Abstract
The optimal method for creating a de-cellularized lung scaffold that is devoid of cells and cell debris, immunologically inert, and retains necessary extracellular matrix (ECM) has yet to be identified. Herein, we compare automated detergent-based de-cellularization approaches utilizing either constant pressure (CP) or constant flow (CF), to previously published protocols utilizing manual pressure (MP) to instill and rinse out the de-cellularization agents. De-cellularized lungs resulting from each method were evaluated for presence of remaining ECM proteins and immunostimulatory material such as nucleic acids and intracellular material. Our results demonstrate that the CP and MP approaches more effectively remove cellular materials but differentially retain ECM proteins. The CP method has the added benefit of being a faster, reproducible de-cellularization process. To assess the functional ability of the de-cellularized scaffolds to maintain epithelial cells, intra-tracheal inoculation with GFP expressing C10 alveolar epithelial cells (AEC) was performed. Notably, the CP de-cellularized lungs were able to support growth and spontaneous differentiation of C10-GFP cells from a type II-like phenotype to a type I-like phenotype.
Keywords: Lung, C10 alveolar cells, De-cellularization, Alveolar epithelial cell, Extracellular matrix, Scaffold
1. Introduction
Lung disease, the third highest cause of death in the United States, is a major public health concern. Almost 400,000 Americans die yearly from pulmonary disease and more than 35 million people are afflicted [1]. Lung transplantation remains a final option but is significantly limited by supply of suitable donor lungs along with the accompanying need for lifelong immunosuppression and the high mortality [2,3]. Though lung transplants are life saving measures, alternative therapeutic options are desperately needed. In an effort to address these issues and improve the therapeutic use of lung transplantation, there has been rapid growth in the development of ex vivo tissue engineering techniques with the goal of creating functional transplantable lungs [4].
In tissue engineering, it is essential for the optimal scaffold to maintain an appropriate three dimensional configuration and retain important extracellular matrix (ECM) proteins. ECM proteins such as laminin, fibronectin, elastin, collagen I and IV have been found to play roles in trans-membrane cell signaling, cellular differentiation, respiratory mechanics and other pulmonary-specific functions [5–8]. The ability of cells to receive organotypic signals from native ECM provides a potential system for functional re-cellularization compared with synthetic constructs [9]. Therefore, the retention of key ECM proteins is critical for a de-cellularized matrix to provide pulmonary-specific cellular signals.
However, the optimal method for de-cellularization of pulmonary tissue while maintaining critical ECM proteins is unclear. The de-cellularization procedure can be performed by different processes, such as physical methods, chemical agents, and enzymatic degradations [3,9,10]. Cellular materials, particularly nucleic acids, proteins, and glycosaminoglycans, are known to be immunostimulatory and should ideally be eliminated by the de-cellularization process [11–15]. Several methods of de-cellularization have been published, including previous work from our laboratory, utilizing detergent and enzymatic washes, as well as physical methods (freeze–thaw) to de-cellularize rodent lung matrices with subsequent maintenance of fetal pulmonary cells within the scaffold [16,17]. However, there is no consensus. One particular issue that remains unclear is whether manual versus mechanized de-cellularization will yield more optimal results. Several papers have described use of manual pressure (MP) de-cellularization approach with generally good results [16,18,19]. However, this process is subject to user variability and no consensus has been reached regarding the optimal de-cellularization procedure.
As automated de-cellularization techniques may provide faster, more reliable scaffolds, we sought to determine whether varying flow or pressure with automation would affect the quality of the scaffold. As such, we compare three different detergent-based de-cellularization approaches utilizing either constant pressure (CP), constant flow (CF) or a previously published method utilizing manual pressure (MP) to instill and rinse out the cellular material and de-cellularization agents. In addition, we compared vascular de-cellularization alone or in combination with airway de-cellularization. Comprehensive assessment of the de-cellularized lungs will include analysis of the matrix composition as well as loss of immunostimulatory material. We will compare the CF and CP techniques to the previously published MP approach in order to assess whether these differences in technique affect cell adherence and viability. We will utilize an alveolar epithelial cell line to evaluate the ability of all of these scaffolds to support epithelial repopulation. This will be compared following 3 days in a physiologic bioreactor system. The data we obtain should help to define and refine optimal approaches to lung de-cellularization.
2. Materials and methods
2.1. Methods for de-cellularization of rat lungs
(Table 1/Fig. 1) 20 week-old Sprague–Dawley rats (Charles River, Wilmington, MA) were euthanized in accordance with University of Connecticut Health Center IACUC approved protocols. A midline incision was made from the upper abdomen to the throat and a median sternotomy was performed. The trachea and heart-lung block were carefully dissected and exposed for cannulation.
Table 1.
Summary of Rat Lung De-cellularization Methods De-cellularization reagent, duration of protocol, levels of nuclear material still present after de-cellularization and proliferation after re-seeding is summarized above. CP de-cellularization appears to remove nucleic acids as well as the MP protocol in a shorter period of time while using a single reagent. CF de-cellularization appears to be ineffective in removing all nuclear material from the matrix during this time point. Following re-seeding, cell proliferation was observed only when utilizing the CP de-cellularized scaffold following 3 days in a bioreactor. Abbreviations: 4′,6-diamidino-2-phenylindole (DAPI), Propidium Iodide (PI), Sodium Deoxycholate (SDC), Sodium Chloride (NaCl), Sodium Dodecyl Sulfate (SDS).
| Decell method | Decell reagent(s) | Airway rinse volume | Vasculature rinse volume | Duration of protocol | Nuclei present | DNA/RNA present (PI) | Nucleic acids present (TOPRO-3) | Proliferation following Re-seeding |
|---|---|---|---|---|---|---|---|---|
| 3 Day method | Triton-X, SDC, NaCl, DNAse via trachea & vasculature | 150 ml | 150 ml | 3 Days | None | Minimal | Minimal | No |
| Perfusion/flow-base | 0.1% SDS via trachea & vasculature | 50 ml | 3,456 ml | 20.5 H | Scarce | Significant retention | Significant retention | No |
| Pressure-base | 0.1% SDS via vasculature | None | 30–40 L | 20.5 H | None | Minimal | Minimal | Yes |
Fig. 1.
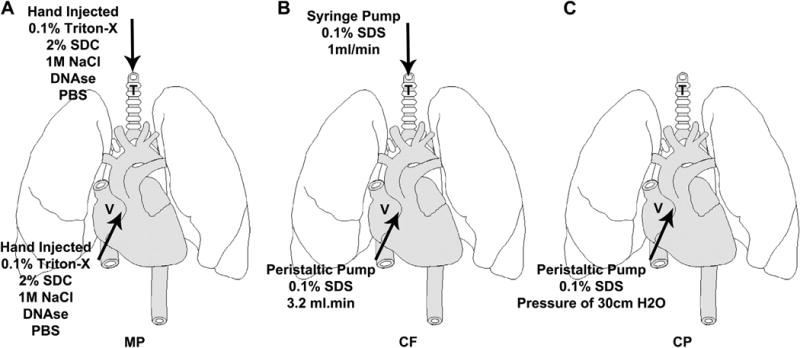
Schematic of De-cellularization Process MP de-cellularization (A) and CF de-cellularization (B) are achieved by infusing detergent through the trachea (T) and vasculature (V), while CP de-cellularization (C) infuses the detergent only through the vasculature (V).
2.1.1. MP de-cellularization
(Fig. 1A) The following procedure was adapted from a previously described protocol [16] and was followed as a comparison to our proposed automated techniques. The heart-lung block of the rat was carefully removed and the trachea was cannulated with an 18 gauge angiocatheter and secured with sterile 2–0 silk ties (Braun Medical, Bethlehem, PA). An 18 gauge needle and a 10cc syringe were used to manually inject 10 ml of phosphate buffered saline (PBS) into the right ventricle of the heart-lung block. The trachea was manually injected with 10 ml PBS to inflate the lungs. This cycle was repeated a total of five times. Triton X-100 (0.1%) in a deionized water (DI) (Sigma, St. Louis, MO) solution was manually injected into the scaffold in the same manner and volume as described above followed by complete submersion in the solution for 24 h at 4° C. The scaffold was rinsed five times through the right ventricle and trachea with PBS as described above. 2% sodium deoxycholate (SDC) in DI (Sigma, St. Louis, MO) was injected as above followed by submersion in the solution for 24 h at 4°C. The scaffold was then rinsed five times as above with PBS. 1 m sodium chloride (NaCl) (Fisher Scientific, Pittsburgh, PA) was injected as previously described and submerged in the NaCl solution for 1 h at 22°C. The scaffold was rinsed five times with PBS as above followed by injection and incubation in a PBS solution containing 30 μg/ml of porcine pancreatic DNAse I (Sigma, St. Louis, MO) for a total of 1 h at 22°C. Lastly, the scaffold was rinsed with PBS as above containing 1× penicillin-streptomycin (Gibco, Grand Island, NY) and 1× amphotericin-b (Gibco Grand Island, NY). The total combined rinse volume was 150 ml via the trachea and 150 ml via the right ventricle (vasculature).
2.1.2. CF de-cellularization
(Fig. 1B) Prior to removing the heart-lung block from the animal, the major vessels were dissected and ligated. An 18-gauge angiocatheter (Braun Medical, Bethlehem, PA) was used to cannulate the pulmonary artery (PA) and secured with 4–0 silk tie. An 18 gauge angiocatheter was also used to cannulate the trachea and was secured with a 2–0 silk tie. Intravenous extension tubing was used to connect the angiocatheter to a four channel peristaltic pump (Fisher FH100M) using Master Flex tubing (Fisher Scientific, Pittsburgh, PA). We tested multiple flow rates and observed damage to the distal airways when flow rates exceeded 3.2 ml/min (data not shown), therefore we chose 3.2 ml/min for the remaining experiments. The scaffold was flushed with PBS or DI through the pulmonary artery catheter with the pump for 30 min. As previously published with perfusion-based de-cellularization [20], we utilized sodium dodecyl sulfate (SDS) as our single de-cellularization agent. Following the rinse, 0.1% SDS (Fisher Scientific, Pittsburgh, PA) solution was perfused in a non-recirculating manner through the vasculature for a total of 2 h at 22°C. The solution was also injected via the trachea using a single channel syringe pump (Braintree Scientific BS-8000, Braintree, MA). To prevent over inflation of the lung, the syringe pump flow rate was set to 1 ml/min and delivered a total of 10 ml of solution. The trachea was uncapped after each injection to allow the lungs to decompress. This procedure was repeated during the vascular perfusion for a total of 5 times. Following the de-cellularization process the scaffold was rinsed with PBS through the vasculature at the same flow rate above for 18 h as well as rinsed 5 times through the trachea utilizing the syringe pump as previously described. The total combined rinse volume was 50 ml via the trachea and 3456 ml via the pulmonary artery (vasculature).
2.1.3. CP de-cellularization
(Fig. 1C) This method of de-cellularization utilizes a Harvard Apparatus dual chamber isolated heart lung – IL2 bioreactor system (Harvard Apparatus Inc., Holliston, MA). Prior to removing the heart-lung block from the animal, all large vessels were carefully ligated. Cannulas for this procedure were provided with the bioreactor system. Prior to euthanizing the animal, heparin sulfate was administered at a dose of 1000 units per rat and allowed to circulate for 3 min. This adjustment was made due to significant variation in flow rates seen if clots were present within the IL-2 bioreactor perfusion loop. This was defined by decreased flow rates and poor lung expansion, or visual identification of clots within the outflow catheter. The trachea was cannulated and the catheter secured with two 2–0 silk ties. The cannulated trachea was attached to the bioreactor to maintain the appropriate orientation of the scaffold in the bioreactor and no detergent or fluids were placed into the trachea prior to seeding the scaffold with cells. The right ventricle was incised and the PA cannula was inserted and secured with a 4–0 silk tie. The apex of the heart was removed revealing the left ventricle, which enabled cannulation of the left atrium. This was secured with a 2–0 silk tie. Precautions were taken to prevent air emboli during the initial inflation of the lung via the vasculature. The perfusion pressure was set to 30 cm H2O and DI water was perfused through the system for 30 min. De-cellularization began by perfusing 0.1% SDS at a rate necessary to maintain a pressure of 30 cm H2O. Following 2 h of de-cellularization, the scaffold was rinsed with DI water for a total of 18 h. The perfused fluid during de-cellularization and rinsing was not allowed to re-circulate back into the scaffold. The total combined rinse volume was approximately 30–40 L via the vasculature.
2.2. Basic histology and immunofluorescence analysis of de-cellularized scaffolds
Scaffolds were fixed in 4% PFA for 12 h prior to being embedded in paraffin. Mounted 5 μm sections were de-paraffinized. Alcian Blue stain was used to evaluate the presence of glycosaminoglycans, while Verhoeff's elastin stain was used to outline elastin filaments. Gomori trichrome stain was used to evaluate the presence of collagen and hematoxylin and eosin (H&E) was used to evaluate the presence of any remaining cells.
For immunofluorescence, mounted 5 μm sections were de-paraffinized and incubated for 35 min in antigen retrieval buffer comprised of sodium citrate and 0.05% Tween-20 (Bio-Rad, Hercules, CA). Both tissue sections and cells were permeabilized and blocked in PBS containing 0.1% Triton X-100, 1% Tween-20 and 3% Fetal Bovine Serum (FBS). Primary antibodies to Laminin (Abcam, Cambridge, MA), Fibronectin (Santa Cruz, Santa Cruz, CA), Collagen IV (Santa Cruz, Santa Cruz, CA), Collagen I (Santa Cruz, Santa Cruz, CA), and Elastin (Santa Cruz, Santa Cruz, CA) were diluted in the blocking buffer above. Cells and sections were incubated overnight at 4°C in primary antibody and rinsed the following day with PBS. Secondary antibody donkey anti-goat Alexa Fluor® 488, goat anti-rabbit Alexa Fluor® 488 (Invitrogen, Grand Island, NY) or donkey anti-Syrian hamster Dyelight 549 (Jackson Immunoresearch, West Grove, PA) was applied in the dark at 22°C for 1 h. Sections were rinsed several times in PBS and counterstained with either Topro-3 (Invitrogen, Grand Island, NY) or DAPI (Invitrogen, Grand Island, NY) for 30 min at 22°C in the dark. Cells or sections were then rinsed with PBS and cover slipped using Immuno-mount (Fisher Scientific, Pittsburgh, PA). Tissue sections were imaged on a Zeiss Axiovert 200M Confocal Microscope with a LSM 510 Meta Laser Module and Carl Zeiss LSM Image Browser Software (Carl Zeiss Microimaging, LLC Thornwood, NY). Cells were imaged on a Zeiss Inverted Fluorescence microscope with Axiovision Software (Carl Zeiss Microimaging, LLC, Thornwood, NJ). Images were compiled and processed using Adobe Photoshop CS5 (Adobe Systems, San Jose, CA). Each de-cellularization method was performed and resultant scaffolds were analyzed in triplicate.
2.3. Assessment of nucleic acid loss following de-cellularization
Paraffin sections were de-paraffinized and prepared as described above. Topro-3 (Invitrogen, Grand Island, NY) is a carbocyanine monomer nucleic acid stain and is one of most sensitive probes for nucleic acid detection. In addition, Propidium Iodide (PI) (Invitrogen, Grand Island, NY) is an intercalating agent and a fluorescent molecule that is the most commonly used dye to quantitatively assess DNA content. Topro-3 and PI were diluted in PBS and sections were allowed to incubate for 30 min in the dark. Sections were then rinsed with PBS, cover slipped and imaged as above. Topro-3 signal was quantified using Image J and the threshold area calculator plugin. Five serial tissue sections were used to produce representative images of the scaffold in each de-cellularization condition. Image J plugin was then utilized to quantify the number of pixels per channel. The controls were normal rat lung treated with the same detection agents. This data was then graphed and analyzed using GraphPad Prism Software (GraphPad, La Jolla, CA).
2.4. Western blot analysis of de-cellularized lung scaffolds
Protein was isolated from de-cellularized scaffolds and adult rat lungs using RIPA buffer, tissue homogenizer and sonication. Electrophoresis was performed with 50 μg of protein loaded onto an 8% acrylamide SDS-PAGE gel. Protein was transferred to equilibrated Polyvinylidene fluoride (PVDF) membrane for 1 h at 100 V at 4°C. Bands were quickly visualized with Ponceau-S prior to blocking with 2% bovine serum albumin (BSA) in either PBS or tris-buffered saline (TBS) for 30 min. Primary antibodies against Histone H1 (Santa Cruz, Santa Cruz, CA), Collagen I (Santa Cruz, Santa Cruz, CA), Collagen IV (Santa Cruz, Santa Cruz, CA), Fibronectin (Santa Cruz, Santa Cruz, CA), Laminin (Abcam, Cambridge, MA), Elastin (Santa Cruz, Santa Cruz, CA) and Beta Actin (Abcam, Cambridge, MA) were diluted in blocking solution. Blots were incubated overnight on a blot rocker (Denville Scientific, South Plainfield, NJ) at 4°C. Blots were then rinsed three times in corresponding buffer (PBS or TBS) and secondary antibodies (goat anti-rabbit HRP or rabbit anti-goat HRP) (Invitrogen, Grand Island, NY) were diluted in blocking buffer and allowed to incubate at 22° C for 1 h. Blots were rinsed three times. Supersignal west pico chemiluminescent substrate (Thermo, Pittsburgh, PA) was applied for 5 min in the dark. Film was exposed to blots in a film cassette, film was developed on an X-ray film processor (Konica SRX101A) and scanned into Adobe Photoshop CS5 (Adobe Systems, San Jose, CA) for image processing. Analysis of the western blots was performed using Image J and GraphPad Prism software (Graphpad Software, La Jolla, CA). Protein bands were quantified and normalized to control values using Image J. These ratios were graphed in GraphPad Prism and statistics were performed (n = 3). Samples were analyzed and compared using a one-way ANOVA analysis with subsequent Bon-ferroni's multiple comparison test with a confidence interval of 95% (p-value of ≤0.05).
2.5. Culturing of C10 epithelial cells
Cells were cultured in maintenance media consisting of Dulbecco's modified eagle medium (DMEM) high glucose (Invitrogen, Grand Island, NY) with 10% FBS, 1× penicillin streptomycin and 2 mm l-glutamine (Gibco, Grand Island, NY). Cells were passaged every 2–3 days and were split at a ratio of 1:3 using TrypLE (Gibco, Grand Island, NY).
2.6. GFP transfection of C10 epithelial cells
PHAGE EF1aL-GFP vector was obtained from Dr. Darrell Kotton's Lab at Boston University School of Medicine. DH5α max efficiency cells (Invitrogen, Grand Island, NY) were transfected with 5 ng of the vector and plated on luria broth (LB) agar and ampicillin (amp) plates overnight. Colonies were picked from the plates and grown in LB + amp overnight. Bacterial culture was expanded and allowed to incubate for another 24 h. Plasmids were extracted from cells using MaxiPrep Kit (Qiagen, Valencia, CA) and quantified using a nanodrop spectrophotometer (Thermo, Pittsburgh, PA). 293T cells were infected with lentivirus and supernatant was collected and concentrated as previously described [21]. C10 cells were transduced with the lentivirus and polybrene agent overnight. Following transduction, cells were expanded and sorted on a BD FACS Aria II cell sorter (BD Biosciences, San Jose, CA) to remove GFP negative cells.
2.7. Characterization of C10-GFP cells after transfection
Cells were fixed with 4% PFA on ice and rinsed two times with PBS. Cells were permeabilized and blocked in a solution containing PBS, 0.1% Triton X-100, 1% Tween-20 and 3% FBS. Primary antibodies for TTF1 (Millipore), Pro-SPC (Santa Cruz) and T1α (University of Iowa Hybridoma Bank) were diluted in permeabilization and blocking buffer overnight at 4°C. As a control, primary antibody was omitted to assess for any non-specific binding of the secondary antibody. Cells were rinsed several times with PBS and secondary antibodies donkey anti-goat Alexa Fluor® 546 (Invitrogen) or donkey anti-Syrian hamster Dyelight 549 (Jackson Immunoresearch) were diluted in PBS and incubated for 1 h at 22°C in the dark. Cells were rinsed several times in PBS and counterstained with DAPI (Invitrogen) for 25 min in the dark. Cells were imaged on a Zeiss Inverted Fluorescence microscope with Axiovision Software (Carl Zeiss Microimaging, LLC). Images were processed and compiled using Adobe Photoshop CS5 (Adobe Systems, San Jose, CA).
2.8. C10-GFP In vitro ECM coating assay
MP, CF, and CP lungs were de-cellularized as above. Lungs were manually minced and placed in 2 mg/ml collagenase/PBS solution at 37° C for 30 min. The slurry was then triturated with an 18 gauge needle, re-incubated in the collagenase solution for 30 min, and triturated again with a 21 gauge needle. The solution was passed through a 40 μm mesh filter and the flow through was centrifuged at 15,000 rpm for 10 min at 4°C. The supernatant was discarded and the pellet was resuspended in sterile DI H2O. The resuspended solution was applied to a 6 well plate and placed under an ultraviolet light (254 nm) for 30 min for sterilization. Following sterilization, the ECM coating solution did not appear to change based on visual inspection. C10-GFP cells were seeded onto the coated plates at a concentration of 5.0 × 104 per well and cultured with maintenance media for three days.
2.9. Re-seeding of de-cellularized lung scaffolds using physiologic bioreactor system
Following de-cellularization and prior to seeding, the lung scaffold produced by all three methods were flushed with sterile PBS containing 5× penicillin streptomycin (Gibco, Grand Island, NY) and 1× amphotericin B (Gibco, Grand Island, NY) for 4 h at a pressure setting of 30 cm H2O. Lung scaffolds were seeded immediately after the rinse step above and were not stored for any period of time prior to seeding. Following this rinse, approximately 6.0 × 107 C10-GFP cells were trypsinized from tissue culture plastic and resuspended in a total volume of 7 ml of media. The scaffold was seeded through the trachea and cells were allowed to adhere for 4 h. Perfusion of C10 medium was then initiated at a pressure of 5, 10, 20, or 30 cm H2O through the vasculature for 3 days prior to harvest. Re-seeding of scaffolds was performed in triplicate.
2.10. Characterization of re-seeded scaffolds
All re-seeded scaffolds were characterized using H&E staining, apoptosis, proliferation, and immunofluorescence staining for markers of type II and type I alveolar epithelial cells. Tissue sections were de-paraffinized as previously described. DeadEnd Fluorometric TUNEL assay (Promega, Madison, WI) was utilized to detect apoptosis in fixed tissue according to manufacturer's instructions. Negative controls were normal rat lung tissue sections and positive controls were normal rat lung tissue sections treated with DNAse I (Sigma, St. Louis, MO).
The antibodies chosen were specific to proliferation and different distal pulmonary cell types: Ki-67 (proliferation, absent in senescent cells; Abcam, Cambridge, MA), TTF1 (distal lung precursor; Millipore, Billerica, MA), Pro-SPC (Type II AEC; Santa Cruz, Santa Cruz, CA), T1α (Type I AEC; University of Iowa Hybridoma Bank, Iowa City, IA) and AQP5 (Type I AEC; Santa Cruz, Santa Cruz, CA). Paraffin sections were stained, imaged and processed as previously described.
3. Results
3.1. Histologic comparison of de-cellularized matrices
The three de-cellularization protocols have been summarized in Table 1 and produce scaffolds that are grossly translucent and possess similar architecture as native lung. Since the MP protocol has been widely reported in the literature, this method was compared to the CF and CP de-cellularization protocols. As a further comparison, the CF and MP protocols utilize airway (trachea) (Fig. 1 “T”) and vasculature-based (Fig. 1 “V”) de-cellularization while the CP protocol utilizes only the vasculature.
H&E staining demonstrate cellular loss across all protocols (Fig. 2M–P). Alcian blue staining qualitatively demonstrates a loss of glycosaminoglycans to varying degrees with all three protocols compared to normal (Fig. 2A–D). Although the CF and CP protocols both retain some elastin, the MP protocol qualitatively retains the most elastin throughout the scaffold (Fig. 2E–H). Collagen retention, as shown by Gomori trichrome staining, is similar across all three protocols (Fig. 2I–L).
Fig. 2.
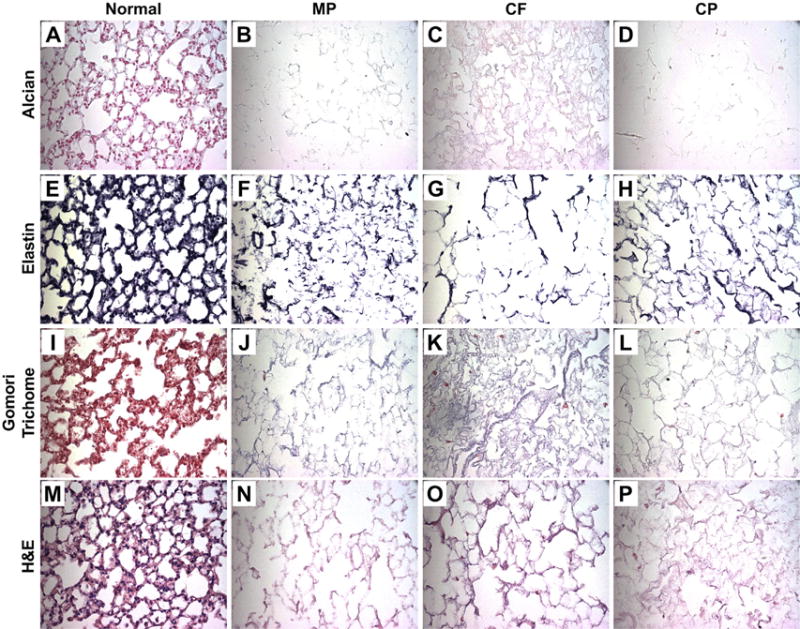
Histologic Evaluation of De-cellularized Scaffolds Representative images for Alcian Blue, a glycosaminoglycan's stain, demonstrate a loss across all three de-cellularization methods (blue) (A–D). Verhoeff's elastin stain, which outlines elastin filaments (black) (E–H), also demonstrates a loss across all three methods. Gomori Trichrome, a collagen stain (blue) (I–L) also indicates a loss across all three methods. Lastly, H&E staining (M–P) confirms loss of nuclei, while maintain structural integrity in all three methods. Representative images are shown from triplicate lungs de-cellularized using each method (All Images 40× Magnification). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Presence of nucleic acids following de-cellularization
Both PI and Topro-3 are absent following the CP protocol and diminished following the MP protocol. However, the CF protocol resulted in substantial retention of DNA and nucleic acids (Fig. 3A). Image quantification of Topro-3 fluorescence via one-tailed t-test (p ≤ 0.05) confirmed the qualitative results from the histologic staining and demonstrated a large and significant increase in nucleic acid content following the CF protocol (Fig. 3B). The MP protocol also resulted in significantly higher levels of residual nucleic acid compared to the CP protocol. Less variance was also observed with the CP protocol, suggesting it is more consistent and reproducible compared to the other protocols.
Fig. 3.
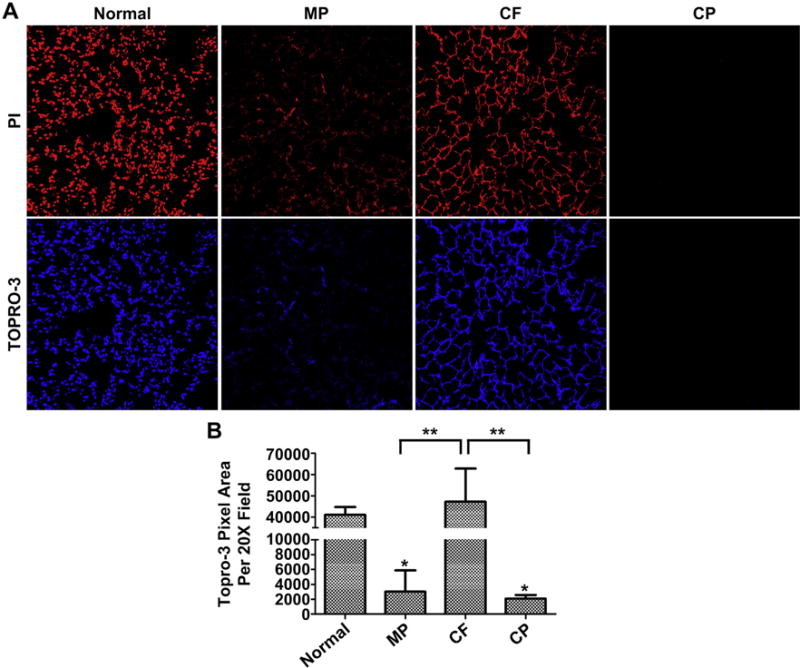
Presence of Nuclear Material Following De-cellularization (A) CP de-cellularization was most effective at removing nucleic acids. The MP protocol was also effective, while CF de-cellularization was very inefficient. Representative images are shown from triplicate lungs de-cellularized using each method (20× Magnification). (B) Quantification of TOPRO-3 signal using Image J and analyzed via one-tailed t-test demonstrates the effectiveness and reproducibility of CP de-cellularization on removing nucleic acids (*P ≤ 0.05 compared to normal lung, **P ≤ 0.005 compared to CF lung). Means + St Dev. from three representative lungs for each method are shown.
3.3. Immunofluorescence of ECM proteins in de-cellularized matrices
Laminin is present in the matrix after de-cellularization using each of the three protocols. The staining qualitatively appears to be greatest in the MP protocol followed by the CF and CP protocols (Fig. 4A–D). The MP protocol retains fibronectin; however, there is significant loss of fibronectin in the CF and CP protocols (Fig. 4E–H). All three protocols resulted in some degree of collagen I and IV loss compared to normal lung (Fig. 4I–P). There was also a significant loss of elastin in all de-cellularization methods (Fig. 4Q–T).
Fig. 4.
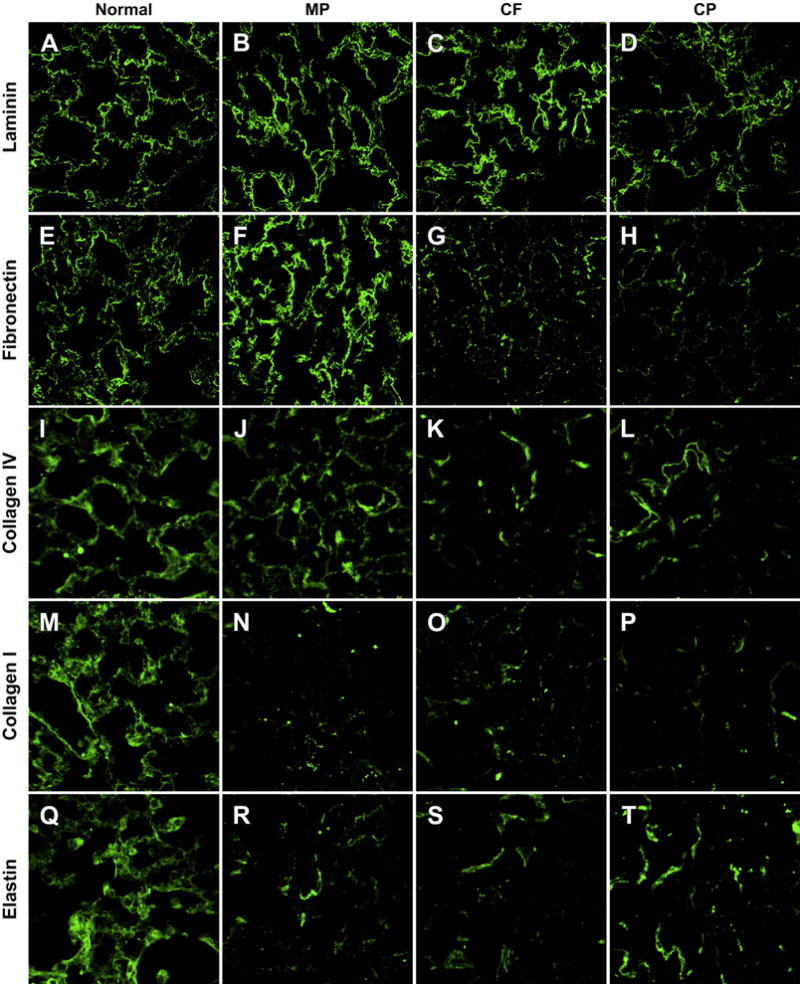
Qualitative Analysis of ECM Proteins by Immunofluorescence Representative images from triplicate lungs de-cellularized using each method and immunostained for laminin (A–D), fibronectin (E–H), collagen IV (I–L), collagen I (M–P), elastin (Q–T) qualitatively demonstrate loss of ECM proteins from the de-cellularization process. The MP protocol appears to retain the most laminin, fibronectin and Collagen IV compared to the CF and CP protocols. The CP method appears to retain the most elastin. (All Images 63× Oil Magnification).
3.4. Protein analysis of ECM by western blotting
The ECM protein composition of acellular scaffolds produced by each de-cellularization protocol was compared qualitatively (Fig. 5A) and quantitatively (Fig. 5B) by western blot analysis. Beta actin, an intracellular cytoskeletal protein, was used to gauge removal of cellular elements. All three protocols remove beta actin completely. Histone H1, an intra-nuclear DNA binding protein, was removed in both the MP and CP protocols, but was retained in the CF protocol. Collagen I and IV levels are decreased in all protocols when compared to normal. Laminin proteins are still present following all three protocols; however, the CP protocol retains the least amount of laminin, while the MP protocol retains the most. Fibronectin is present in all three protocols in equal or greater than normal levels. Lastly, elastin is similarly decreased in the three protocols.
Fig. 5.
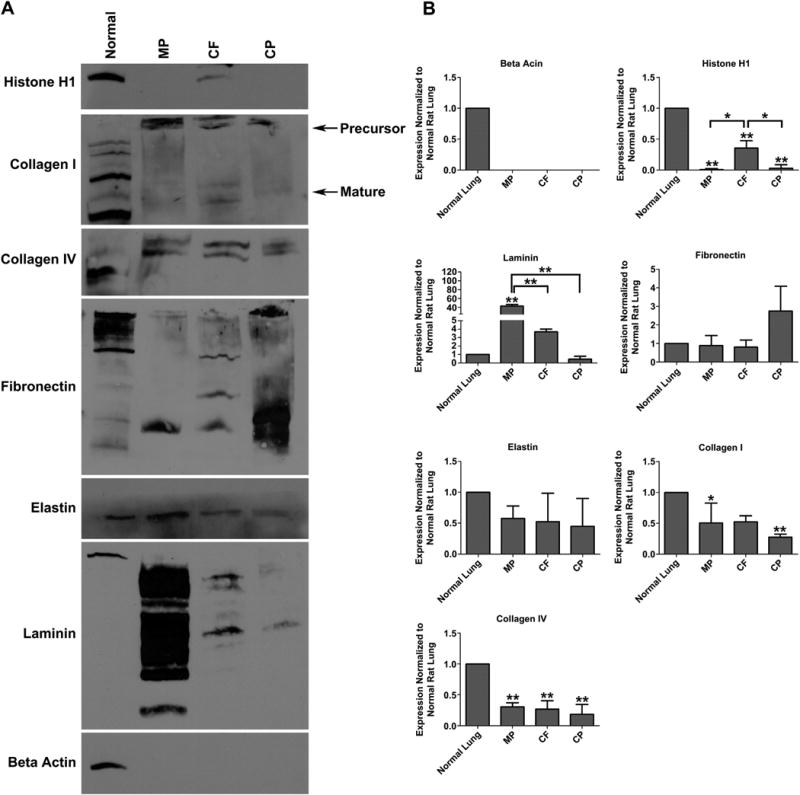
Quantitative Analysis of ECM Proteins by Western Blot (A) Western blot analysis of 50 μg whole cell lysate from MP, CF or CP de-cellularized lung scaffolds relative to normal rat lung for nuclear (histone H1), intracellular (beta actin) and extracellular (collagen I, collagen IV, fibronectin, elastin, and laminin) proteins. (B) Densitometry was performed using Image J and analyzed via one-way ANOVA (* = P ≤ 0.05 ** = P ≤ 0.005 compared to normal lung). There is significant loss of nuclear proteins (Histone H1) in the MP and CP protocols. Laminin is significantly retained in the MP protocol, when compared to the CF and CP protocol. Fibronectin appears to be greatest in the CP protocol; however, it is not statistically significant. There is loss of elastin, collagen I and collagen IV in all three protocols. De-cellularized scaffolds reproduced and analyzed in triplicate.
3.5. Characterization of C10-GFP cells at baseline
GFP-expressing C10 cells readily grew in culture and maintained robust GFP expression over multiple passages (Fig. 6A). C10-GFP cells grown in routine culture in maintenance medium expressed pro-surfactant protein C (Pro-SPC), as well as, thyroid transcription factor-1 (TTF-1). There was minimal expression of type I markers including AQP5 and T1α (Fig. 6B).
Fig. 6.
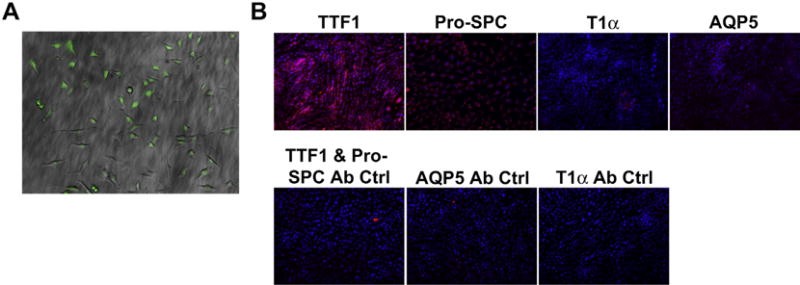
Characterization of C10 Cells After GFP Transfection (A) C10 alveolar epithelial cells express GFP following viral transduction and FACS sorting. C10 cells following flow-based sorting were over 95% GFP positive (10× Magnification). (B) C10-GFP cells cultured on untreated plastic express type II alveolar epithelial markers TTF-1 and Pro-SPC, but do not express type I alveolar epithelial markers such as AQP5 or T1α (20× Magnification).
3.6. C10-GFP In vitro ECM coating assay
To initially assess growth and differentiation on representative lung ECM proteins, scaffolds generated by the MP, CF and CP protocols were homogenized separately and the respective slurries were utilized to coat tissue culture plastic. After three days cultured on ECM protein-coated plastic, C10-GFP cells changed morphology from cobblestone-like to more elongated and fibroblast-like (Fig. 7A). In parallel the cells lost pro-SPC expression and increased expression of AQP5 and T1α. TTF-1 expression was also increased in varying degrees dependent on the de-cellularization protocol (Fig. 7B). This change in morphology and phenotypic expression is not observed in cells cultured long term on uncoated plastic (Fig. 6B).
Fig. 7.
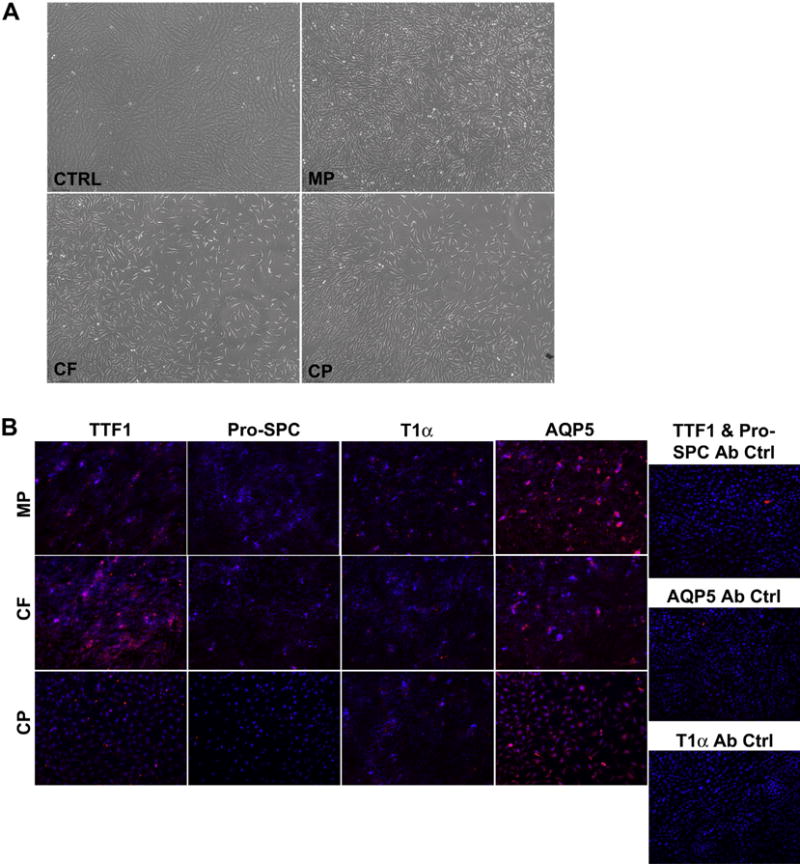
Comparison of C10 Cells Cultured on Homogenized Scaffolds (A) Culture wells coated with homogenized scaffolds and seeded with C10-GFP cells displayed a change in morphology after 3 days compared to those cultured on uncoated plastic (control) after three days (10× Magnification). (B) C10-GFP cells cultured for 3 days in wells coated with homogenized scaffolds and seeded with C10-GFP cells demonstrated loss of pro-SPC expression but increased that of T1α and AQP5. Variable TTF-1 expression was observed. Secondary antibody only controls were used to assess non-specific binding (20× magnification).
3.7. Cell growth in de-cellularized lung matrices
To asses cell growth in the three dimensional de-cellularized matrices, 6.0 × 107 C10-GFP cells were inoculated via the intratracheal route (Fig. 8A) and the seeded matrices were cultured in the Harvard Apparatus bioreactor system for three days. The matrices were perfused with C10-GFP maintenance medium through the vasculature (Fig. 8B,C) at several flow rates in order to investigate the optimal pressure to deliver nutrients. Perfusion at 10 cm H2O in the CP scaffold resulted in mostly apoptotic cells. In contrast, perfusion at 20 cm H2O in the CP scaffold resulted in dense clusters of viable cells. Perfusion at 30 cm H2O in the CP scaffold resulted in some viable cells, however, the number present was significantly lower than 20 cm H2O (Fig. 9A).
Fig. 8.
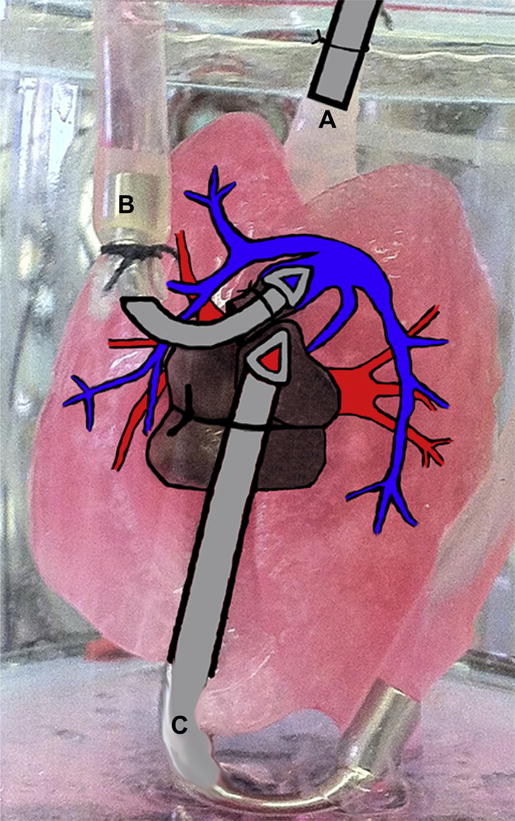
Schematic of Re-cellularization of De-cellularized Scaffolds in Bioreactor System A physiologic heart lung bioreactor system from Harvard Apparatus was utilized to re-cellularize and culture C10-GFP cells on MP CF, and CP de-cellularized lung scaffolds. Following de-cellularization, cells were inoculated via the trachea (A) and allowed to adhere for 4 h. After that time, maintenance media was perfused through the pulmonary artery (B) and exited via the pulmonary vein (C) for 3 days.
Fig. 9.
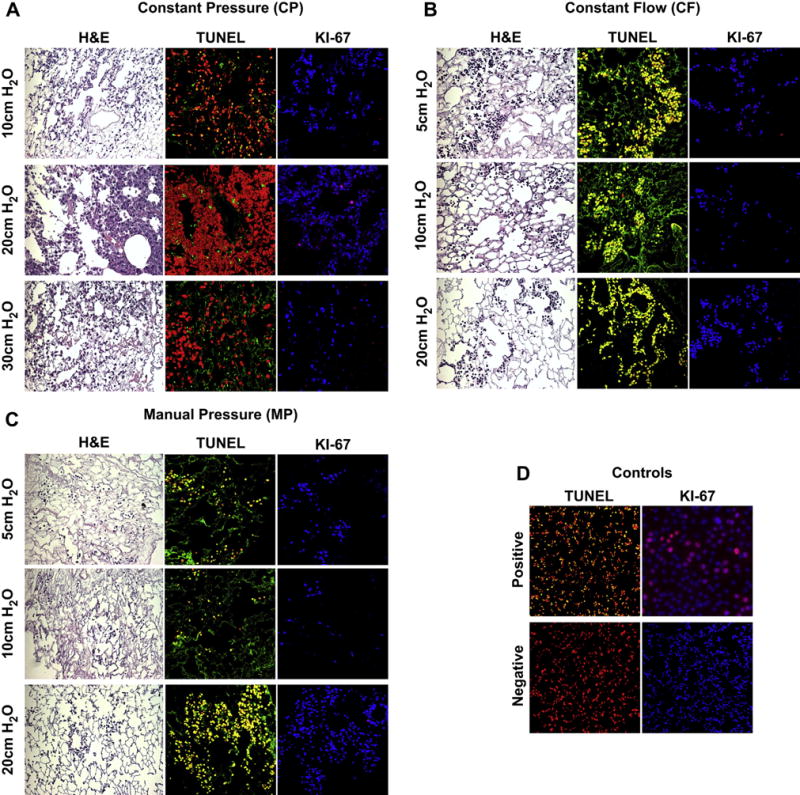
Histology, Apoptosis and Cell Proliferation of Re-cellularized Scaffolds After 3 Days In Culture Delivery pressure of maintenance medium was examined in range of 5–30 cm H2O (A) CP Seeded lungs appear to repopulate the scaffolds by H&E, but only yield proliferative cells when maintenance medium is delivered at a pressure of 20 cm H2O. (B) CF seeded lungs also appear to repopulate the scaffolds by H&E; however, all cells display an apoptotic, non-proliferative phenotype under all perfusion pressure conditions assessed. (C) MP seeded lungs were also apoptotic and non-proliferative under all perfusion pressure conditions assessed. (D) TUNEL negative control was normal lung and TUNEL Positive control was normal lung treated with DNAse (20× magnification). Ki67 negative control was no primary; positive control was C10-GFP cells dividing in culture (20× magnification). Ki67 staining (TOPRO3 = BLUE, Ki67 = RED), TUNEL Staining (PI (Nuclear Counterstain) = RED, TUNEL = Green, CELL DEATH = Yellow). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Assessment of proliferation via Ki-67 demonstrated that 20 cm H2O of perfusion pressure in the seeded CP scaffolds resulted in a few proliferating cells, whereas perfusion at either 10 cm H2O or 30 cm H2O did not display any Ki-67 staining (Fig. 9A). The MP and CF methods did not support the cells, i.e. cells were not viable or proliferative, in any of the perfusion pressures tested (Fig. 9 B,C).
3.8. Further characterization of C10-GFP seeded CP scaffold
After three days in the bioreactor, cells seeded onto the CP scaffold and perfused at 20 cm H2O no longer expressed TTF-1 or pro-SPC but did express high levels of T1α and AQP5 (Fig. 10A) in contrast to in vitro culture on uncoated plastic (Fig. 6B), but similar to in vitro culture on ECM-coated plastic (Fig. 7B). Furthermore, cells expressing type I markers on the scaffold were also GFP positive (Fig. 10B), demonstrating that they are C10 cells and not endogenous cells retained on the scaffold.
Fig. 10.
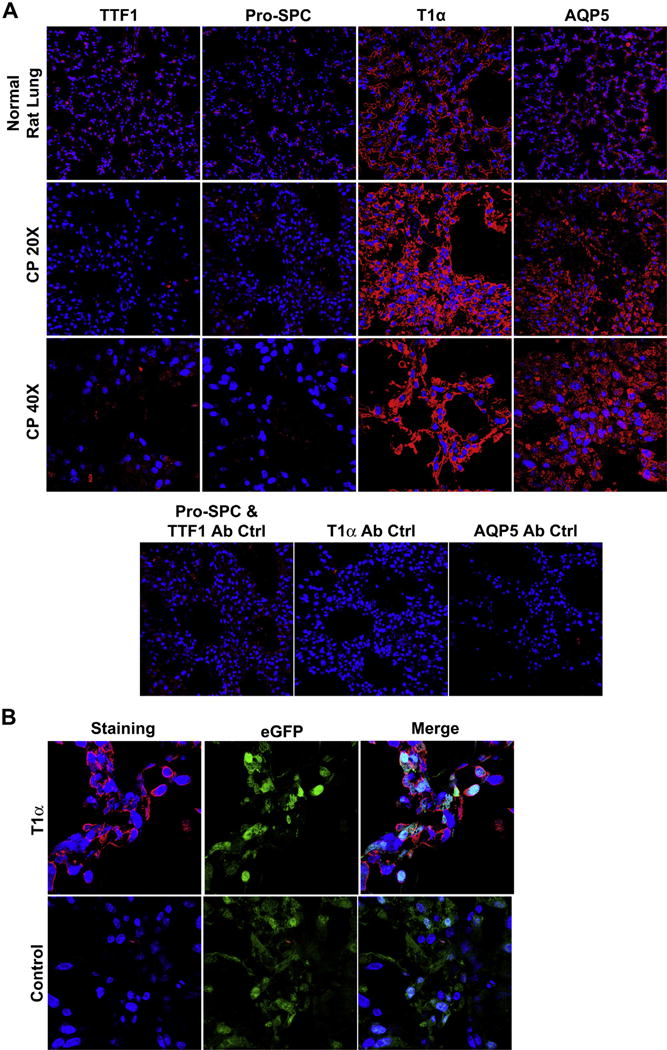
Immunofluorescence Staining of Re-cellularized CP Scaffolds (A) Characterization of CP re-seeded scaffolds indicates the expression of both AQP5 and T1α as well as the loss of pro-SPC expression (40× magnification). Low magnification images help demonstrate the widespread expression of this type I phenotype following re-seeding (20× magnification). No primary controls were used to assess non-specific binding of the secondary antibodies. (B) High magnification images demonstrate GFP positive cells on the scaffold that co-express T1α (Magnification 63× oil). Scaffolds reproduced and analyzed in triplicate.
4. Discussion
De-cellularized scaffolds provide three dimensional spatial cues and are able to maintain an organ's structural anatomy such as the configuration of arteries, veins, airways, and alveoli [9,13]. An ideal de-cellularized scaffold is defined by complete loss of cellular contents and residual cell debris and retention of organ ultra-structure with the preservation of important ECM proteins. Furthermore, the resultant ECM is responsible for influencing cell migration, organ development, and tissue repair [5,6,8,9,13,22]. In addition, for potential use as a transplantable organ, these scaffolds need to be reliably produced and able to support donor cells. For the lung, the optimal method for de-cellularization has not yet been delineated. In this paper, we compared the previously published hand injected (MP) protocol [16,18,23] with automated de-cellularization protocols using constant flow (CF) delivered by a peristaltic perfusion pump or constant pressure (CP) delivered by a Harvard apparatus dual chamber isolated heart lung bioreactor system.
Although manual de-cellularization methods have been widely and successfully utilized in other laboratories, our experience with this method is that it has given a more variable de-cellularized scaffold. An automated process theoretically eliminates variability between investigators and experiments. However, to date, no one has directly compared scaffolds generated by hand injection of de-cellularization detergents versus an automated de-cellularization process. We chose to compare two automated processes either controlling for the pressure or flow rate as these parameters are the most amenable to automation. We tested several flow rates and found that rates higher than 3.2 ml/min resulted in damage to the distal airways of the lung (data not shown), therefore we used 3.2 ml/min for all experiments. Other investigators have utilized constant pressure perfusion methods to de-cellularize a lung with pressures ranging from 20 mmHg (27.19 cm H2O) to 77.4 mmHg (105.22 cm H2O) [20,24]. We chose a pressure of 30 cm H2O because this is physiologic pressure found in the pulmonary artery. For simplicity, we utilized a single de-cellularizing agent, SDS, as data has demonstrated increased ECM retention and less residual cellular proteins when compared to Triton X-100/SDC and CHAPS [18]. In addition, SDS resulted in the most effective removal of cytosolic proteins with less matrix protease activation [18]. This combination appears advantageous as organotypic cues from the lung ECM are better retained, but the more immunogenic cellular elements are removed.
Removal of all cellular elements is vital in creating a scaffold that can potentially be immunologically inert and available for transplant. In comparing the three different protocols, the MP protocol removes histone H1 and β-actin, but there are still remnants of nucleic material left behind. In the automated processes, the CF de-cellularization protocol demonstrates that although the nuclei were grossly removed, there was still evidence of nuclear material left behind. In contrast, the CP protocol completely eliminated β-actin, histone H1, and nucleic acids. We hypothesized that the maintenance of physiologic pressures, as opposed to flow rates, permits uniform distal vessel perfusion, allowing for a more even removal of cellular contents. Although, we did not weigh the lungs, we noted grossly a difference in size between sets of donor lungs. Therefore, the advantage of pressure mediated perfusion is its ability to account for donor lung size discrepancies while ensuring a homogenous de-cellularization throughout the distal portions of the lung.
In addition to differing flow rates and pressure, there are several other differences between each protocol, including the total volume of perfused detergent and the method of delivery. The CF protocol, at 3.2 ml/min, utilized 384 ml of 0.1% SDS solution over the course of 2 h. The CP protocol utilized on average between 2 and 3 L of 0.1% SDS solution over 2 h. The large volume of detergent being perfused through the major blood vessels of the lung, around which the nuclear material is concentrated, contributes to the loss of nuclear material in the automated CP de-cellularization method compared to the MP and CF protocols. In order to achieve the same volume using the CF protocol, it would take approximately 15 h. Since we wanted to generate a faster de-cellularization protocol, we chose to maintain 2 h for each protocol. The flow rates varied during the CP de-cellularization, starting very low and increasing to almost 30 ml/min (data not shown), in order to maintain a vascular pressure of 30 cm H2O. The increase in flow rate is most likely due to the decreased resistance after the disruption of the endothelial network and the increased compliance, which has been previously shown [23]. Although the MP and CF protocols utilize tracheal delivery in addition to vascular delivery of detergent, this was not effective in completely removing nuclear material compared to the CP protocol, which only utilized vascular delivery of detergent. In addition, utilizing only vascular delivery of detergent with the CF protocol resulted in a significant retention of nuclear material (data not shown). Recently it has been described that intermittent tracheal delivery of detergents was superior to that of intravascular or intravascular and tracheal delivery [25]. The bioreactor that we used for CP is designed only for vascular perfusion, therefore we could not compare tracheal versus vascular delivery with this system.
The amount of washing and method of delivery following de-cellularization is also critical in clearing nuclear and intracellular materials. The MP method instills a total rinse volume of 150 ml of PBS into both the trachea and vasculature, in contrast to the CF method which instills a rinse volume of 50 ml of PBS via the trachea and 3456 ml via the vasculature. The CP method delivers 30–40 L of rinse via the vasculature, which is the largest rinse volume across all three methods. We originally hypothesized that a large rinse volume would be essential to clearance of nuclear and cellular material; however, clearance appeared to be more effective in the MP and CP methods, which have vastly different rinse volumes compared to the CF method. We suggest that the pressure generated in both the MP and CP protocols is higher than the CF protocol and contributes to the difference in nuclear clearance. While removing all nuclear material is important, it must be balanced by the retention of ECM proteins. In parallel to what we observed with nuclear material, we also found that the CP protocol, with its large volume of perfused detergent, had the greatest loss of ECM proteins by immunofluorescence and western blot. We noted significant decrease in laminin, fibronectin, and collagen IV in the CP protocol when compared to the MP protocol. Of note, the discrepancy in amount of the fibronectin detected by immunofluorescence versus western blot with the CP protocol can be explained by the fact that protein homogenate in western blotting is normalized to normal lung. Therefore, the western is only comparing relative enrichments of individual proteins as a proportion of the total protein. In summary, the CF protocol is able to retain some critical ECM proteins, however, it fails to remove considerable amounts of nuclear material. The MP protocol is able to retain the most ECM proteins, however, it fails to remove all nuclear material. The CP protocol is able to remove all nuclear material while maintaining some critical ECM proteins, albeit at lesser concentrations than the MP protocol.
To evaluate whether the retained ECM proteins could support epithelial cell growth, we cultured C10-GFP on the remaining ECM proteins either in 2-dimensional cell culture or 3-dimensional culture in a bioreactor. We found that the cells were viable on the ECM from all three protocols in 2-dimensional culture, but were only viable on the CP scaffold in the bioreactor. This finding suggests that the ultrastructure of the de-cellularized scaffold is important to maintain cell viability. The cells were well dispersed on scaffolds from all three protocols following intratracheal inoculation. We found that the amount of continuous pressure of the media delivered through the vasculature to the re-epithelialized scaffold influenced cell survival, with 20 cm H2O being optimal. The fact that the CF and MP protocols were unable to sustain cell viability was a surprising finding, as other investigators have published on cell viability using de-cellularized scaffolds generated by the MP method with A549 cells, fetal pulmonary cells, murine embryonic stem cells, or mesenchymal stem cells [10,16,18–20,23,24,26,27]. Furthermore, other investigators have utilized the MP de-cellularization approach and have been able to support C10 cells for 1 month [18]. In our hands, however, we were unable to maintain C10 cells in the MP derived scaffold using the bioreactor system. This finding supports the idea that there is variability in the scaffolds generated with the MP method that can lead to differences in cell adhesion and viability.
Surprisingly, while the CP protocol retained the least amount of ECM components, it was the only protocol to support cell viability in 3-dimensional culture in our bioreactor system. This could be attributed to the loss of nuclear material, which was greater in the CP protocol compared to the MP and CF protocols. Also, Jensen et al. reported on a hand injected de-cellularization procedure and found that less time in the detergents retained ECM proteins in higher concentrations, however, there was significant activation of undesirable matrix proteases [23]. It is possible that the CF and MP protocols are also activating these matrix proteases therefore affecting cell viability. Further studies are needed to confirm this. It has also been described that C10 cells will synthesize and secrete fibronectin and laminin in response to the ECM proteins [28], therefore it is possible that the C10 cells are producing their own ECM in response to the small amount present on the CP scaffold. Future experiments will be to determine if there are newly synthesized ECM proteins after reseeding the scaffold.
Interestingly, C10-GFP cells, previously TTF-1+/SPC+/T1α -/AQP5- (a type II like AEC phenotype) spontaneously differentiate into TTF-1-/SPC-/T1α+/AQP5+ (a type I like AEC phenotype) when cultured on the remaining ECM proteins either in 2-dimensional cell culture or 3-dimensional culture in a bioreactor. While this phenomenon has been reported in vitro with isolated AECII cells on 2-dimensional cultures [29–31] and on various matrices [32,33], we are not aware of previous reports of C10 cells differentiating into phenotypic type I cells within a three dimensional scaffold. It has been recently described that TGF-β and laminin 5 will promote AEC transdifferentiation from a type II to type I phenotype [34]. The laminin present in our scaffolds following de-cellularization may play a role in the phenotypic change we observed.
Physiologically, the differentiation of type II to type I AEC is also known to occur after alveolar damage from various agents [33,35,36]. Type II AEC hyperplasia after injury and renewal of ECM through inflammation leads to differentiation to and restoration of the type I epithelium [37,38]. The ECM proteins within the de-cellularized matrix appear to not only provide the cues required for the C10-GFP cells to bind, but provide appropriate signals to promote acquisition of a type I AEC phenotype, both in vitro and in vivo.
5. Conclusion
In our comparison of de-cellularization protocols, we found that the automated CP derived acellular lung scaffold can reliably remove nuclear material while retaining key ECM proteins. This reproducible scaffold can be prepared and seeded within 26 h, allowing for quicker time to re-cellularization. Although more ECM is degraded in the CP process, the scaffold is able to support C10-GFP cells. Interestingly, the ECM proteins from all three types of scaffolds promote the differentiation of phenotypic type II C10-GFP cells into a type I like cell phenotype in vitro without the addition of growth factors. Furthermore, seeding the CP scaffold also promotes this same phenotypic conversion. In addition to helping to define more optimal methods of producing de-cellularized lungs, these results demonstrate the critical role of ECM proteins on AECII differentiation.
Acknowledgments
We would like to thank the lab of Darrell Kotton MD for the GFP vector. In addition we wish to thank Andrew Hoffman DVM DVSC, and Bruce Bunnell PhD for their critical review of our results. Lastly, we would like to thank the following institutions and organizations for funding this project: The National Institute of Health [1RO1HL104258-01 (CMF); RC4HL106625 (DJW & CFM)] and Connecticut Children's Medical Center (CMF).
References
- 1.American Lung A. American lung association: lung disease data: 2008; improving life, one breath at a time. New York, N.Y.: American Lung Association; 2008. [Google Scholar]
- 2.Organ procurement and transplantation network and scientific registry of transplant recipients 2010 data report. Am J Transplant. 2012;12(Suppl 1):1–156. doi: 10.1111/j.1600-6143.2011.03886.x. [DOI] [PubMed] [Google Scholar]
- 3.Badylak SF, Weiss DJ, Caplan A, Macchiarini P. Engineered whole organs and complex tissues. Lancet. 2012;379:943–52. doi: 10.1016/S0140-6736(12)60073-7. [DOI] [PubMed] [Google Scholar]
- 4.Vacanti JP. Tissue engineering and the road to whole organs. Br J Surg. 2012;99:451–3. doi: 10.1002/bjs.7819. [DOI] [PubMed] [Google Scholar]
- 5.Warburton D, El-Hashash A, Carraro G, Tiozzo C, Sala F, Rogers O, et al. Lung organogenesis. Curr Top Dev Biol. 2010;90:73–158. doi: 10.1016/S0070-2153(10)90003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu R, Boudreau A, Bissell MJ. Tissue architecture and function: dynamic reciprocity via extra- and intra-cellular matrices. Cancer Metastasis Rev. 2009;28:167–76. doi: 10.1007/s10555-008-9178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shannon JM, Emrie PA, Fisher JH, Kuroki Y, Jennings SD, Mason RJ. Effect of a reconstituted basement membrane on expression of surfactant apoproteins in cultured adult rat alveolar type II cells. Am J Respir Cell Mol Biol. 1990;2:183–92. doi: 10.1165/ajrcmb/2.2.183. [DOI] [PubMed] [Google Scholar]
- 8.Schuger L, Yurchenco P, Relan NK, Yang Y. Laminin fragment E4 inhibition studies: basement membrane assembly and embryonic lung epithelial cell polarization requires laminin polymerization. Int J Dev Biol. 1998;42:217–20. [PubMed] [Google Scholar]
- 9.Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Ann Rev Biomed Eng. 2011;13:27–53. doi: 10.1146/annurev-bioeng-071910-124743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortiella J, Niles J, Cantu A, Brettler A, Pham A, Vargas G, et al. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng A. 2010;16:2565–80. doi: 10.1089/ten.tea.2009.0730. [DOI] [PubMed] [Google Scholar]
- 11.Keane TJ, Londono R, Turner NJ, Badylak SF. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials. 2012;33:1771–81. doi: 10.1016/j.biomaterials.2011.10.054. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–7. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–83. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Hirsiger S, Simmen HP, Werner CM, Wanner GA, Rittirsch D. Danger signals activating the immune response after trauma. Mediators Inflamm. 2012;2012:315941. doi: 10.1155/2012/315941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor KR, Gallo RL. Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. FASEB J. 2006;20:9–22. doi: 10.1096/fj.05-4682rev. [DOI] [PubMed] [Google Scholar]
- 16.Price AP, England KA, Matson AM, Blazar BR, Panoskaltsis-Mortari A. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue Eng A. 2010;16:2581–91. doi: 10.1089/ten.tea.2009.0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lwebuga-Mukasa JS, Ingbar DH, Madri JA. Repopulation of a human alveolar matrix by adult rat type II pneumocytes in vitro. A novel system for type II pneumocyte culture. Exp Cell Res. 1986;162:423–35. doi: 10.1016/0014-4827(86)90347-2. [DOI] [PubMed] [Google Scholar]
- 18.Wallis JM, Borg ZD, Daly AB, Deng B, Ballif BA, Allen GB, et al. Comparative assessment of detergent-based protocols for mouse lung de-cellularization and re-cellularization. Tissue Eng C Methods. 2012;18:420–32. doi: 10.1089/ten.tec.2011.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daly AB, Wallis JM, Borg ZD, Bonvillain RW, Deng B, Baliff BA, et al. Initial binding and re-cellularization of de-cellularized mouse lung scaffolds with bone marrow-derived mesenchymal stromal cells. Tissue Eng A. 2011 doi: 10.1089/ten.tea.2011.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–33. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 21.Sommer C, Stadtfeld M, Murphy G, Hochedlinger K, Kotton D, Mostoslavsky G. Induced pluripotent stem cell generation using a single lentiviral stem cell cassette. Stem Cells. 2009;27:543–9. doi: 10.1634/stemcells.2008-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bissell MJ, Aggeler J. Dynamic reciprocity: how do extracellular matrix and hormones direct gene expression? Prog Clin Biol Res. 1987;249:251–62. [PubMed] [Google Scholar]
- 23.Jensen T, Roszell B, Zang F, Girard E, Matson A, Thrall R, et al. A rapid lung de-cellularization protocol supports embryonic stem cell differentiation in vitro and following implantation. Tissue Eng C Methods. 2012;18:632–46. doi: 10.1089/ten.tec.2011.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–41. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maghsoudlou P, Georgiades F, Tyraskis A, Totonelli G, Loukogeorgakis SP, Orlando G, et al. Preservation of micro-architecture and angiogenic potential in a pulmonary acellular matrix obtained using intermittent intra-tracheal flow of detergent enzymatic treatment. Biomaterials. 2013;34:6638–48. doi: 10.1016/j.biomaterials.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Wong LB, Mao H. Induction of ciliated cells from avian embryonic stem cells using three-dimensional matrix. Tissue Eng C Methods. 2010;16:929–36. doi: 10.1089/ten.tec.2009.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song JJ, Kim SS, Liu Z, Madsen JC, Mathisen DJ, Vacanti JP, et al. Enhanced in vivo function of bioartificial lungs in rats. Ann Thorac Surg. 2011;92:998–1005. doi: 10.1016/j.athoracsur.2011.05.018. discussion-6. [DOI] [PubMed] [Google Scholar]
- 28.Malkinson AM, Dwyer-Nield LD, Rice PL, Dinsdale D. Mouse lung epithelial cell lines–tools for the study of differentiation and the neoplastic phenotype. Toxicology. 1997;123:53–100. doi: 10.1016/s0300-483x(97)00108-x. [DOI] [PubMed] [Google Scholar]
- 29.Bhaskaran M, Kolliputi N, Wang Y, Gou D, Chintagari NR, Liu L. Trans-differentiation of alveolar epithelial type II cells to type I cells involves autocrine signaling by transforming growth factor beta 1 through the smad pathway. J Biol Chem. 2007;282:3968–76. doi: 10.1074/jbc.M609060200. [DOI] [PubMed] [Google Scholar]
- 30.Paine R, 3rd, Simon RH. Expanding the frontiers of lung biology through the creative use of alveolar epithelial cells in culture. Am J Physiol. 1996;270:L484–6. doi: 10.1152/ajplung.1996.270.4.L484. [DOI] [PubMed] [Google Scholar]
- 31.Williams MC. Alveolar type I cells: molecular phenotype and development. Ann Rev Physiol. 2003;65:669–95. doi: 10.1146/annurev.physiol.65.092101.142446. [DOI] [PubMed] [Google Scholar]
- 32.Isakson BE, Lubman RL, Seedorf GJ, Boitano S. Modulation of pulmonary alveolar type II cell phenotype and communication by extracellular matrix and KGF. Am J Physiol Cell Physiol. 2001;281:C1291–9. doi: 10.1152/ajpcell.2001.281.4.C1291. [DOI] [PubMed] [Google Scholar]
- 33.Rannels DE, Rannels SR. Influence of the extracellular matrix on type 2 cell differentiation. Chest. 1989;96:165–73. doi: 10.1378/chest.96.1.165. [DOI] [PubMed] [Google Scholar]
- 34.Zhao L, Yee M, O'Reilly MA. Transdifferentiation of alveolar epithelial type II to type I cells is controlled by opposing TGF-beta and BMP signaling. Am J Physiol Lung Cell Mol Physiol. 2013 doi: 10.1152/ajplung.00032.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warburton D, Wuenschell C, Flores-Delgado G, Anderson K. Commitment and differentiation of lung cell lineages. Biochem Cell Biol. 1998;76:971–95. [PubMed] [Google Scholar]
- 36.Rannels SR, Fisher CS, Heuser LJ, Rannels DE. Culture of type II pneumocytes on a type II cell-derived fibronectin-rich matrix. Am J Physiol. 1987;253:C759–65. doi: 10.1152/ajpcell.1987.253.6.C759. [DOI] [PubMed] [Google Scholar]
- 37.Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol. 1975;22:142–50. doi: 10.1016/0014-4800(75)90059-3. [DOI] [PubMed] [Google Scholar]
- 38.Adamson IY, Bowden DH. Bleomycin-induced injury and metaplasia of alveolar type 2 cells. Relationship of cellular responses to drug presence in the lung. Am J Pathol. 1979;96:531–44. [PMC free article] [PubMed] [Google Scholar]


