Abstract
Background
The internal anal sphincter (IAS) is a major contributing factor to anal canal pressure and is required for maintenance of rectoanal continence. IAS damage or weakening results in fecal incontinence. We have demonstrated that bioengineered intrinsically innervated human IAS tissue replacements possess key aspects of IAS physiology, like generation of spontaneous basal tone and contraction/relaxation in response to neurotransmitters. The objective of this study is to demonstrate the feasibility of implantation of bioengineered IAS constructs in the peri-anal region of athymic rodents.
Methods
Human IAS tissue constructs were bioengineered from isolated human IAS circular smooth muscle cells and human enteric neuronal progenitor cells. Upon maturation of the bioengineered constructs in culture, they were implanted surgically into the perianal region of athymic rats. Growth factor was delivered to the implanted constructs through a microosmotic pump. Implanted constructs were retrieved from the animals 4 weeks post-implantation.
Results
Animals tolerated the implantation well, and there were no early postoperative complications. Normal stooling was observed during the implantation period. Upon harvest, implanted constructs were adherent to the perirectal rat tissue, and appeared healthy and pink. Immunohistochemical analysis revealed neovascularization. Implanted smooth muscle cells maintained contractile phenotype. Bioengineered constructs responded to neuronally evoked relaxation in response to electrical field stimulation and vasoactive intestinal peptide, indicating the preservation of neuronal networks.
Conclusions
Our results indicate that bioengineered innervated IAS constructs can be used to augment IAS function in an animal model. This is a regenerative medicine based therapy for fecal incontinence that would directly address the dysfunction of the IAS muscle.
1. Introduction
Fecal incontinence, or the involuntary loss of stool and flatus, is a devastating problem that afflicts a large number of patients. Fecal incontinence is second only to dementia, as a leading cause of institutionalization in the elderly. In a survey of 3,536 women 30 to 90 years of age demonstrated a population-adjusted prevalence of fecal incontinence of 7.2%. The prevalence also increased linearly with age 1. This embarrassing and socially isolating condition has been associated with documented negative impact on quality of life in several studies 2, 3.
Fecal continence applies to normal stool consistency and volume. Its maintenance requires normal colonic transit time, a compliant rectum as well as innervation of the pelvic floor and anal sphincters. The interplay between the puborectalis muscle, rectum, the internal anal sphincter (IAS) and the external anal sphincter (EAS) is of primary importance in the maintenance of fecal continence 4, 5. Although continence is multifactorial, the IAS contributes to approximately 70% of resting anal canal pressure 6, 7. Damage to the neuromuscular integrity of the IAS, age related weakness of the sphincteric smooth muscle, surgical or obstetric trauma, etc. can result in fecal incontinence. The mainstay of therapy to date has remained non-surgical with pharmacological and dietary manipulations to improve basal IAS tone 8. Surgical interventions have ranged from repeated injections of bulking agents including Deflux® (a complex of dextranomer and hyaluronic acid, Salix Pharmaceuticals, Inc., Raleigh, NC) or implantations of autologous tissue grafts derived from skeletal muscle that unfortunately fail to have the fatigue-resistant characteristics of sphincteric smooth muscles 9. Here, we describe a regenerative medicine based approach using tissue engineered innervated IAS constructs to remedy fecal incontinence.
In our previous studies, we developed bioengineered three-dimensional IAS constructs derived from mouse, rabbit or human IAS smooth muscle cells 10–12. Smooth muscle cells were aligned concentrically around a defined luminal space. Examination of physiologic function of these constructs demonstrated that along with the maintenance of concentric alignment, the bioengineered constructs retained key aspects of IAS physiology including the generation of a spontaneous basal tone and electromechanical coupling integrity 10. We further developed an intrinsic innervation component that included a mouse fetal enteric neuronal cell line that differentiated in the presence of the IAS smooth muscle to form a functional neuronal network. We demonstrated the presence of inhibitory and excitatory motor neurons associated with the bioengineered smooth muscle 12.
As a translational therapy, we implanted bioengineered human IAS constructs subcutaneously in immunocompromised RAG1−/− mice. We demonstrated that the bioengineered constructs preserved both myogenic and neuronal functionality upon implantation, generated spontaneous basal tone and responded to electrical field stimulation12. Additionally, we evaluated angiogenic growth factors including fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), and the FDA-approved Platelet derived growth factor (PDGF) in their ability to maintain viability and improve neo-vascularization of implanted constructs13. These previous efforts, however, did not implant the IAS as an orthotopic transplant, located within the anal canal. In the present studies, we employed an athymic Rnu/Rnu rat model to establish technical feasibility of perianal in-situ implantation of bioengineered human IAS constructs with an intrinsic innervation component derived from enteric neuronal progenitor cells from the adult human intestine. We demonstrated preservation of both intrinsic muscular and neuronal function of the implanted IAS construct.
2. Materials and Methods
2.1 Materials
All tissue culture reagents were purchased from Invitrogen (Carlsbad, CA) unless specified otherwise. Growth media for smooth muscle consisted of Dulbecco’s modified Eagle medium with 10% fetal bovine serum (FBS), 1.5% antibiotics-antimycotics, and 0.6% L-glutamine. Neuronal growth media and neuronal differentiation media have been described previously 14. Type I rat tail collagen was purchased from BD Biosciences (Bedford, MA), and Collagenase was purchased from Worthington Biochemicals (Lakewood, NY).
2.2 Cell Isolation
Human IAS circular smooth muscle was isolated as described before from human IAS obtained after surgery from National Development and Research Institute (NDRI; New York, NY; project code: B1K1 001; protocol code: 001; IRBMED No. 1991-0297) and from organ donors through the Gift of Life Michigan (IRBMED No. HUM00023670) 12. Specimens were collected after all organs for transplantation were procured, between 1 and 3 hours after cross-clamp of the aorta and infusion of organ preservation solution. Most procured specimens were processed within 2 hours of leaving the operating suite.
Enteric neuronal progenitor cells were isolated from samples of human colorectum using a protocol adapted from Raghavan et al. 2013 for rabbit intestines. Briefly, a 4cm2 piece of colorectum was cleaned extensively and washed in ice-cold Hank’s balanced salt solution. The tissue was minced finely and digested in a mixture containing collagenase II, dispase and DNAse I. Following digestions, cells were screened serially through 70μm and 40μm meshes and plated in neuronal growth medium.
2.3 Bioengineering intrinsically innervated human IAS constructs
Bioengineering innervated IAS constructs using dual layered collagen/laminin hydrogels has been described before 12. Two cell types were used in the bioengineering process – i) circular smooth muscle isolated from the human IAS; and ii) enteric neuronal progenitor cells isolated from the human colorectum. A custom-made 35mm culture dish was used, with a central polydimethylsiloxane post defining luminal space. Briefly, 500,000 circular smooth muscle cells isolated from the human IAS was suspended in 0.8mg/ml Collagen I solution. The solution was also supplemented with 1X Dulbecco’s modified Eagle’s Medium, antibiotics/antimycotics, 1% fetal calf serum. In order to promote gelation, the pH of the collagen solution was adjusted to 7.4 using sodium hydroxide. The smooth muscle layer was allowed to form a gel at 37°C. Enteric neuronal progenitor cells were enzymatically dissociated into single cells following treatment with Accutase (Invitrogen). 200,000 neuronal progenitor cells were resuspended in a 0.8mg/ml Collagen gel containing 10μg/ml of laminin. Constructs were examined microscopically, for smooth muscle alignment and neuronal differentiation. Human IAS smooth muscle cells aligned concentrically around a central post defining the luminal space. The concentric alignment was complete in 2–3 days. The smooth muscle retracted the collagen gel, forming a circular structure containing both the hydrogel layers. In the presence of the smooth muscle, enteric neuronal progenitor cells differentiated and demonstrated neurite outgrowth beginning day 3. By day 10, a fibrous neuronal network was associated with the concentric smooth muscle.
2.4 Implantation of innervated human IAS constructs in the Rnu/Rnu athymic rodent rectum
All experiments were performed in accordance to guidelines set forth by the University of Michigan’s University Committee on the Use and Care of Animals. Experimental protocols were approved by this committee (protocol #09714). Male athymic nude rats (rnu/rnu) 6–7 weeks old were purchased from Harlan Laboratories (Indianapolis, IN) and housed in specific pathogen free conditions. Rats had free access to water and standard rat chow, and were allowed to acclimate between 1 to 2 weeks prior to undergoing any surgical procedure.
Human recombinant PDGF BB (20 ng/μL, CYT 501, Prospec Bio, Rehovot, Israel) was sterilely loaded into an Alzet 1004 osmotic pump (Cupertino, CA). Autoclaved Dow-Corning 0.025″ inner diameter silicone tube (Fisher Scientific 11-189-15B) was attached to the pump, and allowed to prime for 3 days prior to implantation in a 37°C incubator. The microosmotic pump was primed to deliver 52.8ng of recombinant PDGF-BB per day, for 21–24 days.
Rats were anesthetized using isoflurane and given a 0.05–0.1mg/kg subcutaneous buprenorphine injection. The rat perineum was prepped with chlorhexidine, and a circumferential incision was made approximately 3-mm away from the anal verge, and approximately 5-mm of the rat’s distal rectum was dissected free. The IAS construct was gently slipped onto the distal-most rectum over the native IAS and rested in place (Figure 1A). A subcutaneous pocket on the abdomen was created for insertion of the osmotic pump (Figure 1B). The attached catheter was then directed towards the distal rectum (Figure 1C), and sutured in place with single non-absorbable polypropylene suture to identify the construct at the time of harvest and to direct the growth factor towards the IAS implant. The perineal skin was closed using a running 3-0 polyglactin suture (Figure 1D).
Figure 1.
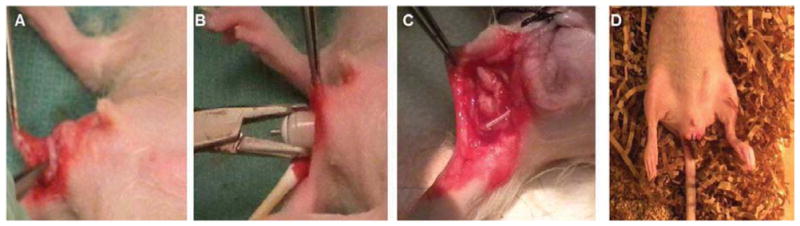
1A) A circumferential incision was made in the rat perineum, and a bioengineered innervated IAS construct was implanted over the rectum. B) A micro-osmotic pump delivering PDNF (52.8ng/day) was implanted subcutaneously, in a pocket created in the abdomen. C) The catheter from the micro-osmotic pump was directed towards the implanted bioengineered innervated human IAS construct. D) Rats recovered from anesthesia and stooling was observed within 30 minutes post-operatively.
2.5 Physiological evaluation of implanted innervated human IAS constructs
Bioengineered constructs were harvested at 4 weeks (range 28–32 days) post implantation after killing rats using CO2 asphyxiation. Constructs were identified using the previously marked polypropylene suture. The implanted construct was identified visually, then separated and dissected free from the underlying rectum and anal sphincter complex. This was performed while applying minimal stretch tension on the construct so as to not influence its post-explantation physiological function. The constructs were explanted as circular rings, from around the rectum. Physiological testing protocols were based on experiments described previously to analyze myogenic and neuronal functionality of bioengineered IAS constructs12. Explanted constructs were hooked between a stationary pin, and a measuring arm of an isometric magnetoresistive force transducer (F10, Harvard Apparatus, Holliston MA). During measurement, explanted constructs were immersed in a tissue bath filled with basal Dulbecco’s modified Eagle’s medium, maintained at 37°C. Basal tone was measured as the active tone generated spontaneously by constructs. Relaxation of basal tone was evaluated with the addition of 1μM Vasoactive Intestinal Peptide (VIP) and electrical field stimulation (EFS). Pretreatment with neuronal blocker Tetrodotoxin (TTX) was used to evaluate inhibitory motor neuronal functionality.
2.6 Immunohistochemical characterization of implanted innervated human IAS constructs
Constructs were fixed in 4% paraformaldehyde after physiological evaluation. Fixed constructs were dehydrated through graded alcohol and embedded within paraffin. 10μm thick cross-sections were cut using a microtome. Cross sections were deparaffinized and immunostained with antibodies directed against von Willebrand’s factor (neovascularization), human specific heat shock protein 27 (HSP27; implanted human smooth muscle), smooth muscle actin (smooth muscle) and smooth muscle specific caldesmon (contractile smooth muscle phenotype). Appropriate fluorophore conjugated secondary antibodies were used to visualize the presence of proteins using an inverted Nikon Ti-E microscope.
3. Results
3.1 Implantation of innervated human IAS constructs
The surgical sites were well-healed, with the rats having normal stooling patterns postoperatively. All rats survived to scheduled harvest time between 28 and 32 days post-implantation. None of the rats demonstrated signs of obstruction or difficulty with defication. And post-mortem examination of the colon failed to show evidence of fecal acculumulation. Post-mortem examination demonstrated neither infections nor abscess formations around the implanted constructs (Figure 2A). Constructs appeared pink and healthy, with no gross inflammation. The constructs were adherent to the perirectal tissue of the rat, and were identifiable by the non-absorbable polypropylene suture placed around them at the time of implantation (Figure 2B). Examination of the proximal bowel indicated no signs of obstruction (Figure 2C).
Figure 2.
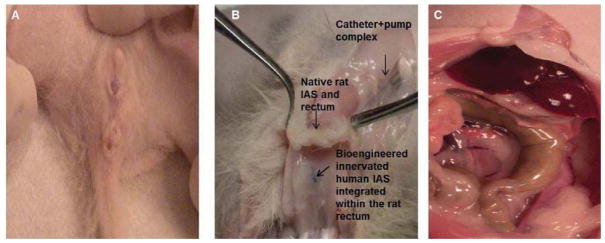
Implanted constructs were retrieved between 28–32 days post operatively. (A) The surgical site had healed very well. (B) Circumferential dissection around the anal verge was performed, and the implanted constructs were identified by the non-absorbable suture placed around them (arrow). The implanted constructs were found integrated within the perirectal soft tissue. (C) Further exploration of the rat abdomen revealed normal proximal bowel without signs of obstruction.
3.2 Immunohistochemical characterization
Histologic cross-sections of implanted constructs were stained for the endothelial specific antigen, von Willebrand’s factor. Several blood vessels were observed (Figure 3A) within the implanted construct, indicating a healthy neo-vascularization. Human HSP27 was used to identify the implanted human IAS smooth muscle, confirming the retention of the original human implanted bioengineered tissue constructs (Figure 3B). Additionally, contractile smooth muscle phenotype was confirmed by positive stains obtained for both α-smooth muscle actin and smooth muscle specific heavy Caldesmon isoform (Figure 3C,D).
Figure 3.
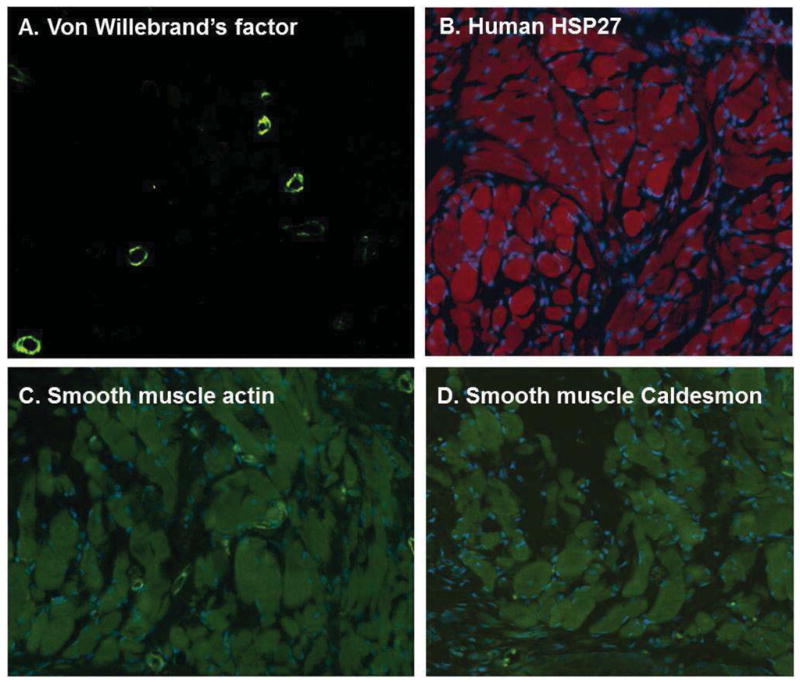
(A) Cross-sections of implanted constructs were neovascularized and several blood vessels were seen embedded within the constructs. (B) Implanted human IAS smooth muscle cells stained positive with human specific HSP27, indicated by the red fluorescence. (C–D). Implanted smooth muscle cells within bioengineered constructs also stained positive for smooth muscle actin and smooth muscle caldesmon, indicating the maintenance of a contractile phenotype in the human smooth muscle.
3.4 Physiological evaluation of implanted innervated human IAS constructs
Constructs established spontaneous basal tone, within 45 minutes. Treatment of 1μM VIP resulted in a reduction of ~90% of basal IAS tone. Relaxation was sustained for 7 minutes, followed by a recovery of 100% of basal tone (Figure 4A). Pre-treatment with neurotoxin TTX resulted in an attenuated magnitude of relaxation with VIP (~25%). TTX-sensitivity implied the presence of a functional neuronal component mediating VIP-ergic relaxation. Additionally, the myogenic mechanisms for mediating VIP-ergic relaxation were preserved upon implantation, including VIP receptor integrity in the implanted smooth muscle and the Protein Kinase A signaling pathway.
Figure 4.
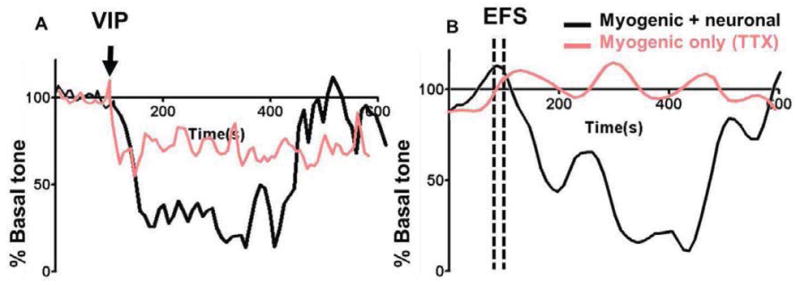
Explanted bioengineered constructs were analyzed for force generation: (A) Treatment with relaxant peptide neurotransmitter VIP resulted in a relaxation of approximately 90% of basal tone. This relaxation was attenuated in the presence of neuronal blocker TTX, indicating neural integrity and survival of inhibitory motor neurons within the implanted construct. (B) Treatment with electrical field stimulation (EFS) resulted in neuronally evoked relaxation of the smooth muscle, which was attenuated completely in the presence of TTX.
Electrical field stimulation (10Hz, 0.3ms) resulted in a neuronally mediated relaxation of basal IAS tone, upto 95%. IAS tone was recovered within 7 minutes of EFS treatment (Figure 4B). EFS-induced relaxation was completely attenuated in the presence of neurotoxin TTX, indicating that the relaxation was neuronally evoked. This further confirmed the presence and preservation of a functional inhibitory motor neuronal population upon in situ implantation.
4. Discussion
The etiology of fecal incontinence is multifactorial. Weakened or reduced IAS pressure is directly responsible for loss of anorectal continence, and is a major contributing factor on a clinical basis. Disruption of the neuromuscular integrity of the IAS can also be responsible for fecal incontinence. Current therapeutic paradigms (both surgical and non-surgical) treat symptoms, and are largely insufficient. We propose a novel regenerative medicine based therapeutic approach, whereby we implant bioengineered innervated human IAS constructs. These constructs are bioengineered using human IAS circular smooth muscle isolated from the Internal Anal Sphincter. The innervation arises from differentiating enteric neuronal progenitor cells isolated from full-thickness human colorectum. Enteric neuronal progenitor cells are neural-crest derived populations that have previously been isolated from fetal, post-natal and adult human gut up to 84 years of age. These cells have even been derived from ganglionic bowel of patients with Hirschsprung disease, and anorectal malformations 15.
In our previous studies, we demonstrated the feasibility of subcutaneously implanting innervated smooth muscle structures in mice. Implanted constructs preserved neuromuscular integrity, smooth muscle alignment and myogenic function, as well as neuronal mediated responses to electrical stimuli. Here, we describe in situ implantation in a larger rodent model, the athymic nude rat. The immune deficient rat was chosen to study implantation of bioengineered constructs of human origin outside of the context of immune rejection. Additionally, the athymic nude rat is a larger animal than the mouse model we described in previous studies. This allowed for accurate surgical dissection of the perineum in order to place a ring of cultured IAS around the distal anal canal in the location of the IAS. This has significant clinical implication in that survival of an IAS construct in this location will be a necessary step towards translating this approach into a novel treatment for human fecal incontinence. We were also able to demonstrate that the procedure was well-tolerated by the animal, the construct did not cause an obstruction, and that IAS-specific properties like contractility were maintained even after surgical manipulation. Equally important, the rats stooled normally, and demonstrated no evidence of excess scarring, contraction or obstruction.
Implanted bioengineered constructs were stained with human specific heat shock protein 27 to demonstrate that the implant area examined post-mortem contained the bioengineered human IAS implant. Histological analysis of adjacent rat rectum showed no expression of human HSP27, indicating no migration of implanted human smooth muscle cells had occurred away from the constructs, as well as the specificity of the selected human antibody. Furthermore, we demonstrated histologically that implanted human IAS smooth muscle cells maintained their contractile smooth muscle phenotype, by expressing smooth muscle actin as well as caldesmon. This is essential when implanted smooth muscle cells are required to augment the physiologic function of the existing native IAS.
We examined the relaxation component of IAS physiology, since transient relaxation upon a rectoanal inhibitory reflex (emulated by electrical field stimulation), is key to IAS function. Our studies indicated that a viable neuronal component was preserved upon implantation into the rat rectum. This neuronal component evoked a TTX-sensitive electrical field stimulated relaxation of the implanted IAS muscle. Additionally, the peptide neurotransmitter VIP also invoked a transient relaxation of IAS basal tone, which was TTX-sensitive. This indicated viable myogenic as well as neuronal contributions to the relaxation of IAS basal tone, with a functional spontaneous recovery.
Similar to the approach used here, we anticipate that in future human applications, the bioengineered sphincter complex will most likely be used in an additive manner rather than in a replacing manner as used in an orthotopic liver transplant. Specifically, the native IAS will be left in place, and the bioengineered construct will be implanted in an additive manner. The best implantation site in humans will need to be determined, but potential sites include the subcutaneous space distal to the IAS, the intersphincteric space, or the distal rectum above or below the levator muscles. These approaches will preserve the patient’s own IAS, and augment it with additional autologous functional muscle.
This current approach offers several critical advantages over current strategies. Artificial anal sphincters have been used with limited success, mainly due to infectious and erosion complications related to the presence of a foreign body in the perineum 16, 17. Gracilis muscle transposition has been used to partially circumvent this problem, but the requisite for a neural stimulator and need for close follow-up is still a challenge 18. The bioengineered IAS has the potential to overcome the problems of foreign bodies in the perineum as it is designed to be made purely from autologous tissue. There are a number of other key issues that need to be addressed in future studies. These include in-situ measurement of contractile forces of the IAS construct, in-situ evaluation of neural responsiveness of the construct and long-term macro-vascularization of constructs in larger animals. Despite these limitations, our data suggests that these constructs represent a promising development in the treatment of fecal incontinence.
Acknowledgments
This work was supported by NIH R01DK071614, 1RC1DK087151 and Training Program for Organogenesis T32HD007505.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Melville JL, Fan MY, Newton K, Fenner D. Fecal incontinence in US women: a population-based study. Am J Obstet Gynecol. 2005;193:2071–6. doi: 10.1016/j.ajog.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 2.Bharucha AE, Zinsmeister AR, Locke GR, Seide BM, McKeon K, Schleck CD, et al. Prevalence and burden of fecal incontinence: a population-based study in women. Gastroenterology. 2005;129:42–9. doi: 10.1053/j.gastro.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Fialkow MF, Melville JL, Lentz GM, Miller EA, Miller J, Fenner DE. The functional and psychosocial impact of fecal incontinence on women with urinary incontinence. Am J Obstet Gynecol. 2003;189:127–9. doi: 10.1067/mob.2003.548. [DOI] [PubMed] [Google Scholar]
- 4.Nygaard IE, Rao SS, Dawson JD. Anal incontinence after anal sphincter disruption: a 30-year retrospective cohort study. Obstet Gynecol. 1997;89:896–901. doi: 10.1016/s0029-7844(97)00119-1. [DOI] [PubMed] [Google Scholar]
- 5.Rasmussen OO. Fecal incontinence. Studies on physiology, pathophysiology and surgical treatment. Dan Med Bull. 2003;50:262–82. [PubMed] [Google Scholar]
- 6.Frenckner B. Function of the anal sphincters in spinal man. Gut. 1975;16:638–44. doi: 10.1136/gut.16.8.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao SS. Pathophysiology of adult fecal incontinence. Gastroenterology. 2004;126:S14–22. doi: 10.1053/j.gastro.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Costilla VC, Foxx-Orenstein AE, Mayer AP, Crowell MD. Office-based management of fecal incontinence. Gastroenterol Hepatol (N Y) 2013;9:423–33. [PMC free article] [PubMed] [Google Scholar]
- 9.Zailani MH, Azmi MN, Deen KI. Gracilis muscle as neoanal sphincter for faecal incontinence. Med J Malaysia. 2010;65:66–7. [PubMed] [Google Scholar]
- 10.Raghavan S, Miyasaka EA, Hashish M, Somara S, Gilmont RR, Teitelbaum DH, et al. Successful implantation of physiologically functional bioengineered mouse internal anal sphincter. Am J Physiol Gastrointest Liver Physiol. 2010;299:G430–9. doi: 10.1152/ajpgi.00269.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hecker L, Baar K, Dennis RG, Bitar KN. Development of a three-dimensional physiological model of the internal anal sphincter bioengineered in vitro from isolated smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G188–96. doi: 10.1152/ajpgi.00335.2004. [DOI] [PubMed] [Google Scholar]
- 12.Raghavan S, Gilmont RR, Miyasaka EA, Somara S, Srinivasan S, Teitelbaum DH, et al. Successful implantation of bioengineered, intrinsically innervated, human internal anal sphincter. Gastroenterology. 2011;141:310–9. doi: 10.1053/j.gastro.2011.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyasaka EA, Raghavan S, Gilmont RR, Mittal K, Somara S, Bitar KN, et al. In vivo growth of a bioengineered internal anal sphincter: comparison of growth factors for optimization of growth and survival. Pediatr Surg Int. 2011;27:137–43. doi: 10.1007/s00383-010-2786-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raghavan S, Gilmont RR, Bitar KN. Neuroglial differentiation of adult enteric neuronal progenitor cells as a function of extracellular matrix composition. Biomaterials. 2013;34:6649–58. doi: 10.1016/j.biomaterials.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindley RM, Hawcutt DB, Connell MG, Almond SL, Vannucchi MG, Faussone-Pellegrini MS, et al. Human and mouse enteric nervous system neurosphere transplants regulate the function of aganglionic embryonic distal colon. Gastroenterology. 2008;135:205–16. e6. doi: 10.1053/j.gastro.2008.03.035. [DOI] [PubMed] [Google Scholar]
- 16.Christiansen J, Rasmussen OO, Lindorff-Larsen K. Long-term results of artificial anal sphincter implantation for severe anal incontinence. Ann Surg. 1999;230:45–8. doi: 10.1097/00000658-199907000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michot F, Costaglioli B, Leroi AM, Denis P. Artificial anal sphincter in severe fecal incontinence: outcome of prospective experience with 37 patients in one institution. Ann Surg. 2003;237:52–6. doi: 10.1097/00000658-200301000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mander BJ, Abercrombie JF, George BD, Williams NS. The electrically stimulated gracilis neosphincter incorporated as part of total anorectal reconstruction after abdominoperineal excision of the rectum. Ann Surg. 1996;224:702–9. doi: 10.1097/00000658-199612000-00006. discussion 9–11. [DOI] [PMC free article] [PubMed] [Google Scholar]


