Abstract
Background
Heart failure with preserved ejection fraction (HFPEF) involves failure of cardiovascular reserve in multiple domains. In HFPEF animal models, dietary sodium restriction improves ventricular and vascular stiffness and function. We hypothesized that the sodium-restricted Dietary Approaches to Stop Hypertension diet (DASH/SRD) would improve left ventricular diastolic function, arterial elastance, and ventricular-arterial (V-A) coupling in hypertensive HFPEF.
Methods and Results
Thirteen patients with treated hypertension and compensated HFPEF consumed the DASH/SRD (target sodium 50 mmol/2100 kcal) for 21 days. We measured baseline and post-DASH/SRD brachial and central BP (via radial arterial tonometry), and cardiovascular function with echocardiographic measures (all previously invasively validated). Diastolic function was quantified via the Parametrized Diastolic Filling formalism, which yields relaxation/viscoelastic (c) and passive/stiffness (k) constants through analysis of Doppler mitral inflow velocity (E-wave) contours. Effective arterial elastance (Ea) end-systolic elastance (Ees), and V-A coupling (defined as the ratio Ees:Ea) were determined using previously published techniques. Wilcoxon matched-pairs tests were used for pre-post comparisons.
The DASH/SRD reduced clinic and 24-hour brachial systolic pressure (155±35 to 138±30 and 130±16 to 123±18 mmHg, both p=.02) and central end-systolic pressure trended lower (116±18 to 111±16 mmHg, p=.12). In conjunction, diastolic function improved (c, 24.3±5.3 to 22.7±8.1 s−1;p=.03; k, 252±115 to 170±37 s−1;p=.03), Ea decreased (2.0±0.4 to 1.7±0.4 mmHg/ml;p=.007), and V-A coupling improved (Ees:Ea, 1.5±0.3 to 1.7±0.4;p=.04).
Conclusions
In hypertensive HFPEF patients, the sodium-restricted DASH diet was associated with favorable changes in ventricular diastolic function, arterial elastance, and V-A coupling.
Keywords: diastolic heart failure, salt sensitivity hypertension, diet, ventricular/vascular coupling hemodynamics, preserved left ventricular function
Introduction
Heart failure with preserved ejection fraction (HFPEF) is becoming more common as the U.S. population ages and the prevalence of associated comorbidities rises1, yet remains without evidence-based therapy.2 Recent studies demonstrate that HFPEF is associated with failure of cardiovascular reserve in additional domains beyond ventricular diastolic function. Systemic hypertension, frequently present in HFPEF, contributes to increased ventricular and arterial stiffness. These factors adversely affect ventricular systolic functional reserve, myocardial work efficiency, and exercise capacity in HFPEF.3–5
Pharmacological treatment of hypertension reduces arterial elastance (Ea), an integrated parameter representing effective arterial afterload6, and improves some markers of left ventricular diastolic7 and systolic8 function. In experimental models of hypertensive HFPEF, dietary sodium restriction or supplementation with potassium, calcium, magnesium, and/or antioxidants reduces blood pressure (BP) and cardiovascular damage.9–12 A recent study demonstrated that the sodium-restricted Dietary Approaches to Stop Hypertension diet (DASH/SRD) reduced BP and oxidative stress in hypertensive HFPEF patients.13 We hypothesized that the DASH/SRD would improve Doppler-based indices of left ventricular diastolic function, arterial elastance, and V-A coupling in human hypertensive HFPEF.
Methods
This investigation conformed with the Declaration of Helsinki and was approved by the University of Michigan Institutional Review Board. All subjects provided written informed consent, and study conduct followed institutional guidelines.
Patient selection and study structure
All patients had a history of systemic hypertension and left ventricular ejection fraction ≥ 50% with no history of ejection fraction < 40%. The remainder of the inclusion and exclusion criteria were patterned after the 2007 European Society of Cardiology HFPEF diagnostic guidelines.14 Specifically, patients were required to have objective evidence of diastolic dysfunction on echocardiography or catheterization, or indeterminate diastolic function and B-type natriuretic peptide ≥ 100 pg/ml.
Exclusion criteria included New York Heart Association class IV symptoms, hospitalization or medication changes within one month, noncardiac limitation of exercise capacity (e.g. due to pulmonary disease), significant valvular heart disease, infiltrative, restrictive, or primary hypertrophic cardiomyopathy, severe anemia (hemoglobin < 9 g/dL), uncontrolled diabetes mellitus (hemoglobin A1C > 9%), and severe uncontrolled hypertension (systolic BP > 180 on current regimen). Patients with severe renal insufficiency (estimated glomerular filtration rate < 30 ml/min/1.73 m2) or risk for hyperkalemia (serum potassium > 5.0 or history of > 6.0 mmol/L) were also excluded.
The study took place over 25 days, with two days of testing prior to and following 21 days of DASH/SRD. Subjects were provided all food and beverages (except water, tea, and/or coffee) for the 21-day study diet period.
On Day 1, patients presented on their habitual diet having taken their usual morning medications. Seated clinic BPs and six-minute walk testing were performed; patients returned home on their habitual diet for ambulatory BP measurement and 24-hour urinary collection. On Day 2, patients presented fasting and prior to taking morning antihypertensives; they underwent transthoracic echocardiography, vascular testing, and blood sampling. Subjects began the DASH/SRD on Day 3, and on Days 24–25 had identical testing to Days 1–2. Further information on the study protocol can be found in our previous publication.13
Characteristics of study diet, tracking of dietary adherence
The study diet was prepared by research dietitians in a metabolic kitchen at the University of Michigan Clinical Research Unit, with goal daily sodium intake of 50 mmol (1150 mg)/2100 kilocalories and energy intake to maintain lean body mass while meeting DASH diet nutritional targets. Adherence was confirmed with three-day food diaries and 24-hour urinary sodium and potassium measurement. Additional description of the study diet can be found in our previous publication.13
Blood pressure and functional testing
Two seated clinic BPs were obtained per Joint National Committee (Seventh Report) recommendations15. Ambulatory BP monitoring was performed per British Society of Hypertension guidelines16 using the Spacelabs 90207 monitor (Spacelabs Healthcare, Issaquah, WA). Transthoracic echocardiography was performed using the Acuson Sequoia C512 (Siemens USA, Malvern, PA) and central BP was measured with the Sphygmocor radial artery tonometer (AtCor Medical, Itasca, IL).17 Six-minute walk testing was performed per American Thoracic Society guidelines.
Echocardiographic measures
All echocardiograms were performed by experienced research sonographers. Ventricular structure and function were assessed using standard techniques according to American Society of Echocardiography recommendations for clinical studies. The left ventricular dimensions were measured in a two-dimensional parasternal long-axis view. Left ventricular mass was calculated using the Devereux formula,18 and left ventricular ejection fraction and volumes by the method of Dumesnil et al.19
The ventricular stroke volume was calculated by integrating the pulse-wave Doppler velocity-time profile of the left ventricular outflow tract (LVOT) in the apical 5-chamber view and multiplying by the LVOT area (determined using the diameter measured immediately proximal to aortic valve leaflet insertion in the parasternal long-axis view). Cardiac output was obtained by multiplying the stroke volume by heart rate, and systemic vascular resistance was estimated by dividing mean arterial pressure by cardiac output (assuming negligible right atrial pressure).
In addition to standard Doppler-derived echocardiographic measures, diastolic function was assessed using the Parametrized Diastolic Filling (PDF) formalism. This method characterizes suction-initiated ventricular filling in terms analogous to damped harmonic oscillatory motion. In this construct, model-based analysis of Doppler mitral inflow velocity (E-wave) contours yields passive/stiffness (k: spring constant), relaxation/viscoelastic (c: damping constant), and load (xo: initial spring displacement) indices that can be used to generate several indices such as available potential energy (ergs) for filling (1/2 kxo2). The PDF formalism has been extensively validated using invasive (high fidelity) hemodynamic methods,20–22 and is highly reproducible in patients with normal left ventricular ejection fraction and elevated filling pressures.23, 24
The Sphygmocor radial artery tonometer uses an invasively validated general transfer function to generate central aortic pressures and waveforms.17 The central end-systolic BP is measured at the incisura of the Sphygmocor-generated aortic pressure wave. Effective arterial elastance (Ea) was calculated as the ratio of central end-systolic pressure to the ventricular stroke volume.3 The end-systolic ventricular elastance (Ees) was measured as per the single-beat echocardiographic method of Chen et al.25. As in other studies of ventricular-arterial interaction, the V-A coupling ratio was defined as Ees:Ea.3–6 In order to further explore the effects of changes in ventricular-vascular interactions, we measured the left ventricular mechanical energetic efficiency as the ratio of left ventricular stroke work to the pressure-volume area as recently described by Lam et al.6
The Ees parameter is sometimes considered a surrogate for ventricular contractile function, but also depends on intrinsic ventricular stiffness. We also assessed ventricular contractility using the maximum rate of change of pressure-normalized stress (dσ*/dtmax), which indexes the maximal flow generated in the LVOT to the ventricular mass. The dσ*/dtmax is reduced in HFPEF, discriminates between HFPEF and controls better than standard diastolic function measures,26 and correlates well with single-beat Ees.27 This parameter has been invasively validated as an integrated and preload-insensitive global contractility measure.
The studies were separately analyzed for LVOT velocity profiles by two investigators (S.L.H and T.J.K.) and for E-wave analysis via the PDF formalism by three investigators (S.Z., E.G., and S.J.K.) who were blinded to additional clinical data; S.L.H. performed standard echocardiographic structural and functional analyses. All analyses involving Ea and Ees were also performed using the standard estimation of end-systolic pressure (0.9 * brachial artery systolic BP).3
Statistical analysis
Data are presented as the mean ± SD. Statistical analyses were performed using STATA 10.0 (STATACorp, College Station, TX). Wilcoxon matched-pairs tests were used for pre-post comparisons, with p < 0.05 considered statistically significant.
Results
Patient characteristics and adherence
The characteristics of the 14 enrolled participants (of 22 screened) are shown in Table 1. All were diagnosed with HFPEF by board-certified cardiologists (in addition to the investigators), most were on loop diuretics, and most had been previously hospitalized for decompensated HFPEF. In addition to meeting study inclusion criteria, 13 of 14 subjects fulfilled 2007 European Society of Cardiology HFPEF diagnostic guidelines (7 by catheterization and 6 via neurohormonal and echocardiographic criteria.14
Table 1.
Baseline demographics and clinical characteristics of subjects
| Demographic/anthropometric data | ||
| Age (years) | 72 ± 10 | |
| Gender (# female/male) | 13/1 | |
| Weight (kg) | 94 ± 30 | |
| BMI (kg/m2) | 35.5 ± 7.9 | |
| Baseline clinical characteristics | n (%) | Comments |
| B-type natriuretic peptide (pg/mL) | 142 ± 33 | |
| Six minute walk distance (m) | 313 ± 86 | |
| Hypertension | 14 (100%) | No known secondary causes |
| Coronary artery disease | 5 (36%) | 1 with prior CABG, 1 with prior PCI |
| Diabetes mellitus | 6 (43%) | All type II; Hgb A1c = 6.8 ± 0.8% |
| Chronic kidney disease | 14 (100%) | eGFR = 60 ± 17 ml/min/1.73 m2 |
| NYHA class II/III | 2 (14%)/12 (86%) | |
| Prior heart failure hospitalization | 10 (71%) | |
| On chronic loop diuretic therapy | 11 (79%) | |
Abbreviations: BMI: body mass index; CABG: coronary artery bypass grafting; NYHA: New York Heart Association; eGFR: estimated glomerular filtration rate per Modification of Diet in Renal Disease; PCI: percutaneous coronary intervention
Thirteen patients completed the study; one was withdrawn due to serum potassium of 5.9 mg/dL at the safety visit. All subjects were highly adherent to the provided DASH/SRD upon review of three-day food diaries (data not shown). A comparison between baseline food frequency questionnaire data and the DASH/SRD menu (final 5 days) predicted a 56% reduction in sodium intake and a 37% increase in potassium intake. These predictions and study diet adherence were strongly corroborated by a 56% reduction in urinary sodium excretion (3,353 ± 1,593 to 1,478 ± 933 mg/24h, p<.001) and a 28% increase in urinary potassium excretion (2,284 ± 793 to 2,925 ± 1,024 mg/24h, p=.04) from baseline.
Blood pressure changes
All clinic and ambulatory BP recordings were adequate for analysis. In most subjects, baseline 24-hour monitoring demonstrated well-controlled BP consistent with current HFPEF guidelines (systolic BP < 130 mmHg).28 As previously demonstrated in this cohort, seated clinic and 24-hour systolic BP significantly decreased (155±35 to 138±30 and 130±16 to 123±18 mmHg respectively, both p=.02) between baseline and post-DASH/SRD measurements.13 The supine brachial BP measured immediately prior to the echocardiogram and Sphygmocor (142±25 to 134±22 mmHg, p=0.07) and the central end-systolic BP (116±18 to 111±16 mmHg, p=0.12) also trended lower after DASH/SRD.
Cardiac and vascular measures
Standard echocardiographic data are summarized in Table 2. As in other hypertensive HFPEF cohorts,29 most patients had left atrial enlargement and left ventricular hypertrophy. Twelve subjects had adequate signals for lateral and nine for septal tissue Doppler velocities. The DASH/SRD was not associated with changes in the mitral inflow E:A ratio or mitral E/e’ ratio.
Table 2.
Echocardiography characteristics
| Structrural and functional data | Pre | Post | p |
| LV mass index (g/m2.7) | 86 ± 28 | 85 ± 27 | .80 |
| Relative wall thickness | .57 ± .07 | .56 ± .08 | .84 |
| Left atrial diameter (mm) | 42 ± 5 | 41 ± 5 | .19 |
| LV end-diastolic volume (ml) | 102 ± 23 | 102 ± 22 | .77 |
| Stroke volume (ml) | 66 ± 16 | 73 ± 16 | .02 |
| Ejection fraction (%) | 66 ± 18 | 74 ± 16 | .03 |
| Cardiac output (l/min) | 4.1 ± 1.0 | 4.3 ± 1.2 | .19 |
| Systemic vascular resistance (dynes s/cm5) | 2,027 ± 501 | 1,817 ± 439 | .03 |
| Standard diastolic function data | Pre | Post | p |
| Mitral E velocity (cm/s) | 92 ± 29 | 84 ± 20 | .20 |
| Mitral A velocity (cm/s) | 79 ± 14 | 83 ± 17 | .25 |
| Mitral E/A ratio | 1.13 ± .31 | .98 ± .14 | .14 |
| Septal e’ velocity (cm/s) | 9 ± 2 | 8 ± 2 | .56 |
| Lateral e’ velocirty (cm/s) | 10 ± 1 | 10 ± 2 | .85 |
| Septal E/e’ ratio | 12 ± 3 | 11 ± 4 | .67 |
| Lateral E/e; ratio | 10 ± 3 | 9 ± 2 | .11 |
Abbreviations: LV, left ventricle
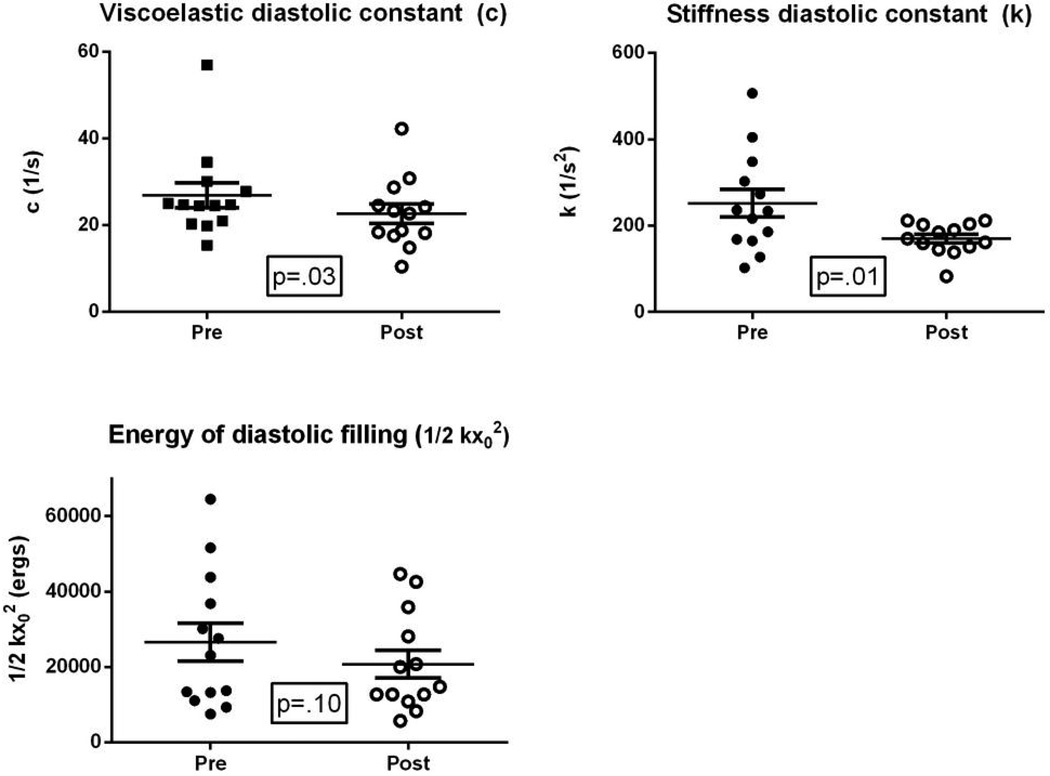
All patients had mitral velocity profile image quality suitable for analysis via the PDF formalism for quantitative diastolic function assessment; the effects of the study diet are shown in Figure 1. The DASH/SRD significantly reduced both the viscoelastic/relaxation (c, 24.3±5.3 to 22.7±8.1 s−1; p=.03) and chamber stiffness (k, 252±115 to 170±37 s−1;p=.03) constants, meaning that left ventricular relaxation improved and diastolic chamber stiffness declined. The load index xo (directly proportional to E-wave velocity-time integral and reflecting early diastolic filling volume) did not significantly change (13.9±4.1 to 15.0±4.4 cm, p=0.35). However, the available energy for diastolic filling trended lower (1/2 kxo2, 26,570±18,050 to 20,720±13,053 ergs, p=0.10).
Figure 1. Effects of DASH/SRD on ventricular diastolic function (PDF formalism).
Abbreviations: DASH/SRD: sodium-restricted Dietary Approaches to Stop Hypertension diet; PDF: Parametrized Diastolic Filling
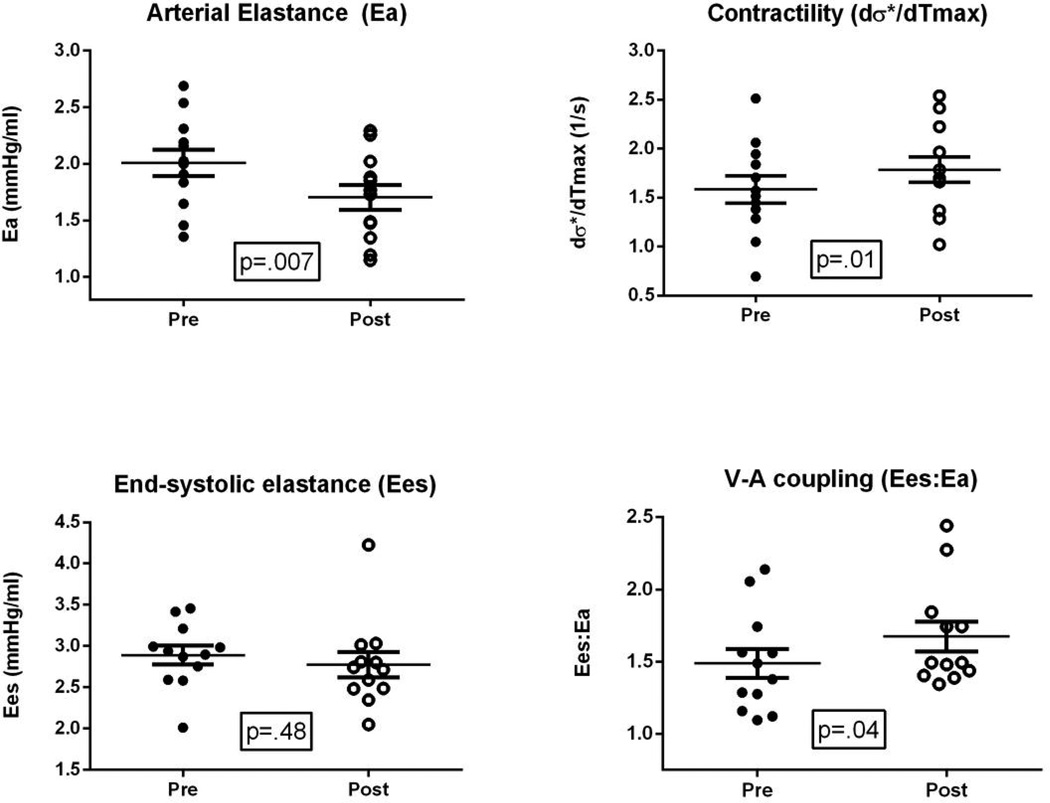
Twelve of 13 patients had adequate LVOT outflow tract velocity profiles for analysis of Ea, Ees, V-A coupling, and dσ*/dtmax; results are displayed in Figure 2. The DASH/SRD was associated with reduced Ea (2.0±0.4 to 1.7±0.4 mmHg/ml; p=.007); the Ees did not significantly change (2.9±0.4 to 2.8±0.5, p=.48), but V-A coupling improved (Ees:Ea, 1.5±0.3 to 1.7±0.4; p=.04) while global contractility increased (dσ*/dtmax (1.6±0.5 to 1.8±0.5 s−1; p=.01). Results for Ea, Ees, the Ees:Ea ratio, and dσ*/dtmax were nearly identical using 0.9 * brachial systolic BP instead of Sphygmocor-measured central end-systolic pressure (not shown).
Figure 2. Effects of DASH/SRD on arterial elastance, end-systolic ventricular elastance, and V-A coupling.
Abbreviations: DASH/SRD: sodium-restricted Dietary Approaches to Stop Hypertension diet; V-A: ventricular-arterial
In conjunction with these changes, the ventricular stroke volume increased and systemic vascular resistance decreased following DASH/SRD. While the heart rate (62±8 to 59±11 beats/min, p=0.23) and cardiac output did not significantly change (Table 2), left ventricular mechanical energetic efficiency increased (76±4 to 78±4%, p=.05). The increase in energy efficiency closely paralleled the changes in Ees:Ea ratio (r=.92, p<.001).
Discussion
In this cohort of patients with hypertensive HFPEF, the sodium-restricted DASH diet (DASH/SRD) improved left ventricular diastolic function, reduced arterial elastance, and shifted V-A coupling to reflect more efficient transfer of blood between the heart and arteries.
Over the past decade, HFPEF has become recognized as a multifactorial disorder of impaired cardiovascular reserve. Left ventricular diastolic dysfunction, the classic paradigm for HFPEF, is common and related to exertional intolerance both via intrinsic stiffness and impaired early diastolic relaxation.28 However, many other factors have also been implicated, such as chronotropic incompetence, endothelial dysfunction, and decreased ventricular systolic reserve.4 Of particular relevance in hypertensive HFPEF are combined increases in large arterial and end-systolic ventricular stiffness.29 These factors contribute to impaired diastolic relaxation and a higher cardiac energy cost to increase cardiac output.3
Several clinical trials have reported improvements in ventricular diastolic function and reductions in Ea and Ees with pharmacological BP management,6, 7, 30, but the effects of dietary modification on ventricular-vascular function in HFPEF have not been established. “Salt-sensitive” animal models develop HFPEF through the shared mechanisms of dietary sodium-induced hypertension, oxidative stress, and perivascular inflammation.10, 31, 32 In humans, the demographic factors and comorbidities that predict BP salt-sensitivity are similar to those of HFPEF,1, 13, 31 and sodium intake and other dietary characteristics may influence the risk of heart failure32, 33 in obese and/or older adults.32, 33 However, few studies have investigated links between dietary factors and HFPEF.13, 34, 35
As revealed by the PDF formalism method, the DASH/SRD improved both viscoelastic/relaxation (c) and passive/stiffness (k) measures of ventricular diastolic function. The energy available for diastolic filling trended lower; since stroke volume was maintained, this finding suggests increased diastolic filling efficiency. We did not vary preload during echocardiographic measurements in this study. However, the lack of change in xo following DASH/SRD supports the interpretation that preload alterations were not the primary reason for changes in diastolic function. The potential impact of ventricular afterload on diastolic function is often under-appreciated.36 For example, during handgrip exercise in HFPEF patients, afterload, systemic BP, and left ventricular end-diastolic pressure increase in tandem.3, 37 In this study, we found no significant correlation between BP or Ea changes and PDF formalism diastolic function measures, but cannot rule out secondary improvement of diastolic function due to reduced arterial afterload.
The significant decrease in Ea following DASH/SRD was related both to lower end-systolic pressure and an increase in ventricular stroke volume. The Ea is a lumped afterload parameter dependent on heart rate, proximal arterial stiffness, and peripheral arterial resistance. In this cohort, the DASH/SRD improved both vascular function components13 without changing heart rate. Our results are consistent with previous studies in older hypertensive adults without heart failure, in which dietary sodium reduction rapidly improved endothelial function and arterial stiffness.38, 39
In patients with hypertensive HFPEF, the Ees and Ea increase in parallel so that the V-A coupling ratio is preserved at rest. However, HFPEF patients have reduced chamber and myocardial contractility when compared with age-matched hypertensives.40 Moderate cycle ergometer exercise reveals a disproportionate impact of proximal arterial stiffness during physical activity in HFPEF patients.5 The resulting unfavorable V-A coupling, or “afterload mismatch,” in the setting of impaired ventricular contractile reserve may be an important factor limiting exercise duration in HFPEF.4
Following the DASH/SRD, the Ees:Ea ratio significantly increased. This observation reflects improved V-A coupling, a conclusion supported by the closely correlated increases in left ventricular mechanical energetic efficiency. These results are similar to a recent pooled study of clinical trial participants with hypertension and ventricular diastolic dysfunction. In that analysis, valsartan ± amlodipine increased the Ees:Ea ratio and ventricular energetic efficiency.6 In our study the dσ*/dtmax also increased, indicating a higher rate of systolic wall stress generation. Since this parameter is indexed for left ventricular mass and does not relate to intrinsic ventricular stiffness, our results suggest that the DASH/SRD was associated with increased global ventricular contractility. While long-term antihypertensive medication treatment improves myocardial contractility, especially in the context of left ventricular mass regression,8 we cannot determine if the DASH/SRD directly affected left ventricular systolic performance or whether the reduction in effective arterial afterload was responsible.
As in other HFPEF cohorts, our study participants were predominantly female and obese. Central obesity is one of the strongest predictors of aortic stiffness in large community cohorts.41 Proximal aortic stiffness is greater in women than men and is associated with ventricular diastolic dysfunction and altered V-A coupling.42 Following antihypertensive medication treatment, women and obese subjects have less improvement in V-A coupling and ventricular systolic efficiency than men and nonobese subjects.6 Our short-term dietary intervention study cannot be directly compared to longer-term drug studies, but it is intriguing that large arterial stiffness13, global left ventricular contractility, and V-A coupling all significantly improved following DASH/SRD in this cohort of elderly, obese women.
Limitations
Our study group was small, but its characteristics closely resembled those in large HFPEF cohort studies2, 34; as such, the cohort had several factors that predict salt-sensitive hypertension (e.g. advanced age, diabetes, obesity, renal insufficiency, post-menopausal state).31 Our findings are hypothesis-generating, and should not be generalized to all HFPEF patients, particularly those with non-hypertensive etiology or different demographics.
We do not have data from a formal control dietary period for comparison with DASH/SRD. However, urinary measures and survey assessments indicated excellent adherence to the study diet and significant alteration from baseline dietary patterns. Other recent studies of ventricular mechanics and V-A coupling in antihypertensive clinical trials also reported pre-post treatment changes without describing findings in the placebo group.6, 8 Nevertheless, given the absence of a control group in this study we cannot exclude the possibility that our findings are influenced by a placebo effect.
We did not observe changes in left ventricular diastolic function by standard methods, in particular the E/e’ ratio. However, the PDF formalism method has been extensively invasively validated and provides several potential advantages over the E/e’ ratio.20–23, 27 A recent study challenged the E/e’ ratio as a measure of diastolic function in the setting of normal left ventricular ejection fraction, finding poor correlation between E/e’ and invasive pulmonary capillary wedge pressure measurements and low discrimination for E/e’ as a predictor of elevated wedge pressure.43 In contrast, the PDF approach accurately predicts elevated ventricular filling pressures in patients with normal ejection fraction and diastolic dysfunction.21–23 This technique is causality based (on the suction pump attribute of the left ventricle), and is unique in its ability to quantify diastolic function in terms of relaxation, stiffness, and load as determinants of transmitral flow.
The DASH/SRD significantly affected several of the key factors that contribute to symptoms in HFPEF. However, our study was performed at rest and the findings cannot be directly extrapolated to physical activity. Future dietary modification studies in HFPEF should evaluate ventricular and vascular function and interactions during exercise.
Conclusions
In HFPEF patients with treated hypertension, the sodium-restricted DASH diet was associated with improvements in left ventricular diastolic function, global contractility, arterial elastance, and V-A coupling. These findings support further dietary modification studies to clarify links between the ‘salt-sensitive’ phenotype and hypertensive HFPEF.
Acknowledgements
The authors would like to acknowledge Joanna Wells, B.S., Theresa Han-Markey, M.S., R.D., Debra Peterman, B.S., R.D., Laura Foess-Wood, B.S., Cynthia Boersma, B.S., Martha Hartdegen, B.S., Elizabeth Meurer, M.S., R.D., and the staff at the Michigan Clinical Research Unit for their invaluable assistance.
Funding:
Work at the University of Michigan was supported by the NIH [K23HL109176 to S.L.H. and UL1TR000433 to the University of Michigan] and the Innovations in Cardiovascular Medicine Award from the Samuel and Jean Frankel Cardiovascular Center at the University of Michigan. Work at Washington University in St. Louis was supported in part by the Alan A. and Edith L. Wolff Charitable Trust (St. Louis, MO) and the Barnes-Jewish Hospital Foundation. E. Ghosh is the recipient of a Heartland Affiliate predoctoral fellowship award (11PRE4950009) from the American Heart Association. S. Zhu is a recipient of an Honorary Scholars Summer Research Grant from Washington University in Saint Louis. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Disclaimer: The manuscript and its contents are confidential, intended for journal review purposes only, and not to be further disclosed.
Disclosures:
None declared
Clinical Trial Registration: http://www.clinicaltrials.gov, NCT00939640
References
- 1.Mohammed SF, Borlaug BA, Roger VL, Mirzoyev SA, Rodeheffer RJ, Chirinos JA, Redfield MM. Comorbidity and ventricular and vascular structure and function in heart failure with preserved ejection fraction: a community-based study. Circ. Heart Fail. 2012;5:710–719. doi: 10.1161/CIRCHEARTFAILURE.112.968594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N. Engl. J. Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [see comment]. [DOI] [PubMed] [Google Scholar]
- 3.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107:714–720. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 4.Borlaug BA, Olson TP, Lam CSP, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2010;56:845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tartière-Kesri L, Tartière J-M, Logeart D, Beauvais F, Cohen Solal A. Increased proximal arterial stiffness and cardiac response with moderate exercise in patients with heart failure and preserved ejection fraction. J. Am. Coll. Cardiol. 2012;59:455–461. doi: 10.1016/j.jacc.2011.10.873. [DOI] [PubMed] [Google Scholar]
- 6.Lam CSP, Shah AM, Borlaug BA, Cheng S, Verma A, Izzo J, Oparil S, Aurigemma GP, Thomas JD, Pitt B, Zile MR, Solomon SD. Effect of antihypertensive therapy on ventricular–arterial mechanics, coupling, and efficiency. Eur. Heart J. 2013;34:676–683. doi: 10.1093/eurheartj/ehs299. [DOI] [PubMed] [Google Scholar]
- 7.Solomon SD, Janardhanan R, Verma A, Bourgoun M, Daley WL, Purkayastha D, Lacourcière Y, Hippler SE, Fields H, Naqvi TZ, Mulvagh SL, Arnold JMO, Thomas JD, Zile MR, Aurigemma GP. Effect of angiotensin receptor blockade and antihypertensive drugs on diastolic function in patients with hypertension and diastolic dysfunction: a randomised trial. Lancet. 2007;369:2079–2087. doi: 10.1016/S0140-6736(07)60980-5. [DOI] [PubMed] [Google Scholar]
- 8.Wachtell K, Palmieri V, Olsen MH, Gerdts E, Papademetriou V, Nieminen MS, Smith G, Dahlöf B, Aurigemma GP, Devereux RB. Change in systolic left ventricular performance after 3 years of antihypertensive treatment: the Losartan Intervention For Endpoint (LIFE) study. Circulation. 2002;106:227–232. doi: 10.1161/01.cir.0000021601.49664.2a. [DOI] [PubMed] [Google Scholar]
- 9.Goodwin K, Ahokas R, Bhattacharya S, Sun Y, Gerling I, Weber K. Preventing oxidative stress in rats with aldosteronism by calcitriol and dietary calcium and magnesium supplements. Am. J. Med. Sci. 2006;332:73–78. doi: 10.1097/00000441-200608000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Klotz S, Hay I, Zhang G, Maurer M, Wang J, Burkhoff D. Development of heart failure in chronic hypertensive Dahl rats: focus on heart failure with preserved ejection fraction. Hypertension. 2006;47:901–911. doi: 10.1161/01.HYP.0000215579.81408.8e. [see comment]. [DOI] [PubMed] [Google Scholar]
- 11.Kido M, Ando K, Onozato ML, Tojo A, Yoshikawa M, Ogita T, Fujita T. Protective effect of dietary potassium against vascular injury in salt-sensitive hypertension. Hypertension. 2008;51:225–231. doi: 10.1161/HYPERTENSIONAHA.107.098251. [DOI] [PubMed] [Google Scholar]
- 12.Seymour EM, Bennink M, Watts S, Bolling S. Whole grape intake impacts cardiac peroxisome proliferator-activated receptor and nuclear factor kappa-B activity and cytokine expression in rats with diastolic dysfunction. Hypertension. 2010;55:1179–1185. doi: 10.1161/HYPERTENSIONAHA.109.149393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hummel SL, Seymour EM, Brook RD, Kolias TJ, Sheth SS, Rosenblum HR, Wells JM, Weder AB. Low-sodium Dietary Approaches to Stop Hypertension diet reduces blood pressure, arterial stiffness, and oxidative stress in hypertensive heart failure with preserved ejection fraction/novelty and significance. Hypertension. 2012;60:1200–1206. doi: 10.1161/HYPERTENSIONAHA.112.202705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur. Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 15.Chobanian A, Bakris G, Black H, Cushman W, Green L, Izzo J, Jones D, Materson B, Oparil S, Wright J, Roccella E. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 16.O'Brien E, Coats A, Owens P, Petrie J, Padfield PL, Littler WA, de Swiet M, Mee F. Use and interpretation of ambulatory blood pressure monitoring: recommendations of the British Hypertension Society. BMJ. 2000;320:1128–1134. doi: 10.1136/bmj.320.7242.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ott C, Haetinger S, Schneider MP, Pauschinger M, Schmieder RE. Comparison of two noninvasive devices for measurement of central systolic blood pressure with invasive measurement during cardiac catheterization. J. Clin. Hypertens. (Greenwich) 2012;14:575–579. doi: 10.1111/j.1751-7176.2012.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gottdiener J, Bednarz J, Devereux R, Gardin J, Klein A, Manning W, Morehead A, Kitzman D, Oh J, Quinones M, Schiller N, Stein J, Weissman N. American Society of Echocardiography recommendations for use of echocardiography in clinical trials. J. Am. Soc. Echocardiogr. 2004;17:1086–1119. doi: 10.1016/j.echo.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Dumesnil JGA. new, simple and accurate method for determining ejection fraction by doppler echocardiography. Can. J. Cardiol. 1995;11:1007–1014. [PubMed] [Google Scholar]
- 20.Lisauskas JB, Singh J, Bowman AW, Kovács SJ. Chamber properties from transmitral flow: prediction of average and passive left ventricular diastolic stiffness. J. Appl. Physiol. 2001;91:154–162. doi: 10.1152/jappl.2001.91.1.154. [DOI] [PubMed] [Google Scholar]
- 21.Mossahebi S, Kovacs SJ. Kinematic modeling-based left ventricular diastatic (passive) chamber stiffness determination with in-vivo validation. Ann. Biomed. Eng. 2012;40:987–995. doi: 10.1007/s10439-011-0458-3. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, Shmuylovich L, Kovács SJ. The E-wave delayed relaxation pattern to LV pressure contour relation: model-based prediction with in vivo validation. Ultrasound in Medicine & Biology. 2010;36:497–511. doi: 10.1016/j.ultrasmedbio.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Shmuylovich L, Kovács SJ. Load-independent index of diastolic filling: Modelbased derivation with in vivo validation in control and diastolic dysfunction subjects. J. Appl. Physiol. 2006;101:92–101. doi: 10.1152/japplphysiol.01305.2005. [DOI] [PubMed] [Google Scholar]
- 24.Hall AF, Kovács SJ. Automated method for characterization of diastolic transmitral doppler velocity contours: early rapid filling. Ultrasound in Medicine & Biology. 1994;20:107–116. doi: 10.1016/0301-5629(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 25.Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA, Kawaguchi M, Kass DA. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J. Am. Coll. Cardiol. 2001;38:2028–2034. doi: 10.1016/s0735-1097(01)01651-5. [DOI] [PubMed] [Google Scholar]
- 26.Zhong L. Attenuation of stress-based ventricular contractility in patients with heart failure and normal ejection fraction. Ann. Acad. Med. Singapore. 2011;40:179–185. [PubMed] [Google Scholar]
- 27.Zhong L, Tan R-S, Ghista DN, Ng EY-K, Chua L-P, Kassab GS. Validation of a novel noninvasive cardiac index of left ventricular contractility in patients. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H2764–H2772. doi: 10.1152/ajpheart.00540.2006. [DOI] [PubMed] [Google Scholar]
- 28.Lindenfeld J, Albert N, Boehmer J, Collins S, Ezekowitz J, Givertz M, Katz S, Klapholz M, Moser D, Rogers J, Starling R, Stevenson W, Tang WHW, Teerlink J, Walsh M. HFSA 2010 comprehensive heart failure practice guideline. J. Card. Fail. 2010;16:e1–194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, Lakatta EG, Najjar SS, Kass DA. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J. Am. Coll. Cardiol. 2007;49:198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 30.Tapp RJ, Sharp A, Stanton AV, O'Brien E, Chaturvedi N, Poulter NR, Sever PS, Thom SAM, Hughes AD, Mayet J. Differential effects of antihypertensive treatment on left ventricular diastolic function: an ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) substudy. J. Am. Coll. Cardiol. 2010;55:1875–1881. doi: 10.1016/j.jacc.2009.11.084. [DOI] [PubMed] [Google Scholar]
- 31.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481–490. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- 32.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Dietary sodium intake and incidence of congestive heart failure in overweight US men and women: First National Health And Nutrition Examination Survey epidemiologic follow-up study. Arch. Intern. Med. 2002;162:1619–1624. doi: 10.1001/archinte.162.14.1619. [see comment]. [DOI] [PubMed] [Google Scholar]
- 33.Levitan EB, Wolk A, Mittleman MA. Consistency with the DASH diet and incidence of heart failure. Arch. Intern. Med. 2009;169:851–857. doi: 10.1001/archinternmed.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hummel SL, DeFranco AC, Skorcz S, Montoye CK, Koelling TM. Recommendation of low-salt diet and short-term outcomes in heart failure with preserved systolic function. Am. J. Med. 2009;122:1029–1036. doi: 10.1016/j.amjmed.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyer TE, Kovács SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J. Am. Coll. Cardiol. 2006;47:398–402. doi: 10.1016/j.jacc.2005.08.069. [DOI] [PubMed] [Google Scholar]
- 36.Chung CS, Strunc A, Oliver R, Kovács SJ. Diastolic ventricular-vascular stiffness and relaxation relation: elucidation of coupling via pressure phase plane-derived indexes. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H2415–H2423. doi: 10.1152/ajpheart.00257.2006. [DOI] [PubMed] [Google Scholar]
- 37.Penicka M, Bartunek J, Trakalova H, Hrabakova H, Maruskova M, Karasek J, Kocka V. Heart failure with preserved ejection fraction in outpatients with unexplained dyspnea: a pressure-volume loop analysis. J. Am. Coll. Cardiol. 2010;55:1701–1710. doi: 10.1016/j.jacc.2009.11.076. [DOI] [PubMed] [Google Scholar]
- 38.Gates PE, Tanaka H, Hiatt WR, Seals DR. Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension. 2004;44:35–41. doi: 10.1161/01.HYP.0000132767.74476.64. [DOI] [PubMed] [Google Scholar]
- 39.Jablonski KL, Racine ML, Geolfos CJ, Gates PE, Chonchol M, McQueen SMB, Seals DR. Dietary sodium restriction reverses vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure. J. Am. Coll. Cardiol. 2013;61:335–343. doi: 10.1016/j.jacc.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borlaug BA, Lam CSP, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease: insights into the pathogenesis of heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2009;54:410–418. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johansen NB, Vistisen D, Brunner EJ, Tabák AG, Shipley MJ, Wilkinson IB, McEniery CM, Roden M, Herder C, Kivimäki M, Witte DR. Determinants of aortic stiffness: 16-year follow-up of the Whitehall II study. PLoS ONE. 2012;7:e37165. doi: 10.1371/journal.pone.0037165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex differences in arterial stiffness and ventricular-arterial interactions. J. Am. Coll. Cardiol. 2013;61:96–103. doi: 10.1016/j.jacc.2012.08.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maeder MT, Karapanagiotidis S, Dewar EM, Gamboni SE, Htun N, Kaye DM. Accuracy of doppler echocardiography to estimate key hemodynamic variables in subjects with normal left ventricular ejection fraction. J. Card. Fail. 2011;17:405–412. doi: 10.1016/j.cardfail.2010.12.003. [DOI] [PubMed] [Google Scholar]