhos Precatalyst 6b (11 mmol scale)
Off-white solid. Yield: 9.59 g, 94%. 1H NMR (500 MHz, CD3OD): δ 7.61 (dd, J = 7.5, 1.6 Hz, 1H), 7.56 (d, J = 1.8 Hz, 1H), 7.52 (d, J = 1.9 Hz, 1H), 7.36 (td, J = 7.5, 1.3 Hz, 1H), 7.32 (td, J = 7.6, 1.6 Hz, 1H), 7.28–7.22 (m, 3H), 7.17 (h, J = 2.6, 2.2 Hz, 3H), 6.99 (dd, J = 7.7, 1.3 Hz, 1H), 3.88 (s, 3H), 3.45 (s, 3H), 3.37 (dq, J = 13.9, 6.8 Hz, 1H), 2.99 (p, J = 6.7 Hz, 1H), 2.95–2.83 (m, 1H), 2.83–2.73 (m, 1H), 2.69 (s, 3H), 2.14 (d, J = 11.2 Hz, 1H), 2.08–1.93 (m, 6H), 1.90 (d, J = 10.1 Hz, 1H), 1.87–1.67 (m, 4H), 1.63–1.18 (m, 11H), 1.18–0.77 (m, 6H), 0.71 (dd, J = 9.2, 6.7 Hz, 5H), 0.41 (qdd, J = 12.8, 6.3, 3.5 Hz, 1H). 13C NMR (126 MHz, CD2Cl2): δ 158.2, 156.4, 156.1, 155.7, 152.3, 152.1, 151.6, 147.1, 142.0, 141.3, 139.7, 135.3, 135.2, 134.6, 130.9, 129.5, 128.5, 128.0, 127.6, 124.2, 123.5, 123.3, 122.1, 120.1, 116.1, 113.2, 56.0, 55.4, 40.7, 40.0, 35.2, 34.7, 34.5, 34.0, 33. 8, 33.4, 31.4, 30.5, 30.2, 28.8, 28.7, 28.4, 28.3, 27.4, 27.3, 27.2, 26.8, 26.5, 26.1, 25.0, 25.0, 24.9, 24.5, 24.4 ppm. 31P NMR (121 MHz, CD3OD): δ 41.61. IR (neat, cm–1): 3236, 2925, 2849, 1422, 1252, 1215, 1201, 1173, 1041, 1011, 763, 747, 739, 727.
BrettPhos Precatalyst 7b (11 mmol scale)
Tan solid. Yield: 9.35 g, 86%. 1H NMR (500 MHz, CD3OD): δ 7.73–7.66 (m, 1H), 7.65 (d, J = 1.8 Hz, 1H), 7.61 (d, J = 1.8 Hz, 1H), 7.56–7.46 (m, 2H), 7.42–7.34 (m, 1H), 7.29–7.23 (m, 1H), 7.19 (d, J = 2.2 Hz, 2H), 7.13–7.06 (m, 1H), 7.06–6.96 (m, 4H), 6.96–6.91 (m, 2H), 6.90–6.78 (m, 3H), 6.65–6.58 (m, 2H), 3.88 (s, 3H), 3.47 (s, 3H), 3.12 (hept, J = 7.0 Hz, 1H), 3.07–2.89 (m, 2H), 2.71 (s, 4H), 2.24 (d, J = 11.2 Hz, 1H), 2.06 (p, J = 6.8 Hz, 1H), 2.02–1.97 (m, 3H), 1.98–1.83 (m, 2H), 1.83–1.46 (m, 5H), 1.46–1.21 (m, 5H), 1.20–0.80 (m, 9H), 0.75 (dd, J = 20.6, 6.7 Hz, 5H), 0.30 (dtd, J = 13.4, 10.8, 10.0, 6.3 Hz, 1H) ppm. 13C NMR (126 MHz, CD2Cl2): δ 156.7, 156.0, 155.6, 155.1, 151.9, 151.6, 144.6, 143.3, 143.3, 140.6, 137.9, 135.5, 135.4, 134.9, 134.8, 129.8, 129.4, 129.2, 129.0, 128.94, 128.88, 128.5, 128.4, 127.1, 126.6, 126.3, 125.4, 125.2, 124.9, 124.8, 123.5, 122.8, 122.0, 121.8, 116.1, 113.3, 56.0, 55.3, 40.2, 35.1, 34.8, 34.6, 34.3, 34.1, 33.4, 31.8, 30.8, 30.43, 30.37, 30.31, 28.73, 28.65, 28.47, 28.3, 28.2, 27.50, 27.45, 27.38, 27.34, 26.73, 26.47, 25.36, 25.22, 24.90, 24.38, 23.73, 23.03, 14.56 ppm (observed complexity due to C–P splitting). 31P NMR (121 MHz, CD3OD): δ 45.87. IR (neat, cm–1): 2926, 1418, 1255, 1144, 1124, 1039, 1012, 1002, 758, 739, 690.
RuPhos Precatalyst 6c
White solid. Yield: 817 mg, 86%. 1H NMR (500 MHz, CD3OD): δ 8.10 (t, J = 8.4 Hz, 1H), 7.85–7.77 (m, 1H), 7.66–7.59 (m, 1H), 7.53 (tt, J = 7.6, 1.5 Hz, 1H), 7.48 (tt, J = 7.4, 1.5 Hz, 1H), 7.39–7.25 (m, 4H), 7.25–7.18 (m, 1H), 7.12–7.04 (m, 2H), 7.02 (d, J = 8.5 Hz, 1H), 6.79 (ddd, J = 7.8, 3.0, 1.3 Hz, 1H), 0.17–0.02 (m, 1H), 4.87–4.79 (m, 1H), 4.54 (hept, J = 6.1 Hz, 1H), 2.70 (s, 3H), 2.45 (tdd, J = 12.6, 9.7, 5.1 Hz, 1H), 2.34 (t, J = 11.4 Hz, 1H), 2.28–2.09 (m, 5H), 2.09–1.88 (m, 1H), 1.82 (d, J = 13.3 Hz, 1H), 1.71 (qt, J = 12.4, 3.2 Hz, 1H), 1.66–1.48 (m, 4H), 1.48–0.96 (m, 8H), 0.94–0.68 (m, 6H) ppm. 13C NMR (126 MHz, CD2Cl2): δ 163.25, 162.16, 151.60, 145.50, 142.43, 141.70, 140.22, 137.26, 135.48, 132.30, 130.86, 129.54, 128.89, 128.58, 127.77, 127.62, 127.31, 122.64, 106.58, 40.51, 40.50, 40.22, 35.9, 35.7, 31.1, 30.2, 28.0, 28.0, 27.7, 27.6, 27.2, 27.1, 26.8, 26.7, 26.6, 26.4, 22.4, 22.3, 21.5 ppm (observed complexity due to C–P splitting). 31P NMR (121 MHz, CD3OD): δ 45.04. IR (neat, cm–1): 3236, 2926, 2843, 1448, 1257, 1204, 1099, 1062, 1039, 786, 761.
RuPhos Precatalyst 7c
Orange solid. Yield: 873 mg, 96%. 1H NMR (500 MHz, CD3OD): δ 7.98 (t, J = 8.3 Hz, 1H), 7.81 (t, J = 7.6 Hz, 1H), 7.74–7.66 (m, 1H), 7.61–7.45 (m, 5H), 7.19–7.05 (m, 3H), 6.97 (dq, J = 31.7, 7.5 Hz, 4H), 6.88–6.78 (m, 2H), 6.72 (dd, J = 7.6, 3.7 Hz, 1H), 6.64 (d, J = 7.9 Hz, 2H), 4.93 (p, J = 6.0 Hz, 1H), 4.60 (p, J = 6.2 Hz, 1H), 2.69 (s, 4H), 2.41 (p, J = 11.8, 11.0 Hz, 2H), 2.23 (q, J = 12.8 Hz, 1H), 2.02 (dd, J = 21.8, 13.1 Hz, 3H), 1.96–1.48 (m, 9H), 1.46–0.83 (m, 14H), 0.72 (dd, J = 12.8, 5.9 Hz, 3H), −0.06 - −0.21 (m, 1H). 13C NMR (126 MHz, CDCl3): complex spectrum—see the Supporting Information (significant peak broadening observed). 31P NMR (121 MHz, CD3OD): δ 62.77, 46.93 ppm. IR (neat, cm–1): 2926, 1459, 1245, 1136, 1111, 1064, 1028, 1000, 761, 756, 738, 690.
SPhos Precatalyst 6d
White solid. Yield: 680 mg, 86%. 1H NMR (500 MHz, CD3OD): δ 8.16 (t, J = 8.4 Hz, 1H), 7.87–7.78 (m, 1H), 7.68–7.61 (m, 1H), 7.56–7.44 (m, 2H), 7.41–7.25 (m, 5H), 7.26–7.19 (m, 1H), 7.14–7.05 (m, 3H), 6.84 (ddd, J = 7.7, 3.1, 1.4 Hz, 1H), 3.96 (s, 3H), 3.41 (s, 3H), 2.69 (s, 3H), 2.54–2.38 (m, 1H), 2.27–2.15 (m, 2H), 2.14 (dd, J = 5.9, 2.5 Hz, 3H), 2.04 (s, 4H), 1.94 (dd, J = 11.0, 7.1 Hz, 1H), 1.81 (d, J = 13.3 Hz, 1H), 1.70 (qt, J = 12.2, 3.1 Hz, 1H), 1.65–1.47 (m, 2H), 1.42 (d, J = 13.1 Hz, 1H), 1.36 (dt, J = 12.8, 3.3 Hz, 2H), 1.30–0.95 (m, 4H), 0.95–0.74 (m, 2H), 0.10 - −0.07 (m, 1H) ppm. 13C NMR (126 MHz, CD2Cl2): complex spectrum—see the Supporting Information (significant peak broadening observed). 31P NMR (121 MHz, CD3OD): δ 46.88. IR (neat, cm–1): 1452, 1288, 1234, 1108, 1094, 1034, 1000, 888, 760, 719.
SPhos Precatalyst 7d
Bright yellow solid. Yield: 788 mg, 92%. 1H NMR (500 MHz, CD3OD): δ 8.05 (t, J = 8.4 Hz, 1H), 7.83 (td, J = 7.7, 1.6 Hz, 1H), 7.73 (dq, J = 5.3, 2.3 Hz, 1H), 7.61–7.46 (m, 5H), 7.18 (dd, J = 20.2, 8.3 Hz, 2H), 7.10 (dd, J = 7.5, 1.6 Hz, 1H), 7.07–7.00 (m, 2H), 6.95 (dddd, J = 16.4, 7.8, 6.6, 0.9 Hz, 2H), 6.89–6.81 (m, 2H), 6.76–6.70 (m, 1H), 6.63 (dq, J = 7.1, 1.1 Hz, 2H), 4.09 (s, 3H), 4.04–3.91 (m, 1H), 3.40 (s, 3H), 2.69 (s, 3H), 2.43 (d, J = 9.8 Hz, 1H), 2.36–2.17 (m, 2H), 2.13–1.97 (m, 3H), 1.91 (d, J = 13.1 Hz, 1H), 1.79 (d, J = 13.3 Hz, 1H), 1.60 (ddt, J = 50.7, 13.1, 3.4 Hz, 3H), 1.49–1.14 (m, 4H), 1.16–0.83 (m, 5H), −0.17 (dd, J = 12.0, 6.3 Hz, 1H) ppm. 13C NMR (126 MHz, CDCl3): δ 144.15, 140.04, 139.52, 134.28, 129.41, 129.14, 128.78, 127.83, 127.42, 126.97, 125.79, 124.78, 121.29, 105.55, 56.48, 56.09, 40.56, 35.94, 35.78, 34.84, 32.01, 28.54, 28.46, 27.04, 23.07, 14.81 ppm (observed complexity due to C–P splitting).31P NMR (121 MHz, CD3OD): δ 47.74 ppm. IR (neat, cm–1): 1231, 1143, 1035, 1001, 763, 740, 571.
tBuXPhos Precatalyst 6e
Light yellow solid. Yield: 720 mg, 89%. 1H NMR (500 MHz, CD3OD): δ 8.15 (t, J = 6.8 Hz, 1H), 7.93–6.80 (m, 13H), 3.44–3.32 (m, 1H), 3.14 (dt, J = 13.6, 6.8 Hz, 1H), 2.71 (m, 4H), 2.29–1.69 (m, 6H), 1.70–0.57 (m, 30H) ppm. 13C NMR (126 MHz, CD3CN): δ 160.4, 157.5, 153.9, 145.5, 145.3, 143.7, 142.0, 141.3, 139.4, 137.4, 137.4, 136.7, 135.5, 135.3, 135.0, 134.9, 132.04, 132.03, 129.8, 129.1, 128.93, 128.90, 128.8, 128.7, 128.5, 128.0, 127.9, 127.86, 127.0, 126.9, 126.4, 125.4, 124.8, 122.59, 122.58, 121.8, 43.84, 40.72, 40.71, 39.95, 39.73, 39.59, 39.47, 39.35, 35.05, 33.73, 32.21, 32.19, 32.15, 32.06, 30.82, 30.78, 26.18, 25.41, 24.28, 24.08, 24.04, 23.81, 23.26, 14.35 ppm (observed complexity due to C–P splitting). 31P NMR (121 MHz, DMSO-d6): δ 56.13 ppm. IR (neat, cm–1): 1247, 1143, 1031, 1018, 1001, 759, 747, 739, 729.
(±) BINAP Precatalyst 6f
Following general procedure A, a mixture of 4 (384 mg, 0.50 mmol, 0.50 equiv), (±)-BINAP (622 mg, 1.00 mmol, 1.00 equiv), and dichloromethane (10 mL) was stirred at room temperature for 1 h. After removal of the solvent, the residue was triturated with pentane to provide the title compound as a yellow solid. Yield: 903 mg, 90%. 1H NMR (500 MHz, CD3OD): δ 7.99–7.91 (m, 2H), 7.84–7.68 (m, 7H), 7.59–7.50 (m, 3H), 7.47–7.38 (m, 2H), 7.38–7.11 (m, 10H), 7.10–7.00 (m, 3H), 7.00–6.86 (m, 4H), 6.84–6.78 (m, 2H), 6.75 (dd, J = 7.8, 1.1 Hz, 1H), 6.71–6.64 (m, 2H), 6.52–6.47 (m, 1H), 6.39 (tdd, J = 7.6, 6.7, 2.6, 1.3 Hz, 1H), 6.35–6.31 (m, 1H), 2.68 (s, 3H), 2.28 (d, J = 2.7 Hz, 3H) ppm. 13C NMR (126 MHz, CD2Cl2): δ 164.86, 164.00, 151.61, 141.05, 141.04, 140.80, 140.30, 138.83, 138.49, 138.41, 138.15, 135.77, 135.68, 135.07, 135.02, 134.97, 134.90, 134.71, 134.69, 134.56, 134.54, 134.06, 133.98, 133.73, 133.66, 133.08, 133.06, 131.75, 131.73, 131.58, 131.08, 131.01, 130.86, 130.24, 130.18, 130.00, 129.96, 129.88, 129.12, 129.04, 128.89, 128.87, 128.70, 128.57, 128.55, 128.26, 128.17, 127.90, 127.68, 127.60, 127.58, 127.54, 127.49, 127.45, 127.25, 126.67, 126.44, 126.38, 126.31, 123.73, 123.39, 122.52, 122.10, 121.51, 41.37, 40.11 ppm (observed complexity due to C–P splitting). 31P NMR (121 MHz, CD3OD): δ 36.35 (d, J = 42.5 Hz), 35.18 (d, J = 43.6 Hz), 13.92 (d, J = 42.4 Hz), 12.42 (d, J = 43.7 Hz). IR (neat, cm–1): 3202, 1225, 1193, 1037, 758, 734, 695, 670.
Suzuki–Miyaura Coupling: General Procedure
A screw-top test tube equipped with a magnetic stir bar was charged with 6a or 7a (0.02 mmol, 2 mol %), arylboronic acid (1.50 mmol, 1.50 equiv), and aryl halide (if solid, 1.00 mmol, 1.00 equiv), and the tube was sealed with a Teflon screw-cap septum. The vessel was evacuated and backfilled with argon (this process was repeated a total of three times), and the aryl halide, if liquid (1.00 mmol, 1.00 equiv) was added at this time. Anhydrous THF (2 mL) and 0.5 M aq K3PO4 solution (4 mL) were then added via syringe, and the reaction mixture was stirred for 30 min at room temperature. The reaction mixture was diluted with EtOAc (10 mL) and H2O (10 mL), and the layers were separated. The aqueous layer was extracted with additional EtOAc (3 × 5 mL). The combined organic layers were dried over Na2SO4 and filtered through a pad of Celite. The filtrate was concentrated, and the resulting residue was purified by flash chromatography using a Biotage Isolera Four system with a SNAP 25 g cartridge to afford the desired product.
2-(Perfluorophenyl)thiophene:
White solid. Yield with 6a: 217 mg, 87%. Yield with 7a: 238 mg, 95%. Mp = 39.4–40.9 °C. 1H NMR (400 MHz, CDCl3): δ 7.56–7.55 (dd, J = 5.2, 1.2 Hz, 1H), 7.54–7.52 (dd, J = 3.8, 1.2, 1H), 7.21–7.18 (dd, J = 4.9, 3.8 Hz, 1H) ppm. 13C NMR (101 MHz, CDCl3): δ 145.3 (m), 142.7 (m), 141 (m), 139.2–138.6 (m), 136.7 (m), 130.2–130.1 (td, J = 5.5, 1 Hz), 128.3–128.2 (t, J = 3.8 Hz), 127.3, 126.3 (m), 110.0 (m) ppm. 19F NMR (282.4 MHz, CDCl3): δ −141.2 (5F) ppm. IR (neat, cm–1): 3110, 2923, 1531. 1477. 1468. 1420, 1391. 1378, 1347, 1222, 1073, 1060, 970, 819, 759, 740, 713, 690, 633. Anal. Calcd for C10H3F5S: C, 48.01; H, 1.21. Found: C, 48.19; H, 1.18.
3-(Thiophene-3-yl)quinoline
White solid. Mp = 88.5–89.0 °C. Yield with 6a: 202 mg, 95%. Yield with 7a: 210 mg, 99%. 1H NMR (400 MHz, CDCl3): δ 9.22–9.21(d, J = 2.3 Hz, 1H), 8.32–8.31 (d, J = 2.2 Hz, 1H), 8.15–8.13 (dd, J = 8.4, 1.2 Hz, 1H), 7.88–7.86 (dd, J = 8.4, 1.2 Hz, 1H), 7.72–7.68 (ddd, J = 8.4, 6.9, 1.5 Hz, 1H), 7.67 (dd, J = 2.9, 1.4 Hz, 1H), 7.58–7.54 (ddd, J = 8.1, 6.8, 1.2 Hz, 1H), 7.53–7.50 (m, 2H) ppm. 13C NMR (101 MHz, CDCl3): δ 149.4, 147.1, 138.8, 131.9, 129.2, 129.1, 128.6, 128.0, 127.8, 127.0, 126.95, 126.0, 121.5 ppm. IR (neat, cm–1): 1599, 1570, 1488, 1124, 968, 951, 876, 859, 844, 693, 667, 647, 641, 616, 603, 600. Anal. Calcd for C13H9NS: C, 73.90; H, 4.29. Found: C, 73.77; H, 4.27.
5-(2,6-Difluorophenyl)-2-methylbenzo[d]thiazole
White solid. Mp = 126–127 °C. Yield from 6a: 248 mg, 95%. Yield from 7a: 255 mg, 98%. 1H NMR (400 MHz, CDCl3): δ 8.07 (q, J = 1.4 Hz, 1H), 7.91–7.89 (d, J = 8.3 Hz, 1H), 7.47–7.43 (dd, J = 8.3, 1.6 Hz, 1H), 7.35–7.28 (tt, J = 8.4, 6.3 Hz, 1H), 7.05–6.99 (m, 2H), 2.87 (s, 3H) ppm. 13C NMR (101 MHz, CDCl3): δ 167.6, 161.4–158.9 (dd, J = 53.3, 26.4 Hz, 2C), 135.6, 129.1–128.9 (t, J = 10.4 Hz), 126.9, 124.2 (dt, J = 253, 2.2 Hz, 2C), 121.1, 118.2–117.9 (t, J = 18.9 Hz), 111.8–111.6 (d, J = 26.3 Hz), 111.8–111.6 (d, J = 12.4 Hz), 20.2 ppm. 19F NMR (282.4 MHz, CDCl3): δ −114.5 (t, J = 6.8 Hz, 2F) ppm. IR (neat, cm–1): 1626, 1586, 1463, 1442, 1411, 1270, 1251,1174, 993, 819, 781, 770, 736, 667, 659, 650, 645, 636. Anal. Calcd for C14H9F2NS: C, 64.35; H, 3.47. Found: C, 64.17; H, 3.63.
Arylation of Amines. Representative Procedure: 4-Methoxydiphenylamine (X = Cl)
A screw-top test tube equipped with a magnetic stir bar was charged with NaOtBu (115 mg, 1.20 mmol, 1.20 equiv), and the tube was sealed with a Teflon screw-cap septum. The tube was evacuated and backfilled with argon (this procedure was performed a total of three times), after which 4-chloroaniline (123 μL, 1.00 mmol, 1.00 equiv), aniline (110 μL, 1.20 mmol, 1.20 equiv), precatalyst solution (0.01 M in THF, 10 μL, 0.01 mol %) and dioxane (1 mL) were added by syringe. The reaction mixture was heated at 110 °C for 24 h, after which it was cooled to room temperature and diluted with ethyl acetate. The crude reaction mixture was then filtered through a pad of Celite, concentrated with the aid of rotary evaporation, and purified by column chromatography, eluting with 10% ethyl acetate in hexanes to provide the title compound as an off-white solid. Yield with 6b: 191 mg, 96%. Yield with 7b: 179 mg, 90%. Mp = 101–102 °C. 1H NMR (500 MHz, chloroform-d): δ 7.32–7.18 (m, 2H), 7.14–7.05 (m, 2H), 6.99–6.91 (m, 2H), 6.91–6.80 (m, 3H), 5.51 (s, 1H), 3.82 (s, 3H) ppm. 13C NMR (126 MHz, CDCl3): δ 156.0, 145.9, 136.5, 130.1, 122.9, 120.3, 116.4, 115.4, 56.3 ppm. IR (neat, cm–1): 3386, 1595, 1500, 1489, 1443, 1297, 1247, 1236, 1032, 749, 694.
4-Methoxydiphenylamine (X = I)
ArI (1 mmol), PhNH2 (1.4 mmol), NaOtBu (1.4 mmol) precatalyst solution (0.01 M in THF, 10 μL, 0.01 mol %), PhMe (1 mL), 5 min. Off-white solid. Yield with 6b: 187 mg, 94%. Yield with 7b: 193 mg, 97%. Characterization data consistent with above case where X=Cl.
5-Fluoro-N2,N4,N4-trimethyl-N2-(pyridin-4-yl)pyrimidine-2,4-diamine
ArCl (1 mmol), amine (1.2 mmol), NaOtBu (1.2 mmol), precatalyst (0.01 mmol), PhMe (1 mL). Yellow, crystalline solid. Yield with 6c: 217 mg, 88%. Yield with 7c: 210 mg, 85%. 1H NMR (500 MHz, chloroform-d) δ 8.48–8.38 (m, 2H), 7.82 (d, J = 6.4 Hz, 1H), 7.40–7.29 (m, 2H), 3.53 (s, 3H), 3.14 (d, J = 2.2 Hz, 6H) ppm. 13C NMR (126 MHz, chloroform-d) δ 156.6, 153.1, 152.6 (d, J = 6.1 Hz), 150.1, 144.2, 142.6 (d, J = 26.2 Hz), 142.2, 117.6, 39.5 (d, J = 7.0 Hz), 37.5 ppm. 19F NMR (282 MHz, CDCl3) δ −154.44 ppm. IR (neat, cm–1): 1602, 1575, 1390, 1371, 1324, 1216, 844, 827, 768, 633, 596.
Acknowledgments
We thank the National Institutes of Health for financial support (GM46059 and GM58160). We thank Dr. Peter Muller (MIT) for X-ray structural analysis. We thank Dr. Aaron C. Sather (MIT) for helpful discussions and assistance in the preparation of this manuscript. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
Supporting Information Available
Spectroscopic data and X-ray data (CIF). This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare the following competing financial interest(s): MIT has patents on the ligands used in this work from which S.L.B. and former co-workers receive royalty payments and on the precatalysts used in this work for which N.C.B. and S.L.B receive royalty payments.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- a Bruno N. C.; Tudge M. T.; Buchwald S. L. Chem. Sci. 2013, 4, 916–920. [DOI] [PMC free article] [PubMed] [Google Scholar]; b For a different precatalyst recently developed in our laboratory based on ligated Pd(0)-1, 5-cod dimers, see:Lee H. G.; Milner P. J.; Buchwald S. L. Org. Lett. 2013, 15, 5602–5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Bruno N. C.; Buchwald S. L. Org. Lett. 2013, 15, 2876–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Cheung C. W.; Surry D. S.; Buchwald S. L. Org. Lett. 2013, 15, 3734–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Senecal T. D.; Shu W.; Buchwald S. L. Angew. Chem., Int. Ed. 2013, 52, 10035–10039. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Zheng B.; Jia T.; Walsh P. J. Org. Lett. 2013, 15, 4190–4193. [DOI] [PubMed] [Google Scholar]
- We have never observed any aminobiphenyls in samples of precatalysts or in reaction mixtures, and 2-aminobiphenyl is reported as being non-carcinogenicGorrod J. W.; Kajbaf M. Eur. J. Drug Metab. 1987, 12, 285–290. [DOI] [PubMed] [Google Scholar]; However, 4-aminobiphenyl is known to cause bladder cancer in humans:Feng Z.; Hu W.; Rom W. N.; Beland F. A.; Tang M.-S. Carcinogenesis 2002, 23, 1721–1727. [DOI] [PubMed] [Google Scholar]
- N-Methyl-2-aminobiphenyl was isolated as a 95:5 mixture of the mono-/dimethylated products, as confirmed by GC and NMR. The mixture was used directly, adjusting the equivalents of MsOH and Pd(OAc)2 to 0.95. N-Phenyl-2-aminobiphenyl contained traces of N-phenylcarbazole and diarylated product and was also used directly.
- The reaction of these ligands and 4 only reach ∼50% conversion. Their reaction with 5 produces a mixture of two species in equilibrium—the desired precatalyst and a related complex without the nitrogen bound to the Pd center.
- When allowed to react with 5, (±)-BINAP produces a mixture of two species in equilibrium—the desired precatalyst and the related complex without the nitrogen coordinated to the Pd center.
- Kinzel T.; Zhang Y.; Buchwald S. L. J. Am. Chem. Soc. 2010, 132, 11278–11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.