Abstract
Robust antibody and CD4 T cell responses are required for the resolution of Salmonella infection in susceptible mice. Here we examined the role of B7-H1 (PD-L1) in resistance to primary Salmonella infection. Infected B7-H1-deficient mice had significantly higher bacterial burdens at day 21 and day 35 after infection compared to wild-type mice, demonstrating that B7-H1 plays an important role in immunity to Salmonella. B7-H1-deficient and wild-type mice both generated Salmonella-specific IgM and IgG2c antibody responses to infection and clonal expansion of endogenous and adoptively transferred Salmonella-specific CD4 T cells was similar in both groups. However, although Salmonella-specific IFN-γ producing Th1 CD4 T cells were generated in Salmonella-infected B7-H1-deficient mice, these cells did not expand to the level observed in wild-type mice. Furthermore, fewer multifunctional Th1 cells that simultaneously secreted IFN-γ, TNF-α, and IL-2 were detected in Salmonella-infected B7-H1-deficient mice. Together, these data demonstrate that B7-H1 is required for the generation of multifunctional Th1 responses and optimal protective immunity to primary Salmonella infection.
Keywords: PD-L1, T cells, Bacterial Infections, Memory, Rodent
Introduction
Salmonella enterica serovar typhi is responsible for human typhoid, a disease that is endemic in the developing world and responsible for over 200,000 deaths every year (1, 2). Although two typhoid vaccines are available (3, 4), neither of these has reduced the incidence of disease in developing nations, due to concerns about vaccine efficacy, safety, or financial cost (5). Thus, development of an effective typhoid vaccine that could lower the burden of typhoid in developing nations remains a global healthcare priority. In order for this objective to be achieved, greater knowledge of the adaptive immune response to Salmonella is required.
Immunity to Salmonella infection is often studied using in-bred strains of mice infected with Salmonella enterica serovar typhimurium (hereafter referred to as S. typhimurium) (6–8). Although S. typhimurium does not usually cause systemic disease in immune competent humans, this pathogen causes a fatal systemic infection, or non-fatal persistent infection in mice. Indeed, several important features of human typhoid are faithfully reproduced in murine S. typhimurium infection, making this the best available model to study systemic Salmonellosis (6). For example, susceptible mice can be infected orally (9), bacteria invade the host intestinal epithelium by targeting Peyer’s patch M cells (10), invasive bacteria replicate within infected macrophages (11), and the primary sites of systemic colonization are the spleen, liver and bone marrow.
Infection of susceptible mice with virulent Salmonella rapidly causes fatal infection, thus protective immunity is often studied following infection with auxotrophic S. typhimurium strains (12, 13). These attenuated Salmonella replicate within the macrophages of the spleen, liver and bone marrow but, in contrast to virulent Salmonella, are eventually cleared from systemic tissues (14). Importantly, the clearance of this primary infection requires the development of Salmonella-specific adaptive immunity (15, 16), and this model has therefore allowed precise dissection of the requirements for immune protection. For example, it has been demonstrated that clearance of primary attenuated Salmonella requires IFN-γ-producing CD4 Th1 cells, and thus mice with deficiencies in CD4 (17), MHC class-II (17), CD28 (18), IFN-γ (19), IFN-γR (17), or the Th1 transcription factor T-bet (20), succumb to fatal infection. Salmonella-specific B cells and CD8 T cells also play an additional protective role, although this is more commonly observed in resistance to secondary infection (21). Therefore, this particular infection model is ideally suited to understanding the development and role of Th1 cells in protective immunity to a natural human pathogen.
Given their important role in bacterial clearance, it is not surprising that Salmonella are capable of inhibiting the development and/or function of CD4 Th1 cells in vivo (22). Indeed, our laboratory recently reported that activated Salmonella-specific CD4 T cells are progressively depleted following Salmonella infection of mice (23). During the completion of these in vivo experiments we noticed that activated Salmonella-specific CD4 T cells expressed higher levels of the inhibitory ligand B7-H1, also known as Programmed cell Death Ligand 1 (PD-L1), suggesting that this molecule may be important for CD4 Th1 cell inhibition by Salmonella. Indeed, several reports using viral infection models have demonstrated that T cell function can be profoundly inhibited by signals delivered via B7-H1 or ligands for this molecule (24, 25).
B7-H1 is constitutively expressed by several cell populations including T cells, dendritic cells, and macrophages, and this expression is increased upon activation (24, 26). PD-1 is the canonical receptor for B7-H1, but B7-H1 also displays affinity for CD80 (27). The role of PD-1 and B7-H1 has been extensively examined in models of autoimmunity and viral infection where signaling through these receptors is thought to deliver a negative signal to T cells and restrain adaptive immune responses (24, 26). Inhibition of PD1-B7-H1 ligation is therefore a promising therapeutic strategy for increasing T cell functionality during chronic viral infection or vaccination (28).
Based on this known inhibitory role for B7-H1, our prediction was that B7-H1-deficient mice would develop an enhanced Th1 response to Salmonella and display evidence of greater protective immunity to infection. However, in contrast, we present data showing that B7-H1 is required for optimal development of multifunctional Th1 cells and protective immunity in the mouse model of Salmonella infection.
Materials and Methods
Mouse and bacterial strains
C57BL/6 mice were purchased from the National Cancer Institute (Frederick, MD) and the Jackson Laboratory (Bar Harbor, ME) and used at 6–12 weeks of age. CD90.1 congenic, RAG-deficient SM1 TCR transgenic mice were originally generated on a C57BL/6 background and express a monoclonal TCR specific for Salmonella flagellin (29, 30). SM1 transgenic, and RAG-, or B7-H1-deficient mice (31), were all maintained on a C57BL/6 background by intercrossing at the University of Minnesota. The initial breeding stock for our B7-H1-deficient colony was kindly provided by Dr. L. Chen (Johns Hopkins University, Baltimore). All mice were cared for in accordance with University of Minnesota Research Animal Resource guidelines. Salmonella BRD509 strain (32) was kindly provided by Dr. D. Xu, University of Glasgow, U.K.
Salmonella infection and bacterial counts
BRD509 (AroA−D−) and SL1344 were grown overnight in LB broth without shaking and diluted in PBS after estimation of bacterial concentration using a spectrophotometer. Mice were infected intravenously in the lateral tail vein with 5×105 BRD509, and monitored daily for signs of infection. Salmonella-infected mice were determined to be moribund if they were unresponsive to gentle prodding. Mice that resolved primary infection with BRD509 were administered 0.1ml 5% sodium bicarbonate to neutralize stomach pH before oral challenge with 5×107 virulent Salmonella (SL1344) and daily monitoring to determine protection. In all experiments the actual bacterial dose administered was confirmed by plating serial dilutions of the original culture onto MacConkey agar plates. To determine bacterial colonization in vivo, spleens and livers from infected mice were homogenized in PBS and serial dilutions were plated onto MacConkey agar plates. After overnight incubation at 37°C, bacterial plates were counted and bacterial burdens calculated for each individual organ.
Salmonella-specific antibody responses
Blood was collected retro-orbitally from mice infected with BRD509 and sera were prepared by centrifugation. High protein binding plates (Costar Inc., Corning, NY) were coated overnight with heat-killed S. typhimurium (HKST) diluted in 0.1M NaHCO3. After incubation in 10% FBS/PBS for one hour at 37°C, these plates were washed twice in PBS/0.5% Tween 20 and serum samples were added in serial dilutions in 10% FBS/PBS. Following incubation for two hours at 37°C, plates were washed four times before the addition of biotin-conjugated antibody specific for the desired antibody isotype (BD Bioscience and eBioscience). After a further incubation for one hour at 37°C, plates were washed six times and incubated for one hour at 37°C with HRP-conjugated streptavidin (Sigma-Aldrich) diluted in 10% FCS/PBS. Plates were then washed eight times and an HRP substrate (OPD, O-Phenylenediamine dihydrochloride, Sigma-Aldrich) was used to develop the plates. After sufficient color-change was observed, the reaction was stopped by adding 50μl of 2N H2SO4 and plates were analyzed using a spectrophotometer (SpectraMax M2, Molecular Devices).
TCR transgenic adoptive transfers
Spleen and lymph node cells (inguinal, axillary, brachial, cervical, mesenteric, and peri-aortic) were harvested from SM1 mice and a single cell suspension was generated. An aliquot of this sample was stained using antibodies to CD4 and the relevant TCR Vβ in order to determine the percentage of TCR transgenic cells. SM1 T cells were labeled with 5μM CFSE (Molecular Probes, Eugene, OR) and incubated at 37°C for 10 minutes. After several washes in cold HBSS, volumes were adjusted based on the percentage of SM1 cells and 1×105–1×106 SM1 were injected intravenously into recipient mice. Three days or one week after infection, spleens were harvested and a single cell suspension generated in Eagle’s Ham’s Amino Acid (EHAA, Invitrogen) medium containing 2% fetal bovine serum. Samples were incubated on ice for 30 minutes in Fc block containing FITC-, PE-, PerCP-, or APC-conjugated antibodies specific for CD4, CD11a, CD90.1, CD62L, CD69, or CD25 (eBioscience and BD Bioscience). After staining, cells were fixed using paraformaldehyde and examined by flow cytometry using a FACS Canto. All flow data were analyzed using FlowJo software (Tree Star, San Carlos, CA).
Examination of endogenous CD4 T cell responses using class-II tetramers
Endogenous Salmonella flagellin-specific CD4 T cell responses were monitored using a previously described methodology (33). Biotinylated I-Ab monomers with an attached peptide derived from Salmonella flagellin (34) (I-Ab-flag), were produced in S2 insect cells cultured using a Wave Bioreactor (GE Healthcare Biosciences, Pittsburg, PA). Purified monomers were tetramerized using fluorochrome-conjugated streptavidin and batch-tested for optimal binding to SM1 T cells, as previously described (35). Spleen and lymph nodes were harvested from infected or uninfected mice and stained with I-Ab-flag and anti-fluorochrome magnetic beads (Miltenyi Biotec, Auburn, CA) before tetramer-specific cells were enriched using magnetic columns. Column-bound and unbound fractions were stained with fluorochrome-conjugated antibodies specific for CD3, CD4, CD8, CD11b, CD11c, CD44, B220, and F4/80 (eBioscience). Cells were then examined using an LSR II (BD Bioscience) and endogenous tetramer-specific T cells identified using a previously described gating strategy (33). All data were analyzed using FlowJo software.
Analysis of cytokine production and transcription factor expression by CD4 T cells
Salmonella-infected or naïve mice were injected i.v. using a mixture of bacterial antigens to activate Salmonella-specific T cells (total of 108 HKST) (14). Four hours later, spleens were harvested in EHAA containing 2% fetal bovine serum and a single-cell suspension was generated. Following rapid surface staining on ice, cells were fixed with formaldehyde, permeabilized using saponin (Sigma-Aldrich), and stained intracellularly using IFN-γ, IL-4, IL-2, IL-10, IL-17A, and/or TNF-α-specific antibodies (eBioscience and BD Bioscience). In some experiments, cells were surface stained and fixed before intracellular staining using antibodies specific for T-bet, GATA-3, or RORgt (eBioscience and BD Bioscience). After intracellular staining, cells were examined by flow cytometry using a FACS Canto (BD Bioscience) and flow data was analyzed using FlowJo software (Tree Star, San Carlos, CA). The percentage of CD4 T cells producing IFN-γ alone or IL-2, IFN-γ, and TNF-α was determined by sequential gating using individual samples stained with antibodies specific for all three cytokines.
Statistical analysis
Statistical differences between groups of normally distributed data were examined using InStat (GraphPad Software, La Jolla, CA). For bacterial burdens, Log10 CFUs are normally distributed and were also compared using InStat. Data in each group were compared using an unpaired t test and were considered significantly different with a p value of <0.05.
Results
B7-H1 is required for optimal protective immunity to Salmonella infection
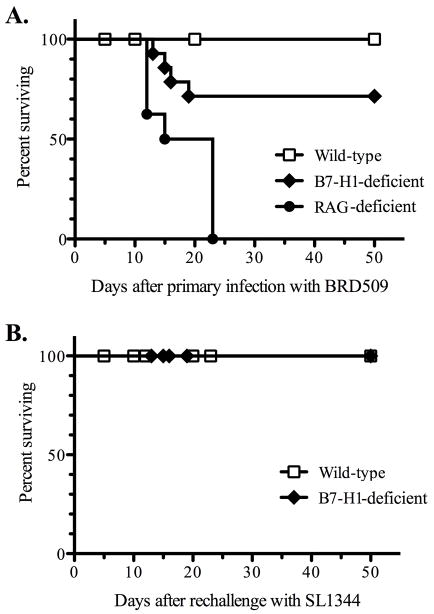
In order to examine the role of the inhibitory molecule B7-H1 in Salmonella infection, wild-type (Wt), RAG-deficient, or B7-H1-deficient mice were infected intravenously with attenuated Salmonella. Institutional animal guidelines do not allow the use of death as an endpoint, therefore Salmonella-infected mice were monitored twice daily and animals in a moribund state (unresponsive to gentle prodding) were sacrificed and considered to have succumbed to infection. As expected, RAG-deficient mice were unable to resolve primary infection with Salmonella while Wt mice survived (Fig. 1). In marked contrast, approximately 30% of B7-H1-defcient mice failed to resolve primary Salmonella infection, with these animals succumbing to infection around the second or third week after challenge (Fig. 1).
Figure 1. B7-H1-deficient mice have a deficiency in immunity to primary, but not secondary Salmonella infection.
(A) C57BL/6 wild-type, RAG-deficient, and B7-H1-deficient mice were infected intravenously with 5×105 attenuated Salmonella, BRD509. (B) C57BL/6 wild-type and B7-H1-deficient mice that had resolved primary infection with Salmonella were infected orally with 5×107 virulent Salmonella, SL1344, 42 days after primary exposure to BRD509. Infected mice were monitored daily for signs of disease and sacrificed following the development of a moribund state. Survival graphs show the percentage of surviving mice in each group and these data were pooled from several experiments and represent 8–12 mice per group.
These survival data suggested that B7-H1 played an unexpected but important protective role during the resolution phase of primary Salmonella infection. We examined this issue more carefully by monitoring bacterial loads in the spleen and liver of Wt and B7-H1-deficient mice at various time points after infection. At 1 week post-infection, no difference in bacterial loads was observed in the spleen or liver of B7-H1-deficient of wild-type mice (Fig. 2). Indeed, at this early time point, control of bacterial replication is mediated primarily by innate immune defense mechanisms (36), and no deficiency is observed in MHC class-II-, or T-bet-deficient mice (17, 20). In contrast, at both 3 and 5 weeks post infection, bacterial loads were significantly higher in the spleens of B7-H1-deficient mice compared to wild-type mice (Fig. 2). Furthermore, at 5 weeks post infection, bacterial colonization of the liver was also significantly higher in B7-H1-deficient mice (Fig. 2). It should be emphasized that these data only show the bacterial burdens in surviving B7-H1-deficient mice, since around 30% had already succumbed to infection prior to these later time points (Fig. 1). Together these data demonstrate an unexpected requirement for B7-H1 during the resolution phase of primary Salmonella infection.
Figure 2. B7-H1-deficient mice have higher bacterial loads at late stage of primary Salmonella infection.
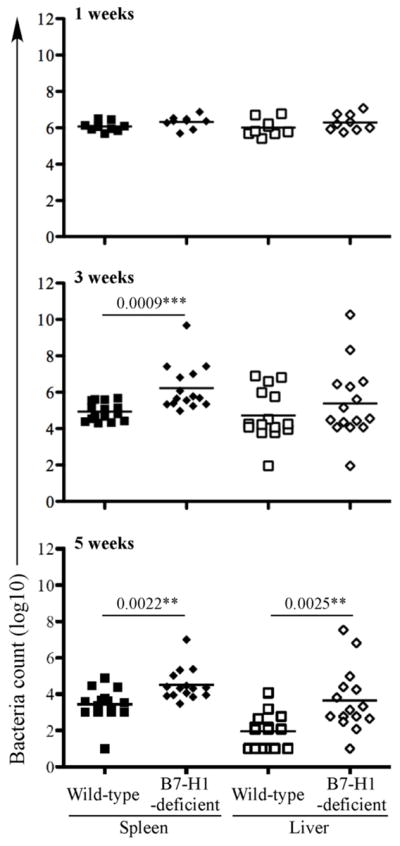
Wild-type and B7-H1-deficient mice were infected intravenously with 5×105 attenuated Salmonella, BRD509. Bacterial burdens were examined at 1, 3, and 5 weeks after infection. Spleens and livers from infected mice were homogenized in PBS and serial dilutions plated onto MacConkey agar plates to determine bacterial numbers at each time point. Data show mean bacterial burden in spleen (left) and liver (right) with individual mice shown as scatter plots. Data are pooled from at least three separate experiments. Numbers above each group indicate statistical significance and show the p value of a comparison between groups.
Despite this deficiency in resolving primary Salmonella infection, the majority of B7-H1-deficient mice eventually cleared attenuated bacteria from the spleen and liver. Wild-type mice that have resolved primary infection with attenuated Salmonella develop robust protective immunity to secondary infection. Although B cells and antibody responses are not required to resolve primary Salmonella infection, they play a major role in protective immunity to secondary challenge (18). Therefore, in order to examine whether immunity to secondary infection was altered by the absence of B7-H1, we reinfected B7-H1-deficient mice with virulent Salmonella strain SL1344. Both wild-type and B7-H1-deficient mice were able to fully resist secondary infection (Figure 1B), indicating that B7-H1 is not required for acquired resistance to Salmonella.
Development of Salmonella-specific antibody responses in B7-H1-deficient mice
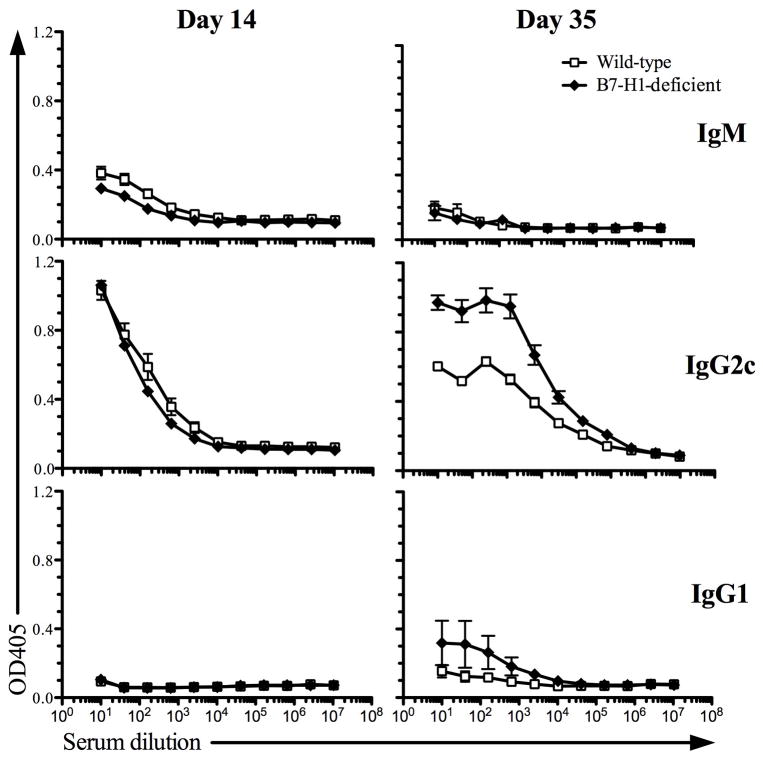
As noted above, despite the fact that Salmonella is an intracellular pathogen, bacterial-specific antibody responses play an important protective role in murine and human Salmonellosis (18, 37–39). We therefore examined Salmonella-specific IgM, IgG1, and IgG2c responses in Wt and B7-H1-deficient mice. Our laboratory has previously reported that these are the major antibody isotypes found in this infection model (18, 40). At both 2-weeks after infection, similar Salmonella-specific IgM and IgG2c responses were detected in both Wt and B7-H1-deficient mice (Fig. 3, day 14). However, at 5-weeks after infection B7-H1-deficient mice had a higher Salmonella-specific IgG2c response and similar or slightly higher IgG1 response (Fig. 3, day 35). Together, these data demonstrate that B7-H1 do not have a deficiency in generating Salmonella-specific antibody responses and in fact develop higher levels of Salmonella-specific IgG2c.
Figure 3. Salmonella-specific antibody responses in B7-H1-deficient mice.
Wild-type and B7-H1-deficient mice were infected intravenously with 5×105 attenuated Salmonella, BRD509. Sera were collected at day 14 and day 35 and examined for the presence of Salmonella specific IgM, IgG2c, and IgG1 by ELISA. Data show absorbance at OD405 of Salmonella-specific IgM (top), IgG2c (middle), and IgG1 (bottom) in Salmonella-infected mice at day 14 (left) and day 35 (right). Data represent the mean ± SEM of six mice per group and two independent experiments were completed with similar results.
Clonal expansion of Salmonella-specific CD4 T cells in B7-H1-deficient mice
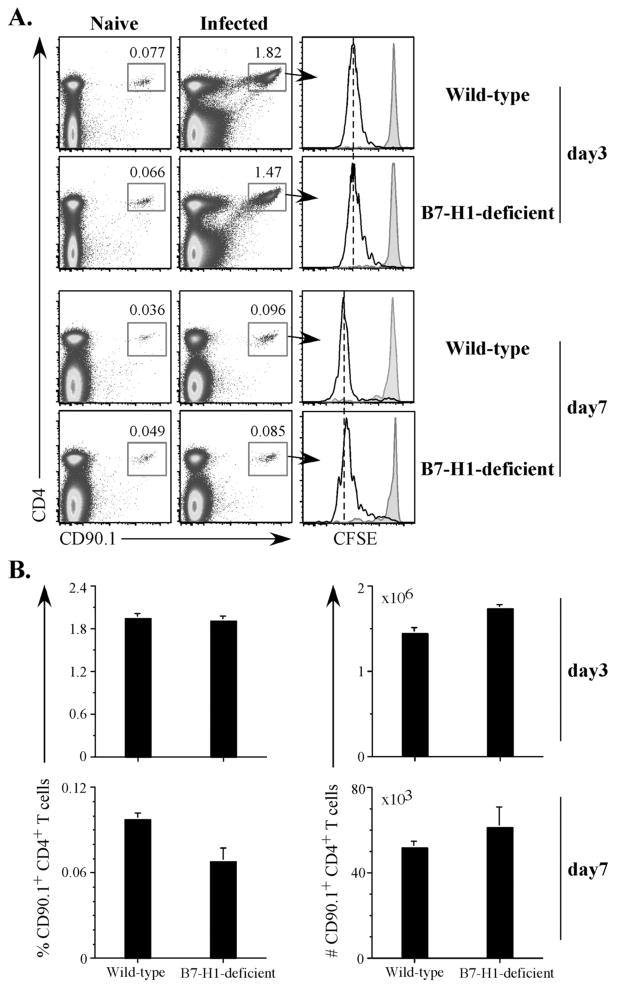
Despite a well-known inhibitory role for B7-H1 in autoimmune and viral infection models (24, 41), there are also reports suggesting that B7-H1 is required for optimal T cell priming and expansion to bacterial infection. In particular, antibody blocking of B7-H1 was shown to reduce T cell expansion to Listeria infection and delay bacterial clearance (42, 43). Therefore, we examined whether B7-H1 was required for optimal expansion of Salmonella-specific T cells after Salmonella infection. Wt and B7-H1-deficient mice were adoptively transferred with Salmonella flagellin-specific SM1 cells and infected the following day. SM1 cells expanded similarly and completed several rounds of CFSE-dye dilution, three days following infection of Wt mice or B7-H1-deficient mice (Fig. 4A). A slightly lower frequency of SM1 T cells was detected at day 7 after infection of B7-H1-deficient mice compared to Wt mice but the absolute number of SM1 T cells in the spleen of infected B7-H1-deficient mice was not statistically different to that detected in Wt mice (Fig. 4B). These data demonstrate that B7-H1 is not required for clonal expansion of Salmonella-specific CD4 T cells.
Figure 4. Clonal expansion of Salmonella-specific SM1 CD4 T cells in B7-H1-deficient mice.
Wild-type and B7-H1-deficient mice were adoptively transferred with 500,000 CFSE-labeled SM1 T cells and infected intravenously the following day with 5×105 attenuated Salmonella, BRD509. On day 3 and day 7, spleens were harvested from uninfected (naïve) or infected mice, and stained with antibodies specific for CD4 and CD901 to detect SM1 T cells. (A) Representative FACS plots showing the percentage of SM1 T cells on day 3 and day 7. Histograms show representative CFSE dilution after gating on SM1 T cells. Black lines show SM1 T cells from infected mice and gray shading shows naïve mice at each time point. (B). Bar graphs show the percentage (left) and absolute number (right) ± SEM of SM1 T cells from spleens of infected Wild-type and B7-H1-deficient mice on day 3 (top) and day 7 (bottom) after Salmonella infection. These data represent two independent experiments with 5 mice per group.
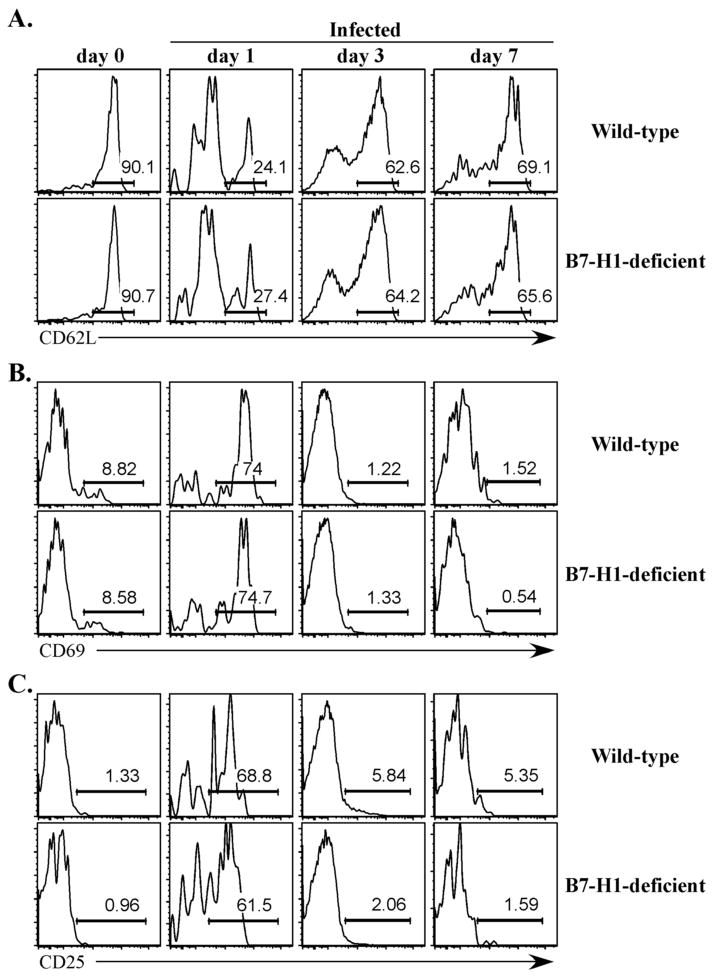
Next we examined SM1 T cell activation in more detail by monitoring the expression of activation markers on SM1 T cells in the first week following Salmonella infection. As expected, naïve SM1 T cells in both Wt and B7-H1-deficient mice expressed high levels of CD62L, but low surface levels of CD25 and CD69 (Fig. 5, day 0). One day after infection, the majority of SM1 T cells in Wt and B7-H1-deficient mice decreased expression of CD62L and increased expression of CD25 and CD69 (Fig. 5, day 1), indicating activation had occurred. At later time points, SM1 T cells in both groups of mice reduced expression of CD25 and CD69 and regained expression of CD62L (Fig. 5, day 3 and 7). Thus, SM1 T cells displayed similar kinetics of activation and expansion in Wt mice and mice lacking expression of B7-H1.
Figure 5. Activation of Salmonella-specific SM1 CD4 T cells in B7-H1-deficient mice.
Wild-type and B7-H1-deficient mice were adoptively transferred with 500,000 CFSE-labeled SM1 T cells and infected intravenously the following day with 5×105 attenuated Salmonella, BRD509. On days 0, 1, 3, and 7, spleens were harvested from infected mice, and stained with antibodies specific for CD4 and CD90.1 (to detect SM1 T cells) and CD62L, CD69, and CD25 to monitor activation. Representative histograms show the percentage of (A) CD62L, (B) CD69, and (C) CD25 after gating on SM1 T cells and are representative of 3 mice per group. Numbers indicate the percentage of cells positive for each surface marker.
In order to confirm our adoptive transfer data and also examine T cells that lacked expression of B7-H1, we used I-Ab-flagellin class-II tetramers and magnetic bead enrichment to examine the endogenous CD4 T cell response to Salmonella. This technique allowed direct tracking of the endogenous response without a requirement for TCR transgenic adoptive transfer. Six days after infection, an expanded population of flagellin-specific CD4 T cells was clearly detected in infected Wt and B7-H1-deficient mice (Fig. 6A). These Salmonella-specific CD4 T cells were uniformly CD44Hi in both groups of mice indicating that they had previously encountered antigen (Fig. 6A). It should be noted that endogenous flagellin-specific cells are still expanding at this time point, whereas the SM1 cells peak at day 3 and are contracting by day 7. This is because of differences in the initial precursor frequency of flagellin-specific T cells following adoptive transfer of SM1 T cells (23). However, as with our analysis of the SM1 response, no difference in peak clonal expansion of this population was detected between Wt versus B7-H1-deficient mice (Fig. 6). Together, our data demonstrate that both adoptively transferred and endogenous Salmonella-specific CD4 T cells are able to expand normally during Salmonella infection of B7-H1-deficient mice.
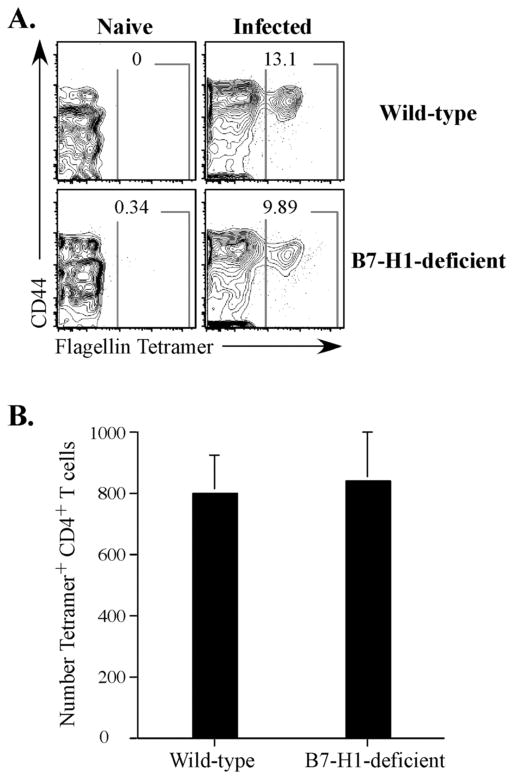
Figure 6. Endogenous Salmonella-specific T cells expand in Salmonella-infected B7-H1-deficient mice.
Wild-type and B7-H1-deficient mice were infected intravenously with 5×105 attenuated Salmonella, BRD509. On day 6, endogenous Salmonella flagellin-specific CD4 T cells were stained with I-Ab-flag tetramer, enriched using magnetic microbeads and stained and examined by flow cytometry. CD4 T cells from uninfected (Naïve, left) and infected (right) mice, were gated from CD11c−CD11b−F4/80−B220−CD3+ cells and further analyzed for CD44 and I-Ab-flag tetramer positive cells. (A) Contour plot show staining from a representative individual mouse and show the percentage of flagellin specific tetramer positive CD4 T cells. (B) Graph shows the mean number ± SEM of endogenous flagellin specific CD4 T cells from wild-type and B7-H1-deficient mice and three mice per group. Differences between these groups are not statistically significant.
Reduced number of Th1 cells in Salmonella-infected B7-H1-deficient mice
Development of Th1 cells with the capacity to produce IFN-γ is absolutely critical for the resolution of primary Salmonella infection (19, 20). We therefore examined whether the expanded Salmonella-specific CD4 T cells in B7-H1-deficient mice had acquired the ability to secrete IFN-γ. In order to examine polyclonal CD4 responses specific for multiple Salmonella target antigens we re-stimulated endogenous effector cells in vivo with Heat-killed Salmonella before direct ex vivo examination of cytokines production by CD4 T cells a few hours later (14, 44).
As expected, CD4 T cells from naïve mice did not produce IFN-γ, while only antigen-experienced CD11aHI CD4 T cells produced IFN-γ in infected Wt or B7-H1-deficient mice (Fig. 7A). In Wt infected mice, IFN-γ-producing CD4 T cells were clearly detected six days after infection, this response peaked at day 12, and then declined steadily thereafter (Fig. 7A and B). In contrast, although IFN-γ-producing CD4 T cells were detected after infection of B7-H1-deficient mice, a lower percentage of these cells was observed after day 6 (Fig. 7A and B). In addition, we examined Th1 lineage commitment in infected mice by monitoring CD4 cell expression of T-bet, the major transcription factor associated with Th1 development. The percentage of T-bet+ CD4 cells increased significantly in Wt infected mice (Fig. 7C and D), indicating effective development of Th1 cells in response to Salmonella infection. In contrast, the percentage of T-bet+ CD4 T cells was lower in B7-H1-deficient mice (Fig. 7C and D). The lower percentage of Th1 cells in B7-H1-deficient mice could not be explained by enhanced Th2 or Th17 development as both GATA-3 and RORgt staining was low in CD4 T cells from infected Wt and B7-H1-deficient mice (data not shown). Furthermore, the production of IL-4, IL-10, and IL-17A was found to be low among endogenous CD4 T cells from Wt or B7-H1-deficient mice (data not shown). Together, these data demonstrate that although CD4 clonal expansion appears to be unaltered by B7-H1 deficiency, the development or maintenance of a Th1 response to Salmonella is significantly reduced in the absence of B7-H1.
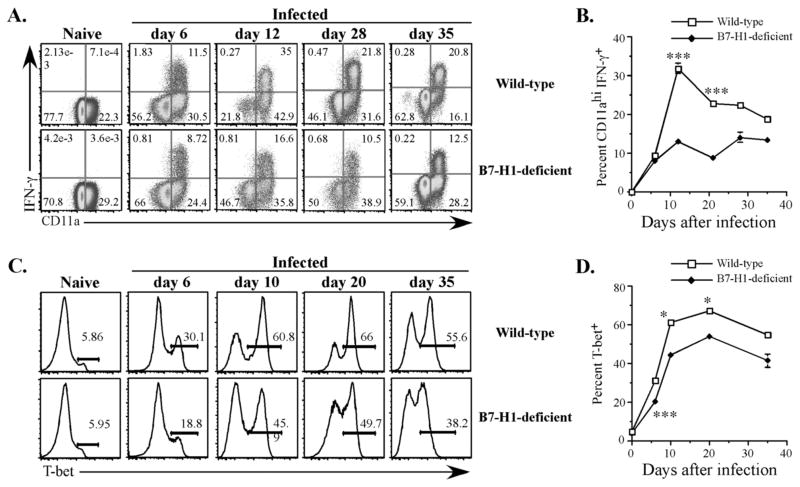
Figure 7. Impaired Th1 development in Salmonella-infected B7-H1-deficient mice.
Wild-type and B7-H1-deficient mice were infected IV with 5×105 attenuated Salmonella, BRD509. On day 6, 12, 21, 28, and 35, mice were injected with HKST for 4 hr and spleen cells analyzed for (A and B) intracellular IFN-γ production or (C and D) T-bet expression. (A) Representative FACS plots show IFN-γ and CD11a staining of CD4 T cells from naïve, day 6, day 12, day 28, and day 35, Salmonella-infected mice. (B) Pooled data showing the mean percentage ± SEM of CD11ahi IFN-γ-producing CD4 T cells in each group and represent at least three mice in each group. Mean IFN-γ production was significantly higher in Wt mice at days 12 and 21 by student t-test, *** p<0.005. (C) Representative histograms showing T-bet expression of CD4 T cells from naïve, day 6, day 10, day 20, and day 35, Salmonella-infected mice. (D) Pooled data showing the mean percentage ± SEM of T-bet expressing CD4 T cells from naïve or infected mice, and represent at least three mice in each group. Mean T-bet expression was significantly higher in Wt mice at days 6, 10, and 20 by student t-test, * p<0.05, *** p<0.005.
Reduced number of multifunctional Th1 cells in Salmonella-infected B7-H1-deficient mice
A recent report indicated that the development of multifunctional Th1 cells secreting IFN-γ, TNF-α, and IL-2, correlated with protective immunity against another intracellular infection pathogen, Leishmania major (45). Multifunctional Th1 cells have not previously been examined in Salmonella infection. Therefore, we examined the development of multifunctional Th1 cells in Salmonella-infected Wt and B7-H1-deficient mice. After Salmonella infection of Wt mice, up to 25% of IFN-γ-producing CD4 T cells simultaneously produced TNF-α (Fig. 8A and B, day 12), although the proportion of these cells varied considerably at different time points. However, only a small proportion of IFN-γ-producing CD4 T cells produced both IL-2 and TNF-α at any time point (Fig. 8C and D). These multifunctional Th1 cells were first detected at low frequency 6 days after infection but became a larger proportion of the memory response around the time that bacterial clearance takes occurs, day 35 (Fig. 8C and D). Among the IFN-γ-producing CD4 T cell population in B7-H1-deficient mice, TNF-α-producers were detected at day 6, but the proportion of these cells did not increase as observed in Wt mice (Fig. 8A and B). Furthermore, a lower frequency of IL-2+/TNF-α+ multifunctional Th1 cells was detected in B7-H1-deficient mice at every time point after day 6 (Fig. 8C and D). Together these data indicate that B7-H1 is required for the development of multifunctional Salmonella-specific Th1 cells.
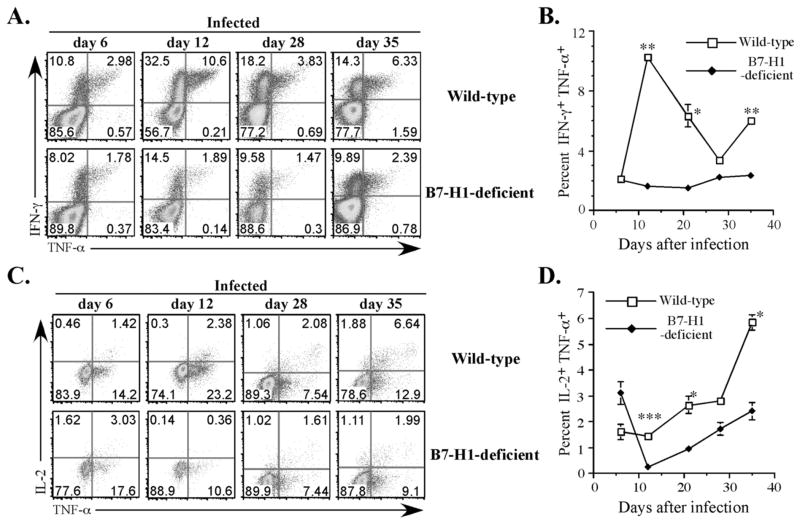
Figure 8. Reduced maturation of multifunctional Th1 cells in Salmonella-infected B7-H1-deficient mice.
Wild-type and B7-H1-deficient mice were infected IV with 5×105 attenuated Salmonella, BRD509. On day 6, 12, 28, and 35, mice were injected with HKST for 4 hr and spleen cells analyzed for IFN-γ, TNF-α, and IL-2 production in a single multi-color analysis. (A) Representative FACS plots show IFN-γ/TNF-α double cytokine producers among CD4 T cells from day 6-, day 12-, day 28-, and day 35-infected mice. (B) Pooled data showing the mean percentage ± SEM of IFN-γ/TNF-α double cytokine producing CD4 T cells, and represent at least three mice per group. The mean percentage of IFN-γ/TNF-α double cytokine producing CD4 T cells was significantly higher in Wt mice at days 12, 21, and 35 by student t-test, *p<0.05, ** p<0.01. (C) Representative FACS plots show IL-2/TNF-α producing multifunctional Th1 cells after gating on IFN-γ-producing CD4 T cells. (D) Pooled data showing the mean percentage of multifunctional Th1 cells ± SEM infected mice and represent at least three mice per group. Mean percentage of multifunctional Th1 cells was significantly higher in Wt mice at days 12, 21, and 35 by student t-test, *p<0.05, ***p<0.005.
Discussion
B7-H1 can bind PD-1 and CD80 and these interactions are thought to deliver a negative signal to T cells and thus inhibit immune responses (24, 26). Strategies to block PD1-B7-H1 interactions are currently being explored as therapeutic strategies for increasing T cell functionality during chronic viral infection or after vaccination (28). Many fewer studies have examined the role of B7-H1 in bacterial infection, but the available data are markedly different from viral infection studies and actually suggest a protective role for B7-H1, at least in Listeria infection. For example, antibody blockade of B7-H1 inhibited T cell expansion and delayed the clearance of bacteria from infected tissues (42, 43). Similarly, our data using the Salmonella infection model suggest a protective role for B7-H1 against a gram-negative bacterial infection and therefore point to an unexpected role for this inhibitory receptor in the development of protective immunity to pathogenic bacteria. It is not yet clear whether this protective role requires the interaction of B7-H1 with PD-1 or with CD80, or whether this requirement is typical of all bacterial infections. However, these findings would suggest that a cautionary approach to B7-H1 blockade in the clinic would be appropriate.
Clearance of primary infection in the Salmonella mouse model requires the development of CD4 Th1 cells, although CD8 T cells are also required for an optimal response during secondary infection (21). B cell play a prominent role in clearance of Salmonella (18, 38), and recent studies suggest that thymus-independent antibody responses can confer significant protection (46). Our data demonstrate a modest increase in primary antibody responses and normal clonal expansion of Salmonella-specific CD4 T cells in the absence of B7-H1. Together, these data would indicate suggest that B7-H1 is not required during initial T cell-APC or T cell-B cell interactions that drive the early adaptive response to Salmonella infection. This conclusion differs markedly from the protective role noted for B7-H1 during Listeria infection where a major effect on clonal expansion was detected (42, 43). It is not clear why these bacterial models should display a divergent role for B7-H1 in clonal expansion but this could be related to the different requirement of CD4 T cells in protective immunity to these pathogens. Indeed, Salmonella infection induces much greater clonal expansion of endogenous CD4 T cells than has been reported in viral or bacterial infection models that depend more heavily on CD8 T cells for clearance (14, 47). Thus, robust clonal expansion in the Salmonella model may simply be less dependent on B7-H1 signals.
Instead, our data point to a major role for B7-H1 in the maturation or survival of effector CD4 Th1 cells after initial clonal expansion has already occurred. The fact that Salmonella-infected B7-H1-deficient mice succumb to infection around 2–3 weeks concurs with a known requirement for effector T cell responses at this time point. The uncoupling of clonal expansion and effector T cell maturation in this model suggests that Th1 cells take several weeks to develop full effector potential. Indeed this conclusion is supported by recent data demonstrating that Th1 cell development required antigen persistence and was impeded by early antibiotic clearance of Salmonella (40). Sustained antigen presentation has also been noted as a requirement for CD4 T cell maturation in other model systems (48, 49). Thus, we propose that B7-H1 most likely plays a major role in maturing Th1 cell effector potential during this period of sustained antigen presentation, but is not required for early CD4 T cell activation and expansion. Given the relatively poor development of multifunctional CD4 T cells in B7-H1-deficient mice, it seems likely that these cells represent a more mature differentiation stage that requires interaction with additional costimulatory molecules, including B7-H1 and perhaps others. Future experiments will examine the role of PD1 and PD-L2 in the development of these multifunctional CD4 T cells.
Our analysis of endogenous flagellin-specific CD4 T cells using class-II tetramers suggests that these cells represent a small fraction of the overall memory response to Salmonella. This stands in contrast to previous studies where flagellin was described as a dominant antigen in the murine typhoid model (34, 50). However, it should be noted that most analysis of flagellin specific CD4 T cells has focused on the very early responses to Salmonella infection (51), in part due to the failure of these cells to survive to seed the memory pool (14, 23). It should also be noted that the CD4 response to epitope 427–441 was previously described to be very low after primary infection with Salmonella (34), raising the possibility that other flagellin epitopes are more dominant in the memory response. Additional tools for examining CD4 responses to Salmonella epitopes are not currently available making it difficult to compare the flagellin-specific memory frequency to the wider Salmonella-specific CD4 repertoire.
Although B7-H1-deficient mice exhibited increased bacterial burdens in the spleen and liver, only around 30% of these B7-H1-deficient mice died after primary infection. Indeed, surviving mice were able to eliminate all remaining bacteria from tissues and fully resisted secondary challenge with virulent Salmonella (data not shown). Thus, the inability of B7-H1-deficient mice to clear primary infection Salmonella is not as profound as that previously reported for class-II-deficient or T-bet-deficient mice. It is therefore likely that other co-stimulatory molecules participate in the maturation of Th1 cells during Salmonella infection and can contribute even in the absence of B7-H1. The ability of B7-H1-deficient mice to resist secondary infection is likely explained by the robust antibody response induced in these mice, since antibody is known to play an important role in secondary protection (18). Thus, in the presence of Salmonella-specific antibody response even the reduced Th1 response noted in B7-H1-deficient mice may be sufficient for pathogen clearance.
In conclusion, our data report a surprising requirement for B7-H1 in the maturation of multifunctional Th1 cells and protective immunity to primary Salmonella infection. Our data differ markedly from previous findings that B7-H1 functions as an inhibitory receptor in chronic viral infections and serve to emphasize the differing roles played by co-stimulatory molecules during infection with different pathogens. Understanding the basis of these differences may suggest future strategies for improving vaccination against intracellular bacterial infections.
Acknowledgments
This work was supported by grants from the National Institutes of Health AI055743 and AI56172.
References
- 1.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82:346–353. [PMC free article] [PubMed] [Google Scholar]
- 2.Bhutta ZA. Current concepts in the diagnosis and treatment of typhoid fever. Bmj. 2006;333:78–82. doi: 10.1136/bmj.333.7558.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Typhoid immunization. Recommendations of the Immunization Practices Advisory Committee (ACIP) MMWR Recomm Rep. 1990;39:1–5. [PubMed] [Google Scholar]
- 4.ACIP updates recommendations for the use of antiviral agents in influenza. Advisory Committee on Immunization Practices. Am Fam Physician. 1995;52:659–660. 663. [PubMed] [Google Scholar]
- 5.DeRoeck D, Clemens JD, Nyamete A, Mahoney RT. Policymakers’ views regarding the introduction of new-generation vaccines against typhoid fever, shigellosis and cholera in Asia. Vaccine. 2005;23:2762–2774. doi: 10.1016/j.vaccine.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 6.Santos RL, Zhang S, Tsolis RM, Kingsley RA, Adams LG, Baumler AJ. Animal models of salmonella infections: enteritis versus typhoid fever. Micrtobes Infect. 2001;3:1335–1344. doi: 10.1016/s1286-4579(01)01495-2. [DOI] [PubMed] [Google Scholar]
- 7.Mastroeni P, Sheppard M. Salmonella infections in the mouse model: host resistance factors and in vivo dynamics of bacterial spread and distribution in the tissues. Microbes Infect. 2004;6:398–405. doi: 10.1016/j.micinf.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Ravindran R, McSorley SJ. Tracking the dynamics of T-cell activation in response to Salmonella infection. Immunology. 2005;114:450–458. doi: 10.1111/j.1365-2567.2005.02140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srinivasan A, McSorley SJ. Activation of Salmonella-specific immune responses in the intestinal mucosa. Arch Immunol Ther Exp (Warsz) 2006;54:25–31. doi: 10.1007/s00005-006-0003-5. [DOI] [PubMed] [Google Scholar]
- 10.Jones BD, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer’s patches. J Exp Med. 1994;180:15–23. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richter-Dahlfors A, Buchan AM, Finlay BB. Murine salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J Exp Med. 1997;186:569–580. doi: 10.1084/jem.186.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoiseth SK, Stocker BA. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 13.Jones BD, Falkow S. Salmonellosis: host immune responses and bacterial virulence determinants. Annu Rev Immunol. 1996;14:533–561. doi: 10.1146/annurev.immunol.14.1.533. [DOI] [PubMed] [Google Scholar]
- 14.Srinivasan A, Foley J, McSorley SJ. Massive Number of Antigen-Specific CD4 T Cells during Vaccination with Live Attenuated Salmonella Causes Interclonal Competition. J Immunol. 2004;172:6884–6893. doi: 10.4049/jimmunol.172.11.6884. [DOI] [PubMed] [Google Scholar]
- 15.Sinha K, Mastroeni P, Harrison J, de Hormaeche RD, Hormaeche CE. Salmonella typhimurium aroA, htrA, and AroD htrA mutants cause progressive infections in athymic (nu/nu) BALB/c mice. Infect Immun. 1997;65:1566–1569. doi: 10.1128/iai.65.4.1566-1569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guilloteau LA, Lax AJ, MacIntyre S, Wallis TS. The Salmonella dublin virulence plasmid does not modulate early T-cell responses in mice. Infect Immun. 1996;64:222–229. doi: 10.1128/iai.64.1.222-229.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hess J, Ladel C, Miko D, Kaufmann SH. Salmonella typhimurium aroA- infection in gene-targeted immunodeficient mice: major role of CD4+ TCR-alpha beta cells and IFN-gamma in bacterial clearance independent of intracellular location. J Immunol. 1996;156:3321–3326. [PubMed] [Google Scholar]
- 18.McSorley SJ, Jenkins MK. Antibody is required for protection against virulent but not attenuated Salmonella enterica serovar typhimurium. Infect Immun. 2000;68:3344–3348. doi: 10.1128/iai.68.6.3344-3348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.VanCott JL, Chatfield SN, Roberts M, Hone DM, Hohmann EL, Pascual DW, Yamamoto M, Kiyono H, McGhee JR. Regulation of host immune responses by modification of Salmonella virulence genes. Nat Med. 1998;4:1247–1252. doi: 10.1038/3227. [DOI] [PubMed] [Google Scholar]
- 20.Ravindran R, Foley J, Stoklasek T, Glimcher LH, McSorley SJ. Expression of T-bet by CD4 T cells is essential for resistance to Salmonella infection. J Immunol. 2005;175:4603–4610. doi: 10.4049/jimmunol.175.7.4603. [DOI] [PubMed] [Google Scholar]
- 21.Moon JJ, McSorley SJ. Tracking the dynamics of salmonella specific T cell responses. Curr Top Microbiol Immunol. 2009;334:179–198. doi: 10.1007/978-3-540-93864-4_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bueno SM, Gonzalez PA, Schwebach JR, Kalergis AM. T cell immunity evasion by virulent Salmonella enterica. Immunol Lett. 2007;111:14–20. doi: 10.1016/j.imlet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Srinivasan A, Nanton M, Griffin A, McSorley SJ. Culling of activated CD4 T cells during typhoid is driven by Salmonella virulence genes. J Immunol. 2009;182:7838–7845. doi: 10.4049/jimmunol.0900382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 25.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 27.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ha SJ, West EE, Araki K, Smith KA, Ahmed R. Manipulating both the inhibitory and stimulatory immune system towards the success of therapeutic vaccination against chronic viral infections. Immunol Rev. 2008;223:317–333. doi: 10.1111/j.1600-065X.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- 29.McSorley SJ, Asch S, Costalonga M, Rieinhardt RL, Jenkins MK. Tracking Salmonella-specific CD4 T cells in vivo reveals a local mucosal response to a disseminated infection. Immunity. 2002;16:365–377. doi: 10.1016/s1074-7613(02)00289-3. [DOI] [PubMed] [Google Scholar]
- 30.Srinivasan A, Foley J, Ravindran R, McSorley SJ. Low-dose salmonella infection evades activation of flagellin-specific CD4 T cells. J Immunol. 2004;173:4091–4099. doi: 10.4049/jimmunol.173.6.4091. [DOI] [PubMed] [Google Scholar]
- 31.Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity. 2004;20:327–336. doi: 10.1016/s1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- 32.Chatfield SN, I, Charles G, Makoff AJ, Oxer MD, Dougan G, Pickard D, Slater D, Fairweather NF. Use of the nirB promoter to direct the stable expression of hetrologous antigens in salmonella oral vaccine strains: Development of a single-dose oral tetanus vaccine. Biotechnology. 1992;10:888–892. doi: 10.1038/nbt0892-888. [DOI] [PubMed] [Google Scholar]
- 33.Moon JJ, Chu HH, Hataye J, Pagan AJ, Pepper M, McLachlan JB, Zell T, Jenkins MK. Tracking epitope-specific T cells. Nat Protoc. 2009;4:565–581. doi: 10.1038/nprot.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McSorley SJ, Cookson BT, Jenkins MK. Characterization of CD4+ T cell responses during natural infection with Salmonella typhimurium. J Immunol. 2000;164:986–993. doi: 10.4049/jimmunol.164.2.986. [DOI] [PubMed] [Google Scholar]
- 35.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wick MJ. Monocyte and dendritic cell recruitment and activation during oral Salmonella infection. Immunol Lett. 2007;112:68–74. doi: 10.1016/j.imlet.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Klugman KP, I, Gilbertson T, Koornhof HJ, Robbins JB, Schneerson R, Schulz D, Cadoz M, Armand J. Protective activity of Vi capsular polysaccharide vaccine against typhoid fever. Lancet. 1987;2:1165–1169. doi: 10.1016/s0140-6736(87)91316-x. [DOI] [PubMed] [Google Scholar]
- 38.Mastroeni P, Simmons C, Fowler R, Hormaeche CE, Dougan G. Igh-6(−/−) (B-cell-deficient) mice fail to mount solid aquired resistance to oral challenge with virulent Salmonella enterica serovar typhimurium and show impaired Th1 T-cell responses to Salmonella antigens. Infect Immun. 2000;68:46–53. doi: 10.1128/iai.68.1.46-53.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mittrucker HW, Raupach B, Kohler A, Kaufmann SH. Cutting edge: role of B lymphocytes in protective immunity against Salmonella typhimurium infection. J Immunol. 2000;164:1648–1652. doi: 10.4049/jimmunol.164.4.1648. [DOI] [PubMed] [Google Scholar]
- 40.Griffin A, Baraho-Hassan D, McSorley SJ. Successful treatment of bacterial infection hinders development of acquired immunity. J Immunol. 2009;183:1263–1270. doi: 10.4049/jimmunol.0900772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang S, Chen L. Co-signaling molecules of the B7-CD28 family in positive and negative regulation of T lymphocyte responses. Microbes Infect. 2004;6:759–766. doi: 10.1016/j.micinf.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 42.Seo SK, Jeong HY, Park SG, Lee SW, Choi IW, Chen L, Choi I. Blockade of endogenous B7-H1 suppresses antibacterial protection after primary Listeria monocytogenes infection. Immunology. 2008;123:90–99. doi: 10.1111/j.1365-2567.2007.02708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rowe JH, Johanns TM, Ertelt JM, Way SS. PDL-1 blockade impedes T cell expansion and protective immunity primed by attenuated Listeria monocytogenes. J Immunol. 2008;180:7553–7557. doi: 10.4049/jimmunol.180.11.7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Srinivasan A, Salazar-Gonzalez RM, Jarcho M, Sandau MM, Lefrancois L, McSorley SJ. Innate immune activation of CD4 T cells in salmonella-infected mice is dependent on IL-18. J Immunol. 2007;178:6342–6349. doi: 10.4049/jimmunol.178.10.6342. [DOI] [PubMed] [Google Scholar]
- 45.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 46.Gil-Cruz C, Bobat S, Marshall JL, Kingsley RA, Ross EA, Henderson IR, Leyton DL, Coughlan RE, Khan M, Jensen KT, Buckley CD, Dougan G, MacLennan IC, Lopez-Macias C, Cunningham AF. The porin OmpD from nontyphoidal Salmonella is a key target for a protective B1b cell antibody response. Proc Natl Acad Sci U S A. 2009;106:9803–9808. doi: 10.1073/pnas.0812431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mittrucker H, Kohler A, Kaufmann SH. Characterization of the murine T-lymphocyte response to salmonella enterica serovar typhimurium infection. Infect Immun. 2002;70:199–203. doi: 10.1128/IAI.70.1.199-203.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bajenoff M, Wurtz O, Guerder S. Repeated antigen exposure is necessary for the differentiation, but not the initial proliferation, of naive CD4+ T cells. J Immunol. 2002;168:1723–1729. doi: 10.4049/jimmunol.168.4.1723. [DOI] [PubMed] [Google Scholar]
- 49.Obst R, van Santen HM, Mathis D, Benoist C. Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response. J Exp Med. 2005;201:1555–1565. doi: 10.1084/jem.20042521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cookson BT, Bevan MJ. Identification of a natural T cell epitope presented by Salmonella-infected macrophages and recognised by T cells from orally immunised mice. J Immunol. 1997;158:4310–4319. [PubMed] [Google Scholar]
- 51.Salazar-Gonzalez RM, Niess JH, Zammit DJ, Ravindran R, Srinivasan A, Maxwell JR, Stoklasek T, Yadav R, Williams IR, Gu X, McCormick BA, Pazos MA, Vella AT, Lefrancois L, Reinecker HC, McSorley SJ. CCR6-Mediated Dendritic Cell Activation of Pathogen-Specific T Cells in Peyer’s Patches. Immunity. 2006;24:623–632. doi: 10.1016/j.immuni.2006.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]