Abstract
Objectives
To determine how all-cause hospitalizations within 12 months preceding an index heart failure (HF) hospitalization affect risk stratification for 30-day all-cause readmission.
Patients and Methods
Early readmission of HF inpatients is challenging to predict, yet this outcome is used to compare hospital performance and guide reimbursement. Most risk models do not consider the potentially important variable of prior admissions. We analyzed Medicare HF inpatients aged ≥66 years admitted to 14 Michigan community hospitals between October 2002 and June 2004. Clinical data were obtained from admission charts, hospitalization dates from Centers for Medicare & Medicaid Services (CMS) claims, and mortality dates from the Social Security Death Index. We used mixed-effects logistic regression and reclassification indices to evaluate the ability of a CMS chart-based readmission risk model, prior admissions, and their combination to predict 30-day readmission in survivors of the index HF hospitalization.
Results
Of 1,807 patients, 43 (2.4%) died during the index admission; 476/1764 survivors (27%) were readmitted ≤30 days after discharge. Adjusted for the CMS readmission model, prior admissions significantly increased the odds of 30-day readmission (1 vs. 0:OR=4.67, 95% CI 3.37–6.46; ≥2 vs. 0:OR=6.49, 95% CI 4.93–8.55; both p<.001), improved model discrimination (c-statistic 0.61 to 0.74, p<.001), and reclassified many patients (net reclassification index 0.40; integrated discrimination index 0.12).
Conclusions
In Medicare HF inpatients, prior all-cause admissions strongly increase all-cause readmission risk and markedly improve risk stratification for 30-day readmission.
Introduction
More than one million Americans are hospitalized each year for heart failure (HF), and one-quarter of these are rehospitalized within 30 days.1 Recurrent hospitalizations predict long-term mortality,2–5 impair quality of life, and drive the high costs of care in HF patients.6 The Medicare Payment Advisory Commission views recurrent hospitalization as a priority area for improvement, and the Centers for Medicare and Medicaid Services (CMS) now financially penalizes hospitals with excessive risk-standardized 30-day all-cause readmission rates in HF patients. Recent work suggests that targeted intervention in high-risk HF patients can reduce 30-day readmission rates within a health system.7 Accurate risk stratification for rehospitalization in older HF patients has never been more important.
Unfortunately, hospital readmission in HF patients is challenging to predict because it often results from multiple intersecting hospital- and patient-level factors.1,8 A history of previous hospitalizations seems a likely marker for early readmission. A recent publication from the Get With The Guidelines-Heart Failure database noted that a history of prior hospitalizations had equivalent discrimination for 30-day readmission as a much more complex clinical model.9 However, most previous HF-specific readmission risk models do not incorporate prior admissions. Those that do predict readmission over a longer timeframe10,11 or require clinical data that are not typically readily available at the time of hospital admission.12
We hypothesized that all-cause hospitalizations within the year prior to an index HF admission would strongly increase the risk of 30-day all-cause readmission, and that including this factor would improve the performance of a widely-recommended CMS readmission risk model.
Methods
Definition of variables used
We used data from the Mid-Michigan Guidelines Applied in Practice – Heart Failure (GAP-HF) study for our analyses; additional details on this study are available in our other publications.2,13,14 In brief, GAP-HF was a collaborative partnership of 15 Michigan community hospitals to assess and improve the quality of inpatient HF care. Each hospital enrolled patients admitted with primary diagnosis of HF during two six-month periods (October 1, 2002–March 31, 2003 and January 1–June 30, 2004). For the purposes of this analysis, all patients were combined into a single cohort. One hospital provided data from only nine HF discharges during the study period and was excluded from our analyses, making the final sample for this study 14 hospitals.
Data on presenting symptoms, vital signs, comorbid conditions, diagnostic studies, and in-hospital therapies were extracted through manual chart review by DynKePRO (York, PA), a CMS Clinical Data Abstraction Center with previously validated high reliability and accuracy.15 Mortality data were collected using the Social Security Death Index more than 12 months after discharge from the index admission. All-cause hospitalization dates during the 12 months prior to and the 30 days following discharge from the index admission (defined as a randomly selected admission during the study period for each specific patient) were obtained from claims data through MPRO, the Michigan Medicare Quality Improvement Organization. Based on our prior work in the GAP-HF database, we categorized the number of all-cause admissions within the year prior to the index hospitalization (hereafter, “prior admissions”) as 0, 1, and 2 or more. We analyzed only Medicare patients aged ≥66 years to ensure ≥12 months of claims data for prior admissions.
Background risk stratification model
For individual readmission risk assessment, the Hospital-to-Home National Quality Initiative and the Veterans Affairs Quality Enhancement Research Initiative endorse a model derived from chart-abstracted data in nearly 80,000 Medicare HF patients from the National Heart Failure Project (CMS readmission model)1. This model was created to validate the claims-based model used by Hospital Compare to risk-standardize 30- day readmission rates in Medicare HF inpatients,1 and is readily available online at http://www.readmissionscore.org and as an iPhone app. The discrimination of the CMS readmission model for patient-level risk is low (c-statistic 0.58),1 albeit comparable to several other previously published models.9,16
Statistical analyses
All variables for the CMS readmission model were present in the GAP-HF database. We used multilevel mixed-effects logistic regression to evaluate the CMS readmission model, prior admissions, and their combination in predicting 30-day readmission, with random effects varying by hospital site to account for case clustering.
We used STATA 10.0 (STATACorp, College Station, TX) for all analyses. Mixed-effects logistic regression (STATA -xtmelogit- command) covariate effects are reported as odds ratios (OR) and 95% confidence intervals (CI). We report overall discrimination with receiver-operating characteristic curves and c-statistics. We investigated the incremental impact of adding prior admissions to the CMS model by comparing areas under the receiver-operating characteristic curve (i.e. c-statistic, using STATA’s-roccomp- command), plotting readmission predicted probability distributions, and calculating net reclassification and integrated discrimination indices.17
The primary outcome was all-cause readmission within 30 days of discharge from the index HF hospitalization; in-hospital deaths were excluded. For patients with multiple hospitalizations in the database, one index admission was randomly selected for analysis. A secondary analysis was performed using the first HF hospitalization for each patient as the index admission. As in the original CMS readmission model derivation,1 patients who were discharged then died prior to being rehospitalized were not counted as “failures,” i.e. were included in the analysis but not assigned a readmission event. The analyses were repeated in the subset of GAP-HF patients with data on ventricular ejection fraction (dichotomized at 40% to define patients with reduced and preserved ejection fraction HF).
Results
Patient characteristics
The GAP-HF cohort contained index hospitalizations in 1,807 unique Medicare HF inpatients aged ≥66. The baseline characteristics (Table 1) were similar to the National Heart Failure Project18 and more recent Medicare HF cohorts.8,9 In GAP-HF, 1,018 (56.3%) patients had not been recently hospitalized, 254 (14.1%) had been admitted once, and 535 (29.6%) had been admitted ≥2 times within the prior 12 months. The median number of patients across the 14 GAP-HF hospitals was 129 (range, 38 to 245). A total of 1,438 (80%) of patients had available data on left ventricular ejection fraction; the baseline characteristics dichotomized at ejection fraction of 40% are also shown in Table 1.
Table 1.
Demographics
| Variables | Overall cohort (n = 1,807) |
Reduced EF (<40%) (n = 669) |
Preserved EF (≥40%) (n = 769) |
|---|---|---|---|
| Age, years | 79.8 ± 7.6 | 78.8 ± 7.2 | 80.0 ± 7.7* |
| Female | 1,010 (56%) | 287 (43%) | 496 (65%)* |
| History of: | |||
| Heart failure | 1,478 (82%) | 662 (86%) | 676 (79%)* |
| Coronary disease | 1,290 (71%) | 540 (81%) | 496 (65%)* |
| Hypertension | 1,447 (80%) | 520 (78%) | 634 (82%)* |
| Stroke | 378 (21%) | 126 (19%) | 169 (22%) |
| Dementia | 205 (11%) | 53 (8%) | 93 (12%)* |
| COPD | 801 (44%) | 283 (42%) | 354 (46%) |
| Diabetes mellitus | 811 (45%) | 296 (44%) | 350 (46%) |
| Systolic BP, mmHg | 147 ± 32 | 139 ± 31 | 153 ± 32 |
| Diastolic BP, mmHg | 75 ± 19 | 75 ± 19 | 75 ± 20 |
| Heart rate, beats/min | 85 ± 21 | 86 ± 22 | 85 ± 22 |
| Respiratory rate/min | 22 ± 6 | 22 ± 7 | 22 ± 6 |
| Hemoglobin, g/dl | 11.9 ± 1.9 | 12.1 ± 1.9 | 11.7 ± 1.8* |
| Urea nitrogen, mg/dl | 31 ± 20 | 33 ± 21 | 30 ± 20* |
| Serum creatinine, mg/dl | 1.7 ± 1.4 | 1.7 ± 1.2 | 1.6 ± 1.2 |
| Serum sodium, mEq/l | 137 ± 5 | 137 ± 5 | 138 ± 5 |
| Ejection fraction, % | 42 ± 17 | 27 ± 10 | 56 ± 10* |
p<.05 for difference between reduced vs. preserved and preserved EF
30-day readmission analyses
Of 1,807 GAP-HF patients, 43 (2.4%) died during the index hospitalization and were excluded. The remaining 1,764 index hospitalizations all had complete data for the CMS readmission model; of these, 476 (27.0%) were rehospitalized within 30 days.
The results for the CMS readmission model, prior admissions, and their combination are displayed in Table 2. The performance of the CMS readmission model in the GAP-HF cohort was similar to that in the original derivation cohort (c-statistic 0.61 vs. 0.58),1 with higher discrimination in HF patients with preserved than in with reduced ejection fraction HF (c-statistic 0.67 vs. 0.60).
Table 2.
Results for 30-day all-cause readmission (logistic regression with random hospital effects)
| CMS readmission model only | CMS readmission model + prior admissions | |||||
|---|---|---|---|---|---|---|
| Variable | OR (95% CI) | z-statistic | p | OR (95% CI) | z-statistic | p |
| Male gender | 1.01 (.81–1.27) | 0.11 | .91 | .98 (.78–1.26) | −0.11 | .92 |
| Age 75–84 years | 1.22 (.93–1.59) | 1.44 | .15 | 1.13 (.85–1.51) | 0.86 | .39 |
| Age ≥85 years | .91 (.67–1.24) | −0.57 | .57 | .87 (.63–1.21) | −0.81 | .42 |
| COPD | 1.17 (.94–1.45) | 1.38 | .17 | 1.05 (.83–1.33) | 0.43 | .67 |
| Dementia | .72 (.49–1.05) | −1.73 | .08 | .68 (.46–1.02) | −1.88 | .06 |
| Diabetes (any type) | .92 (.72–1.17) | −0.70 | .48 | .87 (.67–1.12) | −1.06 | .29 |
| Cerebrovascular accident | .86 (.66–1.12) | −1.13 | .26 | .96 (.72–1.27) | −0.31 | .76 |
| History of heart failure | 2.06 (1.48–2.87) | 4.26 | < .001 | 1.27 (.89–1.81) | 1.30 | .19 |
| History of PTCA | 1.11 (.84–1.48) | 0.74 | .46 | .94 (.69–1.28) | −0.40 | .69 |
| Coronary disease | .89 (.68–1.16) | −0.88 | .38 | .80 (.60–1.06) | −1.53 | .13 |
| Systolic BP <125 mmHg | 1.05 (.82–1.36) | 0.41 | .68 | 1.02 (.78–1.34) | 0.13 | .89 |
| Respiratory rate <12 | 1.81 (.28–11.51) | 0.63 | .53 | 1.25 (.17–9.15) | 0.22 | .83 |
| Respiratory rate >25 | .86 (.65–1.13) | −1.10 | .27 | .83 (.62–1.11) | −1.24 | .22 |
| Heart rate <60 bpm | .85 (.56–1.29) | −0.78 | .44 | .73 (.47–1.13) | −1.41 | .16 |
| Heart rate >100 bpm | 1.13 (.85–1.49) | 0.85 | .40 | 1.05 (.78–1.39) | 0.58 | .56 |
| Cardiac arrest | 2.56 (.50–12.98) | 1.13 | .26 | 6.71 (1.30–34.68) | 2.27 | .02 |
| Ejection fraction < 40% | .99 (.76–1.27) | −0.11 | .91 | .98 (.75–1.29) | −0.15 | .88 |
| Ejection fraction unknown | 1.09 (.82–1.46) | 0.59 | .55 | .93 (.68–1.27) | −0.47 | .64 |
| Aortic stenosis | .88 (.58–1.33) | −0.62 | .53 | .82 (.53–1.29) | −0.85 | .40 |
| Sodium < 130 mg/dl | 1.65 (1.05–2.60) | 2.16 | .03 | 1.76 (1.07–2.88) | 2.24 | .03 |
| Sodium > 145 mg/dl | .78 (.29–2.14) | −0.48 | .63 | .76 (.26–2.24) | −0.49 | .63 |
| Sodium unknown | 1.15 (.37–3.60) | 0.24 | .81 | 1.44 (.42–4.89) | 0.58 | .56 |
| BUN > 40 or creatinine > 2.5 | 1.20 (.93–1.56) | 1.42 | .16 | 1.04 (.79–1.37) | 0.30 | .77 |
| BUN and creatinine unknown | .70 (.35–1.40) | −1.00 | .32 | .83 (.40–1.76) | −0.48 | .63 |
| Glucose > 200 mg/dl | 1.20 (.93–1.56) | 0.96 | .34 | 1.17 (.82–1.66) | 0.85 | .40 |
| Glucose unknown | 1.42 (.73–2.79) | 1.03 | .30 | 1.28 (.63–2.61) | 0.68 | .50 |
| Hematocrit < 30% | 1.38 (1.02–1.86) | 2.11 | .04 | 1.12 (.82–1.55) | 0.71 | .48 |
| Hematocrit unknown | .78 (.30–2.01) | −0.52 | .60 | .63 (.24–1.67) | −0.92 | .36 |
| Prior admissions (last 12 months) | (below as sole variable) | (below combined with CMS model) | ||||
| 0 | (reference) | (reference) | ||||
| 1 | 4.50 (3.28–6.17) | 9.33 | < .001 | 4.67 (3.37–6.46) | 9.26 | < .001 |
| 2 or more | 6.38 (4.94–8.22) | 14.27 | < .001 | 6.49 (4.93–8.55) | 13.33 | < .001 |
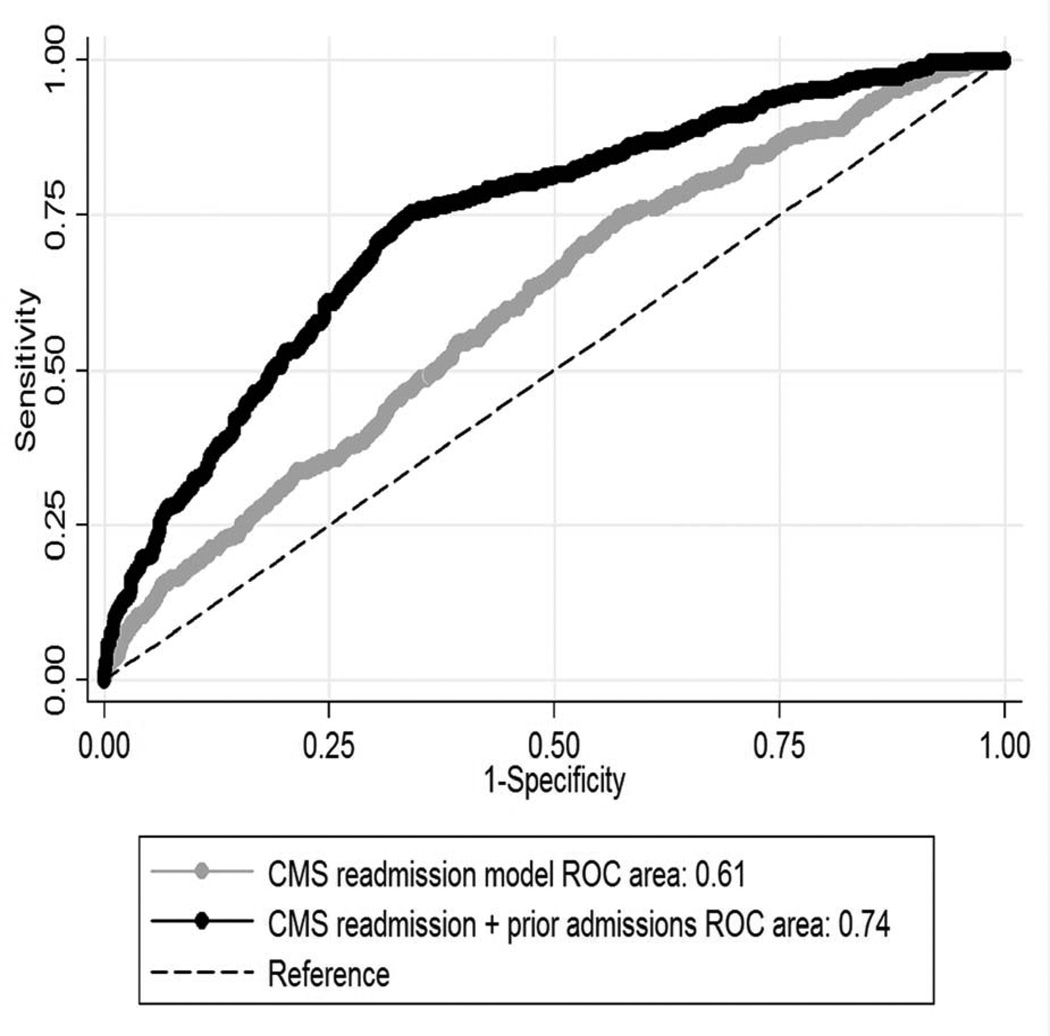
Prior admissions as a sole variable had a significantly higher c-statistic than the CMS readmission model (0.71 vs. 0.61, p<.001). When added to the CMS readmission model, prior admissions improved discrimination markedly (c-statistic 0.74 vs. 0.61, p<.001; Figure 1). Only 13% of patients with no prior admissions were readmitted. In contrast, 39% of patients with 1 prior admission and 48% of those with ≥2 prior admissions were rehospitalized (combined, this 45% of the patients accounted for 74% of 30-day readmissions). There was no significant impact of case clustering within hospitals for the combined CMS readmission + prior admissions model. Using the first HF hospitalization in the GAP-HF database as the index admission for all patients produced little effect on the results (c-statistic for CMS readmission + prior admissions model 0.73 vs. 0.61 for CMS readmission model alone, p<.001; 1 vs. 0 prior admissions: OR 4.49, 95% CI 3.25–6.21; ≥2 vs. 0: OR 5.86, 95% CI 4.50–7.62, both p<.001).
Figure 1. Receiver operating curves for CMS readmission model with and without prior admissions.
Abbreviations: CMS, Centers for Medicare and Medicaid Services; ROC, receiver-operating curve
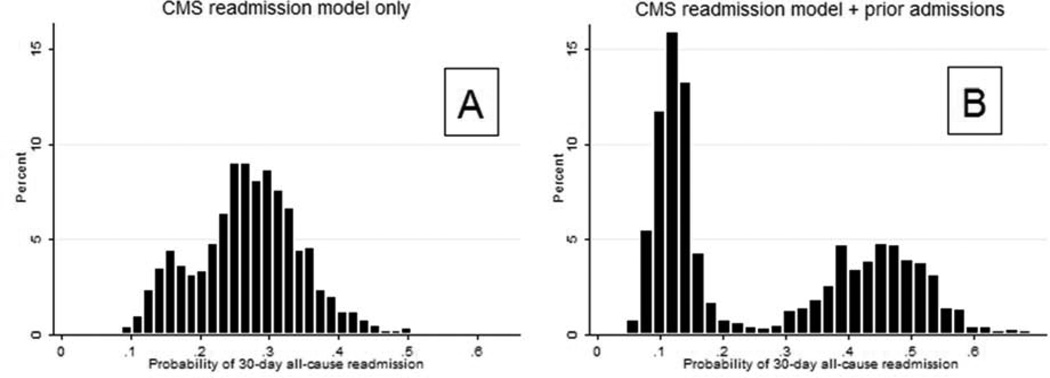
A histogram of the predicted 30-day readmission probabilities from the CMS readmission model in GAP-HF is shown in Figure 2a. Figure 2b illustrates the substantial influence of prior admissions on the probability histogram. Based on expected readmission rates1 and findings in GAP-HF (Figure 2a) we grouped readmission probability: <20%, 20–25%, 25–30%, and > 30%. The net reclassification index for adding prior admissions to the CMS readmission model was 0.40 (SE 0.04, p<.001) and the integrated discrimination index was 0.12 (SE 0.008, p<.001), both indicating a robust reclassification effect.
Figure 2. Probability histograms for CMS readmission model with and without prior admissions.
Abbreviations: CMS, Centers for Medicare and Medicaid Services
The effect of prior admissions was similar in patients with reduced ejection fraction (1 vs. 0 prior admissions: OR 4.07, 95% CI 2.39–6.93; ≥2 vs. 0: OR 7.02, 95% CI 4.40–11.20, both p<.001) and preserved ejection fraction (1 vs. 0: OR 5.98, 95% CI 3.52–10.14; ≥2 vs. 0: OR 7.02, 95% CI 4.54–10.86, both p < .001). Prior admissions significantly improved the CMS readmission model in both groups (reduced ejection fraction, c-statistic 0.60 to 0.74, p<.001; preserved ejection fraction, c-statistic 0.67 to 0.78, p<.001).
Discussion
We found that in Medicare HF inpatients, hospitalizations within the prior 12 months strongly increased the odds for 30-day all-cause readmission, improved the discrimination of a widely-recommended HF readmission risk model, and clearly separated HF patients into high- and low-risk categories for early rehospitalization.
Previous studies
Despite its intuitive appeal, a history of prior admissions has not been extensively explored as a risk factor for 30-day readmission in HF patients. A recent systematic review of HF-specific readmission risk models cited only two that included prior hospitalization.16 Krumholz and colleagues demonstrated that hospitalization within the past 12 months predicted six-month readmission.10 In HF inpatients randomized to milrinone or placebo infusion, HF admissions within the prior 12 months had an odds ratio of 1.14 (per HF hospitalization) for the combined endpoint of death or all-cause readmission within 60 days of discharge.11 Subsequently, our group noted a progressive increase in 30-day readmission risk with the number of prior admissions in GAP-HF patients with preserved ejection fraction.14
In an urban teaching hospital population, Amarasingham and colleagues reported a 30-day readmission odds ratio of 1.17 per all-cause hospitalization within the previous 12 months after adjusting for the Tabak mortality model and other factors.12 The widely used LACE (Length of stay, Acuity of admission, Charlson comorbidity score, Emergency department visits within six months) incorporates recent urgent healthcare utilization. The efficacy of the LACE model in HF patients is uncertain, with similar scores for patients who were and were not readmitted and a c-statistic of 0.58 in one large administrative-data based study.19 Of note, in the LACE score recent emergency department visits are weighted similarly to length of stay and Charlson score, both of which are not strong predictors for HF readmission.19,20 Eapen et al. recently modeled factors predicting 30-day readmission in the Get With The Guidelines-Heart Failure database. They reported a c-statistic of 0.62 for any prior hospitalization within six months of an index HF admission, but did not report its marginal utility when added to the derived risk model (which had a c-statistic of 0.59).9
Comments and Implications
Our analysis builds on the chart-based CMS readmission model, which is widely recommended and was originally developed in a representative Medicare HF population very similar to our cohort. The CMS readmission model encompasses a broad spectrum of illness severity and comorbid conditions, and may be used to predict risk immediately upon hospitalization. Despite its seeming comprehensiveness, the discrimination of the CMS readmission model is modest.1 In addition to outperforming the CMS readmission model alone, the overall discrimination of the CMS readmission + prior admissions model used in GAP-HF (c-statistic 0.74) compared favorably with other previously reported HF-specific models (c-statistic range, 0.58 to 0.72).12,16
Despite intense efforts and some success in reducing HF-specific hospitalization,21 all-cause readmission rates in HF patients have only modestly declined in recent years.22,23 The few strategies that appear effective, such as in-depth discharge education,24,25 early postdischarge clinic follow-up,8 and multidisciplinary home-based interventions24,26 are labor- and resource-intensive. Recent work by Amarasingham and colleagues clearly demonstrates the importance of focusing these efforts. Using only minimal additional personnel, their group implemented a multidisciplinary intervention program in the highest-risk HF patients and reduced 30-day readmission from 26.2% to 21.2%.7 Their impressive work was performed in a relatively young (mean age 57) study cohort with high prevalence of poverty and substance abuse; several related markers also significantly predicted readmission.12 Other previous studies confirm that HF patient characteristics such as race, economic status, social instability, and outpatient healthcare utilization are major drivers for 30-day readmission.16,27,28. We postulate that a history of prior admissions serves as a more easily accessible proxy for important but complex socioeconomic factors, which may differ based on the HF population served by a given hospital. We are unable to confirm this possibility within the GAP-HF cohort, and further study in other populations is needed.
The CMS readmission model and other more recent models produce normally distributed risk scores and do not always sufficiently separate ‘low-risk’ and ‘high-risk’ patients.1,9 Effective risk-stratification presumes an equal distribution of unmeasured confounders across the patients being evaluated. This issue becomes even more important when models with low overall discrimination are used and the unmeasured factors have a large effect.1,9,27,29 In GAP-HF, adding the previously “unobservable” variable of prior admissions to the CMS readmission model strikingly improved its overall discrimination (Figure1) and reclassified many HF patients into high- or low-risk categories (Figure 2B), thereby accomplishing the primary goals of risk stratification. A history of prior admissions is easily obtained information that could be used by clinicians to improve 30-day readmission risk assessment when risk models produce indeterminate results.
Limitations
The GAP-HF hospitals are all located in central or southeast Michigan, and our results may not apply in other geographic areas. However, using patients from a single geographic area avoids regional variation in all-cause Medicare admission rates that may in turn affect readmissions.30 The GAP-HF data were collected from 2002–2004, an identical interval to the data used for the Hospital Compare claims-based readmission model (derived from 2003–2004 Medicare cohorts) and more recent than the data used to derive the CMS chart-based readmission model (National Heart Failure Project, 1998–2001). Accordingly, the GAP-HF cohort provides an appropriate timeframe to evaluate the risk models that are recommended and widely used today.
As in the derivation of CMS model,1 we analyzed only Medicare patients with at least one year of claims data for previous hospitalizations. These prior claims data would likely be unavailable to treating providers during a hospitalization. However, there is no a priori reason to expect that asking HF patients how many times they have been hospitalized within the prior year would produce markedly different results, particularly since our model requires only answering “none, once, or more than once.”
It is unknown whether our results would apply to a younger cohort of HF patients. The GAP-HF dataset has limited information on transitions of care and no data on outpatient management following hospital discharge, and does not contain information on certain demographic and socioeconomic factors known to contribute to readmission risk.12
In GAP-HF, we could not directly investigate the impact of prior admissions on the Hospital Compare claims-based readmission model. However, the CMS model we used was developed to validate the Hospital Compare model, and the state-level correlations between these models are extremely tight (correlation coefficient 0.97, median difference in risk-standardized readmission rates 0.06%).1 It seems likely that prior admissions would similarly affect the performance of claims-based models. Unfortunately, incorporating prior admissions into hospital-to-hospital comparison risk standardization models would pose significant challenges, since facilities with high readmission rates would simultaneously increase their case-mix risk and the negative outcome of interest. The value of our findings is primarily based on improving risk assessment for individual patients.
Conclusions
In Medicare inpatients with HF, all-cause prior admissions within 12 months strongly increased the risk for 30-day all-cause readmission, improved the performance of a widely recommended risk model, and clearly separated patients into high- and low-risk categories for early rehospitalization. This easily obtained information could help focus readmission-prevention efforts in older HF patients.
Acknowledgments
Financial support: The Mid-Michigan GAP-HF study was conducted in conjunction with the Greater Flint Health Coalition, and was funded by unrestricted grants from AstraZeneca Pharmaceuticals, Pfizer, Inc., GlaxoSmithKline, and the Blue Cross Blue Shield of Michigan Foundation. The funding organizations had no role in the design and conduct of this study, analysis of data, or preparation of this manuscript and no authors have conflicts of interest to report. Dr. Hummel is supported by NIH/NHLBI K23HL109176. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- BP
Blood pressure
- BUN
Blood urea nitrogen
- CMS
Centers for Medicare & Medicaid Services
- COPD
Chronic obstructive pulmonary disease
- EF
Ejection fraction
- GAP-HF
Mid-Michigan Guidelines Applied in Practice – Heart Failure study (source of study data)
- HF
Heart failure
- PTCA
Percutaneous transluminal coronary angioplasty
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keenan PS, Normand S-LT, Lin Z, et al. An administrative claims measure suitable for profiling hospital performance on the basis of 30-day all-cause readmission rates among patients with heart failure. Circ Cardiovasc Qual Outcomes. 2008;1(1):29–37. doi: 10.1161/CIRCOUTCOMES.108.802686. [DOI] [PubMed] [Google Scholar]
- 2.Kommuri NVA, Koelling TM, Hummel SL. The impact of prior heart failure hospitalizations on long-term mortality differs by baseline risk of death. Am J Med. 2012;125(2):209.e9–209.e15. doi: 10.1016/j.amjmed.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Lee DS, Austin PC, Stukel TA, et al. "Dose-dependent" impact of recurrent cardiac events on mortality in patients with heart failure. Am J Med. 2009;122(2):162–169. e1. doi: 10.1016/j.amjmed.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 4.Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154(2):260–266. doi: 10.1016/j.ahj.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 5.Solomon S, Dobson J, Pocock S, et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116(13):1482–1487. doi: 10.1161/CIRCULATIONAHA.107.696906. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd-Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121(7):948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 7.Amarasingham R, Patel PC, Toto K, et al. Allocating scarce resources in real-time to reduce heart failure readmissions: a prospective, controlled study. [published Online First June 30, 2013];BMJ Qual Saf. 2013 doi: 10.1136/bmjqs-2013-001901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716–1722. doi: 10.1001/jama.2010.533. [DOI] [PubMed] [Google Scholar]
- 9.Eapen ZJ, Liang L, Fonarow GC, et al. Validated, electronic health record deployable prediction models for assessing patient risk of 30-day rehospitalization and mortality in older heart failure patients. JCHF. 2013;1(3):245–251. doi: 10.1016/j.jchf.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Krumholz HM, Chen YT, Wang Y, Vaccarino V, Radford MJ, Horwitz RI. Predictors of readmission among elderly survivors of admission with heart failure. Am Heart J. 2000;139(1 Pt 1):72–77. doi: 10.1016/s0002-8703(00)90311-9. [DOI] [PubMed] [Google Scholar]
- 11.Felker GM, Leimberger JD, Califf RM, et al. Risk stratification after hospitalization for decompensated heart failure. J Card Fail. 2004;10(6):460–466. doi: 10.1016/j.cardfail.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Amarasingham R, Moore B, Tabak Y, et al. An automated model to identify heart failure patients at risk for 30-day readmission or death using electronic medical record data. Med Care. 2010;48(11):981–988. doi: 10.1097/MLR.0b013e3181ef60d9. [DOI] [PubMed] [Google Scholar]
- 13.Hummel S, Skorcz S, Koelling T. Prolonged electrocardiogram QRS duration independently predicts long-term mortality in patients hospitalized for heart failure with preserved systolic function. J Card Fail. 2009;15(7):553–560. doi: 10.1016/j.cardfail.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hummel SL, DeFranco AC, Skorcz S, et al. Recommendation of low-salt diet and short-term outcomes in heart failure with preserved systolic function. Am J Med. 2009;122(11):1029–1036. doi: 10.1016/j.amjmed.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eagle KA, Montoye CK, Riba AL, et al. Guideline-based standardized care is associated with substantially lower mortality in Medicare patients with acute myocardial infarction: the American College of Cardiology's Guidelines Applied in Practice (GAP) projects in Michigan. J Am Coll Cardiol. 2005;46(7):1242–1248. doi: 10.1016/j.jacc.2004.12.083. [DOI] [PubMed] [Google Scholar]
- 16.Ross JS, Mulvey GK, Stauffer B, et al. Statistical models and patient predictors of readmission for heart failure: a systematic review. Arch Intern Med. 2008;168(13):1371–1386. doi: 10.1001/archinte.168.13.1371. [DOI] [PubMed] [Google Scholar]
- 17.Pencina MJ, D' Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 18.Havranek EP, Masoudi FA, Westfall KA, et al. Spectrum of heart failure in older patients: results from the National Heart Failure Project. Am Heart J. 2002;143(3):412–417. doi: 10.1067/mhj.2002.120773. [DOI] [PubMed] [Google Scholar]
- 19.Au AG, McAlister FA, Bakal JA, et al. Predicting the risk of unplanned readmission or death within 30 days of discharge after a heart failure hospitalization. Am Heart J. 2012;164(3):365–372. doi: 10.1016/j.ahj.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Eapen ZJ, Reed SD, Li Y, et al. Do countries or hospitals with longer hospital stays for acute heart failure have lower readmission rates?: findings from ASCEND-HF. Circ Heart Fail. 2013;6(4):727–732. doi: 10.1161/CIRCHEARTFAILURE.112.000265. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Normand S-LT, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998–2008. JAMA. 2011;306(15):1669–1678. doi: 10.1001/jama.2011.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joynt KE, Jha AK. Thirty-day readmissions — truth and consequences. N Engl J Med. 2012;366(15):1366–1369. doi: 10.1056/NEJMp1201598. [DOI] [PubMed] [Google Scholar]
- 23.Heidenreich PA, Sahay A, Kapoor JR, et al. Divergent trends in survival and readmission following a hospitalization for heart failure in the Veterans Affairs health care system 2002 to 2006. J Am Coll Cardiol. 2010;56(5):362–368. doi: 10.1016/j.jacc.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 24.Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.[see comment] N Engl J Med. 1995;333(18):1190–1195. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]
- 25.Koelling TM, Johnson ML, Cody RJ, Aaronson KD. Discharge education improves clinical outcomes in patients with chronic heart failure. Circulation. 2005;111(2):179–185. doi: 10.1161/01.CIR.0000151811.53450.B8. [DOI] [PubMed] [Google Scholar]
- 26.Stewart S, Marley JE, Horowitz JD. Effects of a multidisciplinary, home-based intervention on unplanned readmissions and survival among patients with chronic congestive heart failure: a randomised controlled study. Lancet. 1999;354(9184):1077–1083. doi: 10.1016/s0140-6736(99)03428-5. [DOI] [PubMed] [Google Scholar]
- 27.Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698. doi: 10.1001/jama.2011.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joynt KE, Orav EJ, Jha AK. Thirty-day readmission rates for Medicare beneficiaries by race and site of care. JAMA. 2011;305(7):675–681. doi: 10.1001/jama.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenberg AL, Hofer TP, Strachan C, et al. Accepting critically ill transfer patients: adverse effect on a referral center's outcome and benchmark measures. Ann Intern Med. 2003;138(11):882–890. doi: 10.7326/0003-4819-138-11-200306030-00009. [DOI] [PubMed] [Google Scholar]
- 30.Epstein AM, Jha AK, Orav EJ. The relationship between hospital admission rates and rehospitalizations. N Engl J Med. 2011;365(24):2287–2295. doi: 10.1056/NEJMsa1101942. [DOI] [PubMed] [Google Scholar]