Abstract
New oxidative dehydrogenative couplings of pyrazol-5-amines for the selective synthesis of azopyrrole derivatives have been described. The former reaction simultaneously installs C–I and N–N bonds through iodination and oxidation, whereas the latter involved a copper-catalyzed oxidative coupling process. The resulting iodo-substituted azopyrroles were employed by treatment with various terminal alkynes through Sonogashira cross-coupling leading to new azo compounds.
Introduction
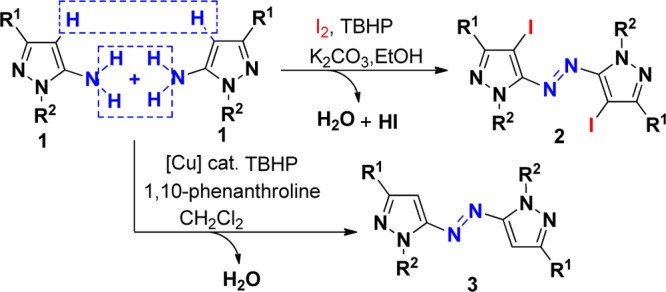
Aromatic azo compounds have been playing a pivotal role in organic and biomedical sciences for several decades.1−5 They serve as therapeutic agents, radical reaction initiators, food additives, etc.1 and can be utilized in research on photochemical molecular switches,2 self-assembly of liquid crystal materials,3 biomedical imaging,4 and light-driven molecular motors.5 Many efforts have been devoted to the synthesis of azo derivatives, which made it more powerful and applicable.6 Among them, the oxidation of anilines has taken a dominant position,7 and various stoichiometric oxidants such as manganese salts,7a lead salts,7b,7c mercury salts,7d,7e ferrates,7f,7g or N-chloroacetanilide7h have been utilized for oxidation. The reduction of nitro aromatic derivatives by stoichiometric amounts of metals was previously reported.8,9 However, these catalytic procedures are focused on the synthesis of aromatic azo compounds, and the construction of heteroaryl azo derivatives was seldom investigated. Salaheldin and co-workers have developed a protocol for the oxidation of pyrazol-5-amines to pyrazolyl azo derivatives using NBS as the oxidant, but narrow substrate scopes and the employment of environmentally malignant oxidants has limited its application.10 Therefore, the development of alternative step-economical routes to azo derivatives by minimizing waste and byproducts continues to be of great interest and importance. Herein, we demonstrate a novel method for the construction of azo compounds from inexpensive and commercially available pyrazol-5-amines under mild conditions using readily available molecular iodine (I2) with copper catalyst and tert-butyl hydroperoxide (TBHP)11 as an oxidant (Scheme 1). To the best of our knowledge, this is the first catalytic approach to azopyrazole compounds through iodination of pyrazol-5-amines using TBHP as the oxidant.
Scheme 1. Selective Formation Azopyrrole Derivatives.
Results and Discussion
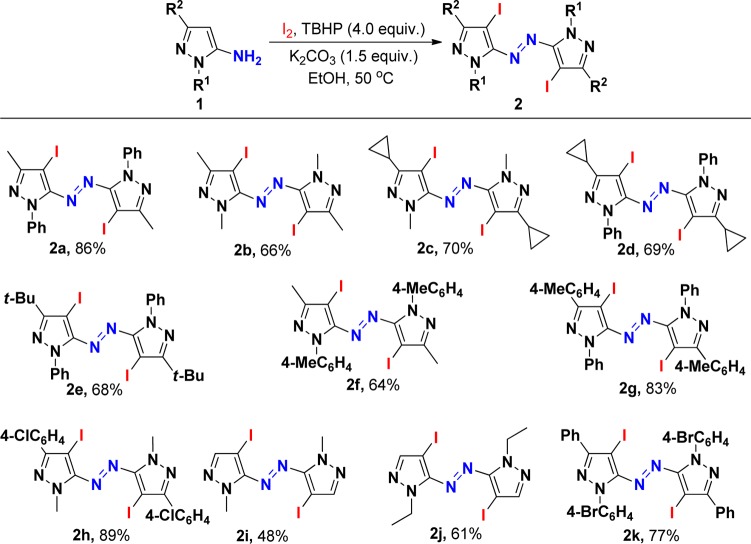
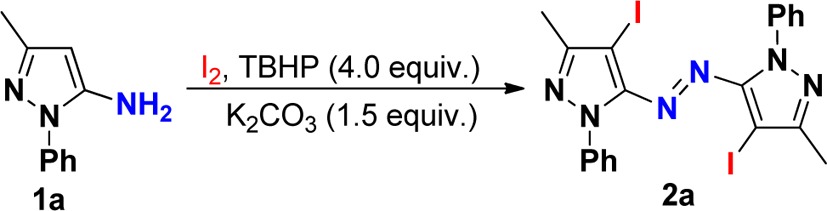
The use of iodine and TBHP as a catalytic system to construct active molecules has attracted considerable attention.12 Our investigation began with the reaction of pyrazol-5-amines (1a) in the presence of 1.1 equiv of iodine and TBHP (4.0 equiv). The results were summarized in Table 1. First, the solvent effect was examined in this reaction. It was found that the reaction cannot proceed in toluene. When CHCl3 or DMF was employed as solvent, low yields of the desired product were isolated. The identical reaction in CH2Cl2 under refluxing conditions afforded 2a in slightly higher yield (46%).13 The best yield of 67% was obtained when the reaction was performed in EtOH. We then examined the effect of different reaction temperatures on the reaction. This reaction worked more efficiently in EtOH at an enhanced temperature of 50 °C, leading to the corresponding product 2a in 86% yield (entry 7). With substrate 1a, a lower yield of the product was obtained when the reaction was performed at lower (rt) or higher (60 °C) reaction temperature (entries 6 and 8). Finally, the effect of different amounts of iodine was examined. When the amount of iodine was changed to 1.1 equiv from 1.0 equiv, the corresponding yield was improved to 86% from 62%. A further increase in the amount of iodine to 1.3 equiv failed to improve the yield of desired product 2a. Moreover, without TBHP, the reaction of 1a with 1.1 equiv of iodine in the presence of K2CO3 (1.5 equiv) did not proceed at 50 °C, using ethanol as a solvent (see the control experiment). The structure of 2a was determined by X-ray diffraction analysis (see the Supporting Information). It was shown that in this reaction iodine behaved as both Lewis acid catalyst and iodination reagent for this oxidative dehydrogenative coupling.
Table 1. Optimization for the Synthesis of Product 2a.
| entry | I2 (equiv) | solvent | temp (°C) | yielda (%) |
|---|---|---|---|---|
| 1 | 1.1 | toluene | 40 | trace |
| 2 | 1.1 | CHCl3 | 40 | 38 |
| 3 | 1.1 | DMF | 40 | 22 |
| 4 | 1.1 | CH2Cl2 | reflux | 46 |
| 5 | 1.1 | EtOH | 40 | 67 |
| 6 | 1.1 | EtOH | rt | 42 |
| 7 | 1.1 | EtOH | 50 | 86 |
| 8 | 1.1 | EtOH | 60 | 79 |
| 9 | 1.0 | EtOH | 50 | 62 |
| 10 | 1.3 | EtOH | 50 | 84 |
Isolated yield.
With these optimized reaction conditions in hand, the scope of pyrazol-5-amines in the direct oxidative dehydrogenative coupling of iodine with a variety of pyrazol-5-amines was investigated. The results were summarized in Scheme 2. As aniticipated, the substituents on the aromatic ring of pyrazol-5-amines 1 did not hamper the reaction process. Reactions of methyl- (1f,g), chloro- (1h), or bromophenyl-substituted (1k) pyrazol-5-amines proceeded efficiently to afford the desired products in good to excellent yields. Variation of nitrogen-tethered substituents on the pyrazole ring 1 including methyl cyclopropyl, ethyl, and tert-butyl groups afforded heteroaryl azo compounds in moderate to good yields through oxidative dehydrogenative coupling and iodination using TBHP as the oxidant. Importantly, iodination of heteroaromatics is an electrophilic substitution reaction, which has immense synthetic and industrial value.14
Scheme 2. Synthesis of Azopyrrole Derivatives 2.
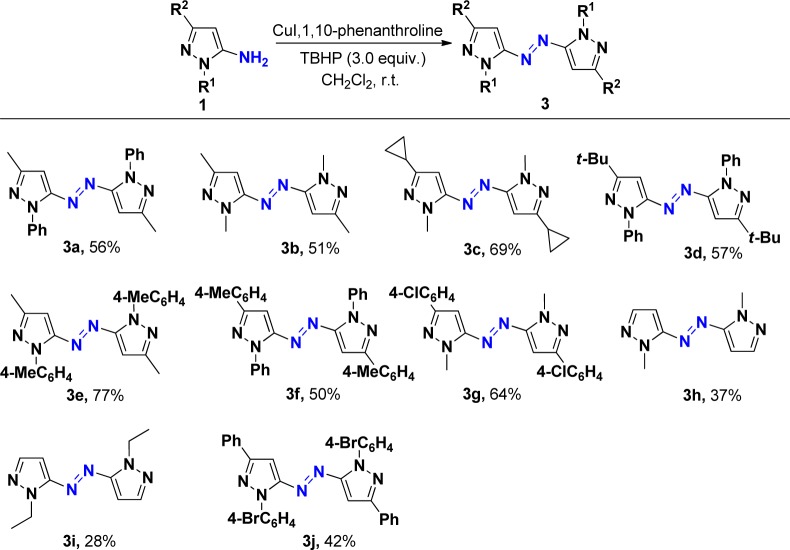
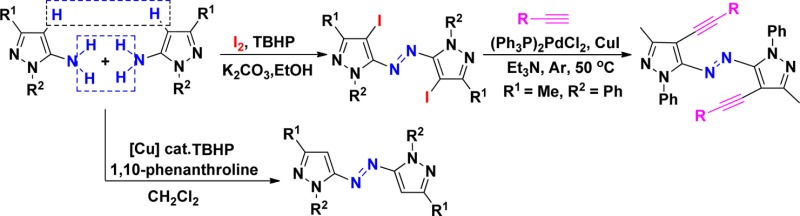
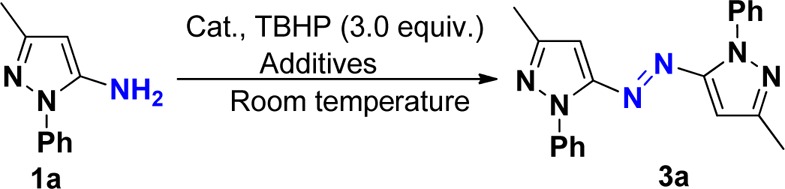
After the above reactions were accomplished, we then turned our attention toward examining the oxidative dehydrogenative coupling of pyrazol-5-amines without iodination. We envisaged that the above oxidative iodination is occurring due to excess usage of iodine (1.1 equiv) and examined whether the products 2 or 3 can be obtained with the decrease in the amount of iodine to 0.1 equiv. On the basis of this analysis, oxidative dehydrogenative coupling of pyrazol-5-amines 2a was attempted using 0.1 equiv of iodine as catalyst and 3.0 equiv of TBHP as the oxidant, and the reaction was performed in EtOH. However, the product 2 was still obtained. Without iodine, this coupling reaction proceeds with no desired product obtained. We then planned to use the copper(I) reagent as a catalyst to succeed in this oxidative dehydrogenative coupling. The coupling reaction of pyrazol-5-amines 1a was carried out in EtOH using CuI (10 mol %) as a catalyst without any additive, but no desired product 3a was detected. When pyridine was used as a ligand in this reaction, product 3a was obtained in 18% yield (Table 2, entry 1), which indicated that ligand is the key for this efficient transformation. Encouraged by the above results, we then investigated the effect of different solvents: poor yields of product 3a were obtained in CHCl3 (30%) and DMF (35%), respectively. After optimization, CH2Cl2 was found to be the best solvent for this reaction, affording the product in 45% yield. Attempts to employ other metal catalysts such as CuBr (27%), CuCl (25%), and AuCl (trace) were unsuccessful. Further studies indicated that the efficiency of this transformation was improved when 1,10-phenanthroline was used as a ligand (Table 2, entry 3). After extensive screening of other reaction parameters, 10 mol % of CuI and 30 mol % of 1,10-phenanthroline in CH2Cl2 at room temperature under air were found to be the best conditions for this reaction affording the product in 56% yield (Table 2, entry 3).
Table 2. Optimization for the Synthesis of Product 3a.
| entry | cat. (mol %) | additive (mol %) | solvent | yielda (%) |
|---|---|---|---|---|
| 1 | CuI (10) | pyridine (30) | EtOH | 18 |
| 2 | CuI (10) | pyridine (30) | CH2Cl2 | 45 |
| 3 | CuI (10) | 1,10-phen (30) | CH2Cl2 | 56 |
| 4 | CuI (5) | 1,10-phen (15) | CH2Cl2 | 42 |
| 5 | CuI (20) | 1,10-phen (60) | CH2Cl2 | 56 |
Isolated yield.
With the optimized conditions in hand, we next explored its scope using various readily available pyrazol-5-amines. A series of substituted pyrazol-5-amines 1 were successfully transformed into the corresponding heteroaryl azo compounds 3 through oxidative dehydrogenative coupling, and the results are summarized in Scheme 3. It should be noted that the reaction of pyrazol-5-amines 1i–j gave the corresponding product 3h–i in lower yields, respectively, due to its relatively lower reactivity. It should be noted that the iodination of aryl ring is special and useful in the formation of carbon–carbon bonds through Sonogashira cross-coupling.15 To further expand the scope of this reaction and construct new azo compounds, the coupling of the resulting azopyrroles 2a with various terminal alkynes 4 was performed with a palladium catalyst and a copper(I) cocatalyst, using Et3N as base at 50 °C under argon. The expected coupling products 5 were isolated in 28–51% chemical yields (Scheme 4). 4-Methyl- and 4-t-butyl-substituted arylalkynes showed higher reactivity than those substituents in the meta position, therefore providing better yields. Hex-1-yne was suitable for this Sonogashira cross-coupling. The structure of 5b was determined by X-ray diffraction analysis (see the Supporting Information).
Scheme 3. Synthesis of Azopyrrole Derivatives 3.
Scheme 4. Application of Azopyrroles 2a.
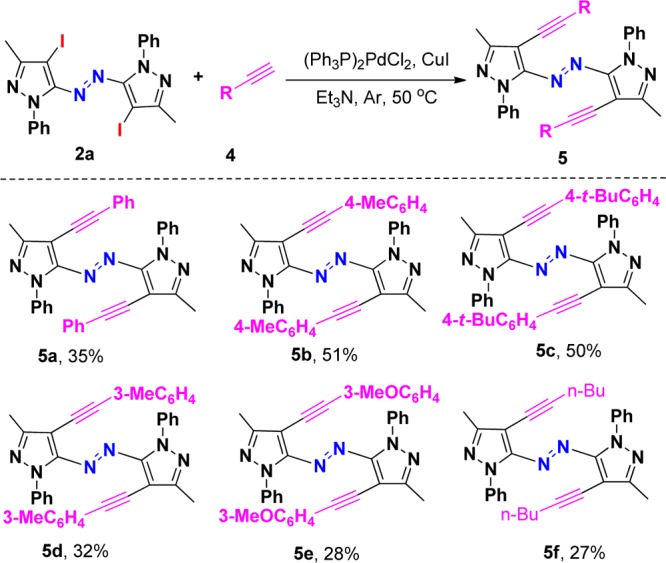
Conditions: (Ph3P)2PdCl2 (5 mol %), CuI (10 mol %), Et3N (4.0 mL), 50 °C, 12 h.
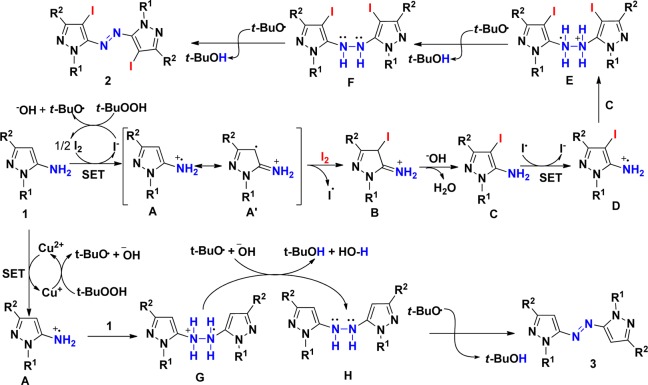
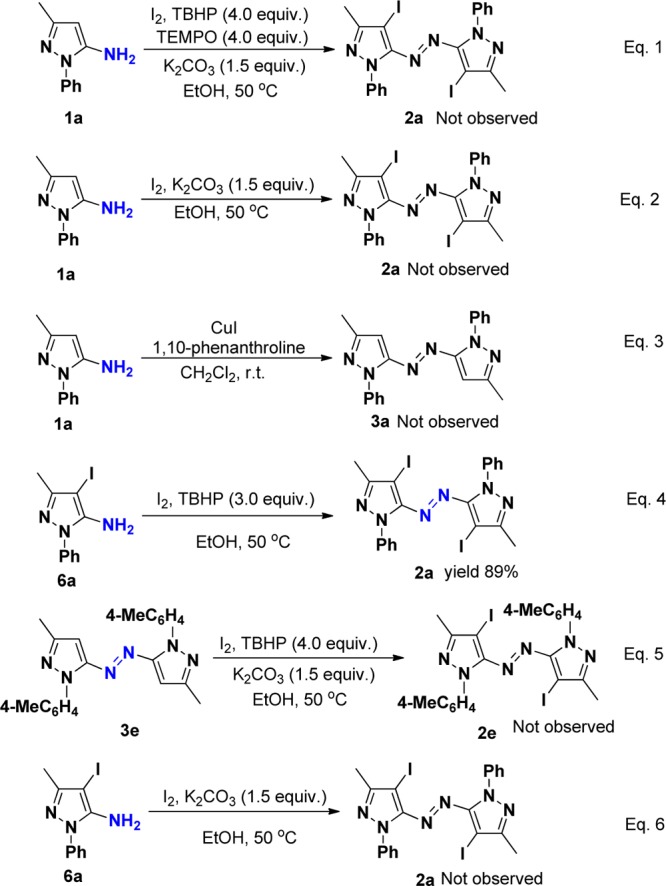
As a probe to the mechanism (Scheme 5), when the mixture of pyrazol-5-amines 1a with 1.1 equiv of iodine and TBHP (4.0 equiv) was treated in the presence of radical scavenger TEMPO (4 equiv), the reaction gave complex mixtures and the desired product 2a was not observed, indicating the possibility of a radical mechanism (eq 1). As a further support to the radical path of the mechanism, the reactions were carried out independently with I2 and CuI under air conditions without the use of aq TBHP. The desired products 2a and 3a were not observed in either of these reactions (eqs 2 and 3). These control experiments suggest that neither I2 nor CuI alone is sufficient to form the radical cation and the radical initiator TBHP is essential for this transformation. Furthermore, to further confirm the iodination sequence, the reaction of 4-iodopyrazoles was carried out to generate the desired azopyrrole product 2a in 89% yield (eq 4),16 but the reaction of azopyrroles 3e did not give the desired azopyrrole product 2e (eq 5). These controlled experiments suggest that TBHP is essential for this transformation and the iodination occurred prior to coupling step. To determine the oxidative dehydrogenation process, the reaction of 4-iodopyrazoles 6a in the presence of 1.1 equiv of iodine was performed, which did not provide the desired azopyrrole 2a (eq 6). It was shown that iodine cannot promote the coupling of the NH2 group. Thus, we reasoned that in situ generated tert-butoxy radical from TBHP catalyzed by I2 may take part in the oxidative dehydrogenation process.
Scheme 5. Control Experiments.
On the basis of literature reports17 and from the observations of the control experiments, a mechanism analogous to the one recently proposed18 was envisaged for these reactions. Since peroxides are the most common source of spontaneously induced free radicals, which was in combination with I211or copper salt19 to serve as a single-electron oxidant, it was proposed that a single-electron transfer (SET) process might be involved in the dehydrogenative coupling. The proposed mechanism for forming azopyrroles 2 and 3 is depicted in Scheme 6. In the former reaction, the single-electron oxidation of pyrazol-5-amines 1,19 mediated by TBHP in the presence of I2, results in radical cation A, followed by resonance to radical cation A′, which was subjected with molecular iodine to form iodo-substituted imine cation B. Then, the imine cation B underwent tautomerization to give iodo-substituted enamines C mediated by TBHP in the presence of I2, forming the corresponding radical cation D. Subsequent coupling of D with C gives a three-electron σ bond E,6a,19 which consecutively donates two protons and one electron providing hydrazine F. Hydrazine F is further converted into the corresponding aromatic azo product 2 mediated by in situ generated tert-butoxy radical from TBHP catalyzed by I2. Peroxides spontaneously induced by copper(I) catalyst are the most common source of free radicals,20 and the copper(II) catalyst, generated by the initiator peroxides, oxidizes arylamino group into arylamino radical cation by single-electron transfer (SET) process.18 Similar to the former, the latter involves a single-electron oxidation mediated by copper(II) catalyst generated by the initiator TBHP (1 to A), intermolecular coupling (A to G), and oxidization (G to H) to give aromatic azo product 3 (Scheme 6).
Scheme 6. Proposed Mechanisms for the Direct Transformation.
In conclusion, we have developed a novel oxidative dehydrogenative coupling strategy of pyrazol-5-amines for the selective formation of highly functionalized heteroaromatic azo compounds by controlling the catalytic system. The former reaction simultaneously installs C–I and N–N bonds through intermolecular iodination and oxidation, showing that the synthetic route allows us to build blocks of iodo-substituted azopyrroles 2 with a wide diversity in substituents. The latter involved a copper-catalyzed oxidative coupling of pyrazol-5-amines, which were directly converted into azopyrroles 3. The mild reaction conditions, selective modification of pyrrole skeleton, and high bond-forming efficiency (BFE) make this oxidative coupling strategy highly viable for future applications. A detailed study of this oxidative dehydrogenative mechanism and the application of this reaction are currently in progress.
Experimental Section
General Methods
HRMS (APCI or ESI) was measured with a miroTOF HRMS instrument.
Example for the Synthesis of 2a
(E)-1,2-Bis(4-iodo-3-methyl-1-phenyl-1H-pyrazol-5-yl)diazene
3-Methyl-1-phenyl-1H-pyrazol-5-amine (1a, 1 mmol, 173 mg) was introduced in a 25 mL reaction flask, and I2 (1.1 mmol, 279.4 mg), K2CO3 (1.5 mmol, 207 mg), and EtOH (3.0 mL) as well as aq TBHP (TBHP 70% solution in water, 0.2 mL, 2.0 mmol) were then successively added and the mixture stirred at 50 °C for 3 h. After completion of the reaction (monitored by TLC), the reaction mixture was then cooled to room temperature and diluted with cold water (50 mL). The solid product was collected by Büchner filtration and was purified by flash column chromatography (silica gel, mixtures of petroleum ether/EtOAc, 10:1, v/v) to afford the pure product 2a: yellow solid, 0.255 g; yield 86%; mp 243–244 °C; IR (KBr, ν, cm–1) 1595, 1504, 1458, 1422, 1035, 824, 768, 688, 648; 1H NMR (400 MHz, CDCl3) (δ, ppm) 8.37–5.86 (m, 10H, ArH), 2.40 (s, 3H, CH3), 2.37 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3) (δ, ppm) 153.7, 138.9, 129.5, 129.3, 128.9, 128.2, 125.90, 125.0, 14.5; HRMS (APCI) m/z calcd for C20H17I2N6 594.9604 [M + H]+, found 594.9599.
(E)-1,2-Bis(4-iodo-1,3-dimethyl-1H-pyrazol-5-yl)diazene (2b):
yellow solid, 0.155 g; yield 66%; mp 289–290 °C; IR (KBr, ν, cm–1) 1635, 1456, 1302, 1036, 882, 700; 1H NMR (400 MHz, CDCl3) (δ, ppm) 4.21 (s, 6H, CH3), 2.34 (s, 6H, CH3); 13C NMR (100 MHz, CDCl3) (δ, ppm) 152.4, 148.9, 55.9, 40.1, 14.7; HRMS (APCI) m/z calcd for C10H12I2N6: 470.9291 [M + H]+, found: 470.9291.
(E)-1,2-bis(3-cyclopropyl-4-iodo-1-methyl-1H-pyrazol-5-yl)diazene (2c)
Yellow solid, 0.183 g, yield 70%; mp 248–250 °C; IR (KBr, ν, cm–1) 3091, 1541, 1493, 1477, 1311, 1021, 832, 698; 1H NMR (400 MHz, CDCl3) (δ, ppm) 4.16 (s, 6H, CH3), 1.99–1.79 (m, 2H, CH), 1.08–0.70 (m, 8H, CH2); 13C NMR (100 MHz, CDCl3) (δ, ppm) 154.6, 147.7, 57.4, 49.2, 8.4, 6.6; HRMS (APCI) m/z calcd for C14H17I2N6 522.9604 [M + H]+, found 522.9586.
(E)-1,2-Bis(3-cyclopropyl-4-iodo-1-phenyl-1H-pyrazol-5-yl)diazene (2d):
orange red solid, 0.223 g; yield 69%; mp 91–92 °C; IR (KBr, ν, cm–1) 1594, 1506, 1415, 1331, 1003, 833, 765, 692; 1H NMR (400 MHz, CDCl3) (δ, ppm) 7.58–7.55 (m, 2H, ArH), 7.50–7.39 (m, 5H, ArH), 7.38–7.29 (m, 3H, ArH), 2.05–1.83 (m, 2H, CH), 1.09–0.93 (m, 8H, CH2); 13C NMR (100 MHz, CDCl3) (δ, ppm) 129.4, 129.3, 128.8, 128.1, 125.9, 125.0, 52.5, 9.9, 9.6, 8.2, 7.9; HRMS (APCI) m/z calcd for C24H21I2N6 646.9917 [M + H]+, found 646.9916.
(E)-1,2-Bis(3-tert-butyl-4-iodo-1-phenyl-1H-pyrazol-5-yl)diazene (2e):
red solid, 0.231 g; yield 68%; mp 186–189 °C; IR (KBr, ν, cm–1) 1542, 1507, 1458, 1418, 999, 756, 685, 669; 1H NMR (400 MHz,) (δ, ppm) 8.47 (d, J = 8.3 Hz, 4H, ArH), 7.57 (t, J = 7.8 Hz, 4H, ArH), 7.39–7.16 (m, 2H, ArH), 1.72 (s, 18H, CH3); 13C NMR (100 MHz, CDCl3) (δ, ppm) 153.1, 141.9, 139.9, 132.4, 129.2, 125.1, 119.4, 34.3, 29.2; HRMS (APCI) m/z calcd for C26H28I2N6 679.0543 [M + H]+, found 679.0543.
(E)-1,2-Bis(4-iodo-3-methyl-1-p-tolyl-1H-pyrazol-5-yl)diazene (2f):
red solid, 0.200 g; yield 64%; mp 201–203 °C; IR (KBr, ν, cm–1) 1559, 1542, 1509, 1457, 1173, 1037, 816, 780, 669; 1H NMR (400 MHz, CDCl3) (δ, ppm) 7.48 (d, J = 7.4 Hz, 4H, ArH), 7.37–7.09 (m, 4H, ArH), 2.42 (s, 6H, CH3), 2.38 (s, 6H, CH3); 13C NMR (100 MHz, CDCl3) (δ, ppm) 153.4, 149.6, 138.1, 136.6, 129.4, 125.6, 52.6, 21.2, 14.5; HRMS (APCI) m/z calcd for C22H22I2N6 625.0074 [M + H]+, found 625.0089.
(E)-1,2-Bis(4-iodo-1-phenyl-3-p-tolyl-1H-pyrazol-5-yl)diazene (2g):
orange red solid, 0.310 g, yield 83%; mp 142–144 °C; IR (KBr, ν, cm–1) 1595, 1499, 1422, 1299, 1200, 962, 822, 688; 1H NMR (400 MHz, CDCl3) (δ, ppm) 7.79–7.71 (m, 4H, ArH), 7.65 (d, J = 7.9 Hz, 3H, ArH), 7.51 (d, J = 5.8 Hz, 2H, ArH), 7.49–7.38 (m, 7H, ArH), 7.29 (s, 2H, ArH), 2.42 (s, 3H, CH3), 2.41 (s, 3H,CH3); 13C NMR (100 MHz, CDCl3) (δ, ppm) 149.9, 129.7, 129.4, 129.3, 129.2, 129.0, 128.9, 128.6, 128.5, 126.0, 124.9, 21.4; HRMS (APCI) m/z calcd for C32H24I2N6 747.0230 [M + H]+, found 747.0252.
(E)-1,2-Bis(3-(4-chlorophenyl)-4-iodo-1-methyl-1H-pyrazol-5-yl)diazene (2h):
yellow solid, 0.277 g, yield 89%; mp 146–147 °C; IR (KBr, ν, cm–1) 1596, 1508, 1432, 1397, 1089, 981, 830; 1H NMR (400 MHz, CDCl3) (δ, ppm) 7.83–7.70 (m, 4H, ArH), 7.45 (d, J = 7.8 Hz, 4H, ArH), 4.31 (s, 6H, CH3); 13C NMR (100 MHz, CDCl3) (δ, ppm) 151.6, 135.4, 129.9, 129.7, 129.6, 128.7, 128.6, 59.6, 42.3; HRMS (APCI) m/z calcd for C20H15Cl2I2N6 662.8825 [M + H]+, found 662.8853.
(E)-1,2-Bis(4-iodo-1-methyl-1H-pyrazol-5-yl)diazene (2i):
yellow solid, 0.106 g; yield 48%; mp 99–101 °C; IR (KBr, ν, cm–1) 1541, 1436, 1329, 1023, 967, 876, 829; 1H NMR (400 MHz, CDCl3) (δ, ppm) 7.62 (s, 2H, CH), 4.28 (s, 6H, CH3); 13C NMR (100 MHz, CDCl3) (δ, ppm) 146.3, 144.9, 59.78, 42.3; HRMS (APCI) m/z calcd for C8H9I2N6 442.8972 [M + H]+, found 442.8968.
(E)-1,2-Bis(1-ethyl-4-iodo-1H-pyrazol-5-yl)diazene (2j):
red solid, 0.143 g; yield 61%; mp 179–181 °C; IR (KBr, ν, cm–1)1541, 1436, 1304, 1064, 993, 946, 842, 742, 679; 1H NMR (400 MHz, CDCl3) (δ, ppm) 7.69 (s, 2H, CH), 4.69 (q, J = 7.2 Hz, 4H, CH2), 1.54 (t, J = 7.1 Hz, 6H, CH3); 13C NMR (100 MHz, CDCl3) (δ, ppm) 147.7, 146.4, 47.0, 45.6, 15.8; HRMS (APCI) m/z calcd for C10H13I2N6 470.9286 [M + H]+, found 470.9287.
(E)-1,2-Bis(1-(4-bromophenyl)-4-iodo-3-phenyl-1H-pyrazol-5-yl)diazene (2k):
orange red solid; 0.337 g; yield 77%; mp 173–174 °C; IR (KBr, ν, cm–1) 1634, 1491, 1420, 1321, 1072, 1011, 965, 828, 771, 696; 1H NMR (400 MHz, CDCl3) (δ, ppm) 7.92–7.79 (m, 4H, ArH), 7.69–7.54 (m, 6H, ArH), 7.54–7.44 (m, 6H, ArH), 7.33 (d, J = 8.2 Hz, 2H, ArH); 13C NMR (100 MHz, CDCl3) (δ, ppm) 132.6, 132.1, 129.6, 129.1, 128.8, 128.7, 128.6, 128.4, 127.2, 126.5,64.3; HRMS (APCI) m/z calcd for C30H19Br2I2N6 876.8103 [M + H]+, found 876.8123.
Example for the Synthesis of 3a
(E)-1,2-Bis(3-methyl-1-phenyl-1H-pyrazol-5-yl)diazene
3-Methyl-1-phenyl-1H-pyrazol-5-amine (1a, 1 mmol, 173 mg) was introduced in a 25 mL reaction flask. CuI (0.05 mmol, 9.5 mg), 1,10-phenanthroline (0.15 mmol, 27 mg), and CH2Cl2 (2.0 mL) as well as aq TBHP (TBHP 70% solution in water, 0.15 mL, 1.5 mmol) were then successively added and the mixture stirred at room temperature for 2 h. After the completion of the reaction (monitored by TLC), the solvent was removed under vacuum. The residue was separated by column chromatography on silica gel (eluent, petroleum ether/ethyl acetate 10:1 v/v) to afford the pure product 3a: oil, (eluent, petroleum ether/ethyl acetate 10:1 v/v), 0.096 g; yield 56%; IR (KBr, ν, cm–1)1597, 1517, 1422, 1343, 1141, 1021, 831, 764, 693; 1H NMR (400 MHz, CDCl3) (δ, ppm) 7.49 (d, J = 5.6 Hz, 6H, ArH), 7.41–7.35 (m, 4H, ArH), 6.99 (s, 2H, CH), 2.38 (s, 6H, CH3); 13C NMR (100 MHz, CDCl3) (δ, ppm) 148.9, 138.7, 129.34, 129.1, 128.8, 125.7, 125.0, 124.8, 106.6, 13.8; HRMS (APCI) m/z calcd for C20H19N6 343.1666 [M + H]+, found 343.1701.
(E)-1,2-Bis(1,3-dimethyl-1H-pyrazol-5-yl)diazene (3b):
yellow solid (eluent, petroleum ether/ethyl acetate 10:1 v/v), 0.056 g; yield 51%; mp 140–144 °C; IR (KBr, ν, cm–1) 1598, 1521, 1444, 1341, 1028, 993, 837, 742; 1H NMR (400 MHz, CDCl3) (δ, ppm) 6.33 (s, 2H, CH), 4.11 (s, 6H, CH3), 2.32 (s, 6H, CH3); 13C NMR (100 MHz, CDCl3) (δ, ppm) 130.9, 128.9, 93.1, 35.6, 19.2, 13.9; HRMS (APCI) m/z calcd for C10H15N6 219.1358 [M + H]+, found 219.1358.
(E)-1,2-bis(3-cyclopropyl-1-methyl-1H-pyrazol-5-yl)diazene (3c):
red oil (eluent, petroleum ether/ethyl acetate 10:1 v/v), 0.093 g; yield 69%; IR (KBr, ν, cm–1)2926, 1653, 1517, 1448, 1289, 1008, 785, 679; 1H NMR (400 MHz, CDCl3) (δ, ppm) 6.72 (s, 2H, CH), 4.16 (s, 6H, CH3), 1.99–1.82 (m, 2H, CH), 1.00–0.93 (m, 4H, CH2), 0.81–0.70 (m, 4H, CH2); 13C NMR (100 MHz, CDCl3) (δ, ppm) 163.9, 153.5, 102.6, 40.5, 9.0, 8.1; HRMS (APCI) m/z calcd for C14H19N6 271.1671 [M + H]+, found 271.1673.
(E)-1,2-Bis(3-tert-butyl-1-phenyl-1H-pyrazol-5-yl)diazene (3d):
brown oil (eluent, petroleum ether/ethyl acetate 20:1 v/v), 0.121 g; yield 57%; IR (KBr, ν, cm–1) 2955, 1596, 1522, 1419, 1345, 1234, 985, 815, 774, 697; 1H NMR (400 MHz, CDCl3) (δ, ppm) 7.51–7.45 (m, 6H, ArH), 7.42–7.38 (m, 4H, ArH), 7.06 (s, 2H, CH), 1.37 (s, 18H, CH3); 13C NMR (100 MHz, CDCl3) (δ, ppm) 161.8, 138.9, 129.2, 129.1, 128.8, 125.8, 103.8, 32.6, 30.3; HRMS (APCI) m/z calcd for C26H31N6 427.2610 [M + H]+, found 427.2623.
(E)-1,2-Bis(3-methyl-1-p-tolyl-1H-pyrazol-5-yl)diazene (3e):
yellow solid (eluent, petroleum ether/ethyl acetate 20:1 v/v), 0.143 g; yield 77%; mp 82–83 °C; IR (KBr, ν, cm–1) 2924, 1698, 1509, 1434, 1349, 1020, 840, 814, 740; 1H NMR (400 MHz, CDCl3) (δ, ppm) 7.28 (d, J = 8.6 Hz, 6H, ArH), 7.25 (d, J = 8.8 Hz, 2H, ArH), 6.97 (s, 2H, CH), 2.43 (s, 6H, CH3), 2.37 (s, 6H, CH3); 13C NMR (100 MHz, DMSO-d6) (δ, ppm) 143.5, 134.3, 131.0, 124.4, 120.2, 101.1, 25.7, 16.0, 8.6; HRMS (APCI) m/z calcd for C22H25N6 373.2141 [M + H]+, found 373.2153.
(E)-1,2-Bis(1-phenyl-3-p-tolyl-1H-pyrazol-5-yl)diazene (3f):
yellow solid (eluent, petroleum ether/ethyl acetate 15:1 v/v), 0.124 g; yield 50%; mp 157–158 °C; IR (KBr, ν, cm–1) 1595, 1506, 1423, 1332, 1012, 950, 806, 765, 689; 1H NMR (400 MHz, CDCl3) (δ, ppm) 7.75 (d, J = 8.0 Hz, 4H, ArH), 7.59–7.50 (m, 7H, ArH), 7.49–7.40 (m, 7H, ArH), 7.27 (s, 2H, CH), 2.40 (s, 6H, CH3); 13C NMR (100 MHz, CDCl3) (δ, ppm) 151.2, 139.3, 138.9, 129.8, 129.6, 129.5, 129.2, 128.2, 125.9, 125.7, 103.7, 21.4; HRMS (APCI) m/z calcd for C32H27N6 495.2297 [M + H]+, found 495.2287.
(E)-1,2-Bis(3-(4-chlorophenyl)-1-methyl-1H-pyrazol-5-yl)diazene (3g):
yellow solid (eluent, petroleum ether/ethyl acetate 15:1 v/v), 0.131 g; yield 64%; mp 132–133 °C; IR (KBr, ν, cm–1) 1600, 1543, 1494, 1437, 1333, 1091, 951, 837, 819, 743; 1H NMR (400 MHz, CDCl3) (δ, ppm) 7.72 (d, J = 8.4 Hz, 4H, ArH), 7.41 (d, J = 8.4 Hz, 4H, ArH), 7.30 (s, 2H, CH), 4.30 (s, 6H, CH3); 13C NMR (100 MHz, CDCl3) (δ, ppm) 148.4, 134.9, 129.8, 129.1, 126.8, 102.7, 41.0; HRMS (APCI) m/z calcd for C20H16Cl2N6 411.0886 [M + H]+, found 411.0848.
(E)-1,2-Bis(1-methyl-1H-pyrazol-5-yl)diazene (3h):
yellow solid (eluent, petroleum ether/ethyl acetate 30:1 v/v), 0.035 g; yield 37%; mp 77–78 °C; IR (KBr, ν, cm–1)1730, 1571, 1447, 1335, 1287, 1124, 1075, 968, 873, 833, 744; 1H NMR (400 MHz, CDCl3) (δ, ppm) 7.71 (dd, J = 9.0, 4.0 Hz, 1H, CH), 7.61 (s, 2H, CH), 7.53 (dd, J = 5.4, 3.0 Hz, 1H, CH), 4.27 (s, 6H, CH3); 13C NMR (100 MHz, CDCl3) (δ, ppm) 144.9, 130.9, 128.8, 42.2; HRMS (APCI) m/z calcd for C8H11N6 191.1045 [M + H]+, found 191.1040.
(E)-1,2-Bis(1-ethyl-1H-pyrazol-5-yl)diazene (3i):
yellow solid (eluent, petroleum ether/ethyl acetate 30:1 v/v), 0.031 g; yield 28%; mp 91–92 °C; IR (KBr, ν, cm–1)1520, 1464, 1363, 1190, 1082, 968, 855, 819, 722; 1H NMR (400 MHz, CDCl3) (δ, ppm) 7.51 (s, 2H, CH), 7.06 (s, 2H, CH), 4.66 (q, J = 7.2 Hz, 4H, CH2), 1.50 (t, J = 7.2 Hz, 6H, CH3); 13C NMR (100 MHz, CDCl3) (δ, ppm) 137.8, 128.8, 106.7, 48.6, 15.2; HRMS (APCI) m/z calcd for C10H15N6 219.1358 [M + H]+, found 219.1388.
(E)-1,2-Bis(1-(4-bromophenyl)-3-phenyl-1H-pyrazol-5-yl)diazene (3j):
yellow solid (eluent, petroleum ether/ethyl acetate 30:1 v/v), 0.131 g; yield 42%; mp 161–163 °C; IR (KBr, ν, cm–1)1588, 1526, 1505, 1422, 1345, 1069, 1006, 834, 765, 685; 1H NMR (400 MHz, CDCl3) (δ, ppm) 7.84 (d, J = 7.7 Hz, 4H, ArH), 7.67 (d, J = 8.6 Hz, 4H, ArH), 7.53–7.37 (m, 10H, ArH), 7.35 (s, 2H, CH); 13C NMR (100 MHz, CDCl3) (δ, ppm) 151.5, 137.8, 132.4, 130.7, 129.4, 129.0, 127.5, 125.8, 123.7, 119.6, 104.2; HRMS (APCI) m/z calcd for C30H21Br2N6 625.0170 [M + H]+, found 625.0174.
Example for the Synthesis of 5a
(E)-1,2-Bis(3-methyl-1-phenyl-4-(phenylethynyl)-1H-pyrazol-5-yl)diazene
Under an argon atmosphere, (E)-1,2-bis(4-iodo-3-methyl-1-phenyl-1H-pyrazol-5-yl)diazene (2a) (593 mg, 1 mmol) and CuI (19 mg, 0.1 mmol) as well as bis(triphenylphosphine)palladium(II) chloride (35 mg, 0.05 mmol) were introduced into a 25 mL Schlenk reaction flask, and ethynylbenzene 3a (208 mg, 2 mmol) and TEA (4 mL) were then successively added into this reaction mixture. The reaction system was stirred at 50 °C for 12 h. After completion of the reaction (monitored by TLC), the TEA was removed under vacuum. The residue was purified by flash column chromatography (silica gel, mixtures of petroleum ether/ethyl acetate, 40:1, v/v) to afford the pure product 5a: red solid, 0.190 g; yield 35%; mp 192–193 °C; IR (KBr, ν, cm–1) 2287, 1594, 1500, 1464, 1366, 1117, 807, 750, 686. 1H NMR (400 MHz, CDCl3) (δ, ppm) 7.82 (d, J = 8.0 Hz, 4H, ArH), 7.29–7.28 (m, 2H, ArH), 7.24–7.19 (m, 8H, ArH), 7.12–7.08 (m, 6H, ArH), 2.49 (s, 6H, CH3); 13C NMR (100 MHz, CDCl3) (δ, ppm) 153.1, 152.5, 138.7, 131.5, 128.8, 128.2, 128.1, 127.5, 124.4, 123.2, 95.3, 93.9, 81.9, 12.8; HRMS (ESI) m/z calcd for C36H27N6 543.2297 [M + H]+, found 543.2306.
(E)-1,2-Bis(3-methyl-1-phenyl-4-(p-tolylethynyl)-1H-pyrazol-5-yl)diazene (5b):
red solid, 0.291 g, yield 51%; mp 204–205 °C; IR (KBr, ν, cm–1) 2203, 1594, 1505, 1420, 1364, 1116, 815, 755, 690. 1H NMR (400 MHz, CDCl3) (δ, ppm) 7.84 (d, J = 8.0 Hz, 4H, ArH), 7.24–7.20 (m, 4H, ArH), 7.15–7.12 (m, 2H, ArH), 7.04–6.98 (m, 8H, ArH), 2.49 (s, 6H, CH3), 2.36 (d, J = 6.4 Hz, 6H, CH3); 13C NMR (100 MHz, CDCl3) (δ, ppm) 153.0, 152.5, 138.7, 138.3, 131.4, 128.9, 128.8, 127.5, 124.4, 120. 1, 95.5, 94.1, 81.3, 21.6, 12.8; HRMS (ESI) m/z calcd for C38H31N6 571.2610 [M + H]+, found 571.2638.
(E)-1,2-Bis(4-((4-tert-butylphenyl)ethynyl)-3-methyl-1-phenyl-1H-pyrazol-5-yl)diazene (5c):
red solid, 0.327 g; yield 50%; mp 208–209 °C; IR (KBr, ν, cm–1) 2210, 1594, 1499, 1461, 1363, 1101, 833, 756, 688. 1H NMR (400 MHz, CDCl3) (δ, ppm) 7.81 (d, J = 8.2 Hz, 4H, ArH), 7.24–7.22 (m, 4H, ArH), 7.19–7.16 (m, 4H, ArH), 7.08–7.04 (m, 6H, ArH), 2.48 (s, 6H, CH3), 1.32 (s, 18H, CH3); 13C NMR (100 MHz, CDCl3) (δ, ppm) 152.9, 152.5, 151.5, 138.7, 131.2, 128.7, 127.3, 125.1, 124.4, 120.2, 95.6, 94.1, 81.3, 34.8, 31.2, 12.8; HRMS (ESI) m/z calcd for C44H43N6 655.3549 [M + H]+, found 655.3550.
(E)-1,2-Bis(3-methyl-1-phenyl-4-(m-tolylethynyl)-1H-pyrazol-5-yl)diazene (5d):
red solid, 0.182 g, yield 32%; mp 161–163 °C; IR (KBr, ν, cm–1) 2273, 1596, 1499, 1409, 1366, 1158, 784, 752, 687. 1H NMR (400 MHz, CDCl3) (δ, ppm) 7.84 (d, J = 8.2 Hz, 4H, ArH), 7.20–7.18 (m, 4H, ArH), 7.14–7.06 (m, 6H, ArH), 6.97–6.90 (m, 4H, ArH), 2.50 (s, 6H, CH3), 2.28 (s, 6H, CH3); 13C NMR (100 MHz, CDCl3) (δ, ppm) 153.1, 152.5, 138.7, 137.8, 131.9, 129.1, 128.8, 128.7, 128.1, 127.4, 124.3, 123.0, 95.6, 94.0, 81.5, 21.3, 12.8; HRMS (ESI) m/z calcd for C38H31N6 571.2610 [M + H]+, found 571.2611.
(E)-1,2-Bis(4-((3-methoxyphenyl)ethynyl)-3-methyl-1-phenyl-1H-pyrazol-5-yl)diazene (5e):
red solid, 0.169 g; yield 28%; mp 174–176 °C; IR (KBr, ν, cm–1) 2246, 1594, 1499, 1420, 1368, 1116, 844, 757, 682. 1H NMR (400 MHz, CDCl3) (δ, ppm) 7.82 (d, J = 8.2 Hz, 4H, ArH), 7.22–7.07 (m, 8H, ArH), 6.85 (d, J = 8.4 Hz, 2H, ArH), 6.77–6.69 (m, 4H, ArH), 3.76 (s, 6H, CH3), 2.49 (s, 6H, CH3); 13C NMR (100 MHz, CDCl3) (δ, ppm) 159.2, 153.1, 152.6, 138.6, 129.2, 128.8, 127.5, 124.3, 124.2, 116.2, 114.8, 95.3, 93.8, 81.7, 55.3, 12.8; HRMS (ESI) m/z calcd for C38H31N6O2 603.2508 [M + H]+, found 603.2501.
(E)-1,2-Bis(4-(hex-1-yn-1-yl)-3-methyl-1-phenyl-1H-pyrazol-5-yl)diazene (5f):
red solid, 0.136 g; yield 27%; mp 238–240 °C, IR (KBr, ν, cm–1) 2238, 1595, 1504, 1422, 1340, 1186, 824, 768, 688, 760, 688; 1H NMR (400 MHz, CDCl3) (δ, ppm) 7.84–7.79 (m, 4H, ArH), 7.46–7.42 (m, 4H, ArH), 7.33 (m, 2H, ArH), 2.38 (s, 6H, CH3), 2.05 (m, 4H, CH2), 1.33 (m, 8H, CH2), 0.87 (d, J = 7.2 Hz, 6H, CH3); 13C NMR (100 MHz, CDCl3) (δ, ppm) 153.0, 152.5, 139.1, 128.7, 127.2, 124.6, 96.9, 94.4, 72.4, 29.7, 22.0, 19.5, 13.6, 12.6; HRMS (ESI) m/z calcd for C32H34N6 503.2923 [M + H], found 503.2927.
Acknowledgments
We are grateful for financial support from the NSFC (Nos. 21232004, 21272095, and 21102124) and PAPD of Jiangsu Higher Education Institutions, Jiangsu Science and Technology Support Program (No. SBE2011045), the Qing-Lan Project (12QLG006), the Robert A. Welch Foundation (D-1361), and the NIH (R33DA031860). We thank Dr. Venkatesh Kattamuri for his generous assistance.
Supporting Information Available
1H and 13C NMR spectra for all pure products, and X-ray crystal data (CIF) for 2a and 5b. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- For selected reviews, see:; a Hunger K.Industrial Dyes: Chemistry, Properties, Applications; Wiley-VCH: Weinheim, 2003. [Google Scholar]; b Anderson R. G.; Nickless G. Analyst 1967, 92, 207. [DOI] [PubMed] [Google Scholar]; c Ashutosh P. N. D.; Mehrotra J. K. Colourage 1979, 26, 25. [Google Scholar]; d Athey R. D. Jr. Eur. Coatings J. 1998, 3, 146. [Google Scholar]; e Sheppard C. S. Encycl. Polym. Sci. Eng. 1985, 2, 143. [Google Scholar]; f Hoult J. R. S. Drugs 1986, 32, 18. [DOI] [PubMed] [Google Scholar]; g Sandborn W. J. Am. J. Gastroenterol. 2002, 97, 2939. [DOI] [PubMed] [Google Scholar]; h Cation S. C.; Farris E.. Concise Encyclopedia of Chemical Technology; Wiley: New York, 1985. [Google Scholar]
- Cisnetti F.; Ballardini R.; Credi A.; Gandolfi M. T.; Masiero S.; Negri F.; Pieraccini S.; Spada G. P. Chem.—Eur. J. 2004, 10, 2011. [DOI] [PubMed] [Google Scholar]
- a Bandara D.; Burdette C. S. Chem. Soc. Rev. 2012, 41, 1809. [DOI] [PubMed] [Google Scholar]; b Ikeda T.; Tsutsumi O. Science 1995, 268, 1873. [DOI] [PubMed] [Google Scholar]; c Tamaoki N. Adv. Mater. 2001, 13, 1135. [Google Scholar]
- Beharry A. A.; Wong L.; Tropepe V.; Woolley G. A. Angew. Chem., Int. Ed. 2011, 50, 1325. [DOI] [PubMed] [Google Scholar]
- Murakami H.; Kawabuchi A.; Kotoo K.; Kutinake M.; Nakashima N. J. Am. Chem. Soc. 1997, 119, 7605. [Google Scholar]
- a Grirrane A.; Corma A.; Garca H. Science 2008, 322, 1661. [DOI] [PubMed] [Google Scholar]; b Corma A.; Concepcin P.; Serna P. Angew. Chem. 2007, 119, 7404. [DOI] [PubMed] [Google Scholar]; Angew. Chem., Int. Ed. 2007, 46, 7266. ; c Zhu Z.; Espenson J. H. J. Org. Chem. 1995, 60, 1326. [Google Scholar]; d Lim Y.-K.; Lee K.-S.; Cho C.-G. Org. Lett. 2003, 5, 979. [DOI] [PubMed] [Google Scholar]; e Drug E.; Gozin M. J. Am. Chem. Soc. 2007, 129, 13784. [DOI] [PubMed] [Google Scholar]
- a Baumgarten H. E.; Staklis A.; Miller E. M. J. Org. Chem. 1965, 30, 1203. [Google Scholar]; b Firouzabadi H.; Mostafavippor Z. Bull. Chem. Soc. Jpn. 1983, 56, 914. [Google Scholar]; c Birchall J. M.; Haszeldine R. N.; Kemp J. E. G. Chem. Commun. 1970, 449. [Google Scholar]; d Wenkert K.; Wickberg B. J. Am. Chem. Soc. 1962, 84, 4914. [DOI] [PubMed] [Google Scholar]; e Farhadi S.; Zaringhadam P.; Sahamieh R. Z. Acta Chim. Slov. 2007, 54, 647. [Google Scholar]; f Goldstein S. L.; McNelis E. J. Org. Chem. 1973, 38, 183. [Google Scholar]; g Huang H.; Sommerfeld D.; Dunn B. C.; Lloyd C. R.; Eyring E. M. J. Chem. Soc., Dalton Trans. 2001, 1301. [Google Scholar]; h Kumar A.; Bhattacharjee G. J. Indian Chem. Soc. 1991, 68, 523. [Google Scholar]
- Wang M. X.; Funabiki K.; Matsui M. Dyes Pigments 2003, 57, 77. [Google Scholar]
- Dabbagh H. A.; Teimouri A.; Chermahini A. N. Dyes Pigments 2007, 73, 239. [Google Scholar]
- Salaheldin A. M.; Oliveira-Campos A. M. F.; Rodrigues L. M. Tetrahedron Lett. 2007, 48, 8819. [Google Scholar]
- a Hill J. G.; Rossiter B. E.; Sharpless K. B. J. Org. Chem. 1983, 48, 3607. [Google Scholar]; b Li Z.; Li C.-J. J. Am. Chem. Soc. 2004, 126, 11810. [DOI] [PubMed] [Google Scholar]; c Li Z.; Li C.-J. J. Am. Chem. Soc. 2006, 128, 56. [DOI] [PubMed] [Google Scholar]
- a Zhang J.; Zhu D.; Yu C.; Wan C.; Wang Z. Org. Lett. 2010, 12, 2841. [DOI] [PubMed] [Google Scholar]; b Wan C.; Gao L.; Wang Q.; Zhang J.; Wang Z. Org. Lett. 2010, 12, 3902. [DOI] [PubMed] [Google Scholar]; c Dhineshkumar J.; Lamani M.; Alagiri K.; Prabhu K. R. Org. Lett. 2013, 15, 1092. [DOI] [PubMed] [Google Scholar]
- Crystal data for 2a (CCDC-953161).
- Frota C.; Casagrande G. A.; Pizzuti L.; Raminelli C. Curr. Org. Synth. 2013, 10, 265. [Google Scholar]
- a Chinchilla R.; Najera C. Chem. Rev. 2007, 107, 874. [DOI] [PubMed] [Google Scholar]; b Tykwinski R. R. Angew. Chem., Int. Ed. 2003, 42, 1566. [DOI] [PubMed] [Google Scholar]; c Diederich F.; Stang P. J.. Metal-Catalyzed Cross-Coupling Reactions; Wiley-VCH: Weinheim, 1998. [Google Scholar]; d Miyaura N.Cross-Coupling Reaction; Springer: Berlin, 2002. [Google Scholar]; e de Meijere A.; Diederich F.. Metal-Catalyzed Cross-Coupling Reactions; Wiley-VCH: Weinheim, 2004. [Google Scholar]
- For the preparation of 4-iodopyrazoles, see:Kim M. M.; Ruck R. T.; Zhao D.; Huffman M. A. Tetrahedron Lett. 2008, 49, 4026. [Google Scholar]
- a Hub W.; Schneider S.; Doerr F.; Oxman J. D.; Lewis F. D. J. Am. Chem. Soc. 1984, 106, 701. [Google Scholar]; b Scaiano J. C.; Garca S.; Garca H. Tetrahedron Lett. 1997, 38, 5929. [Google Scholar]; c Brede O.; Maroz A.; Hermann R.; Naumov S. J. Phys. Chem. A 2005, 109, 8081. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Jiao N. Angew. Chem., Int. Ed. 2010, 49, 6174. [DOI] [PubMed] [Google Scholar]
- a Gogoi A.; Guin S.; Rout S. K.; Patel B. K. Org. Lett. 2013, 15, 1802. [DOI] [PubMed] [Google Scholar]; b Xia X.-F.; Zhang L.-L.; Song X.-R.; Niu Y.-N.; Liu X.-Y.; Liang Y.-M. Chem. Commun. 2013, 1410. [DOI] [PubMed] [Google Scholar]
- Cheng K.; Huang L.; Zhang Y. Org. Lett. 2009, 11, 2908. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.