Abstract
Novel self-assembled monolayers (SAMs) designed to present homogenous surface chemistries were utilized to further investigate the material surface chemistry dependent macrophage and foreign body giant cell (FBGC) behaviors including macrophage adhesion, fusion, and apoptosis. Contact angle analysis revealed instabilities in the –CH3 and –COOH terminate SAM surfaces upon incubation in serum-free media at 37oC or under dry, room temperatureconditions. Further analysis indicated that the –CH3 terminated SAM surface degraded rapidly within 2 hours and loss of sufficient SAM units to be comparable to the gold (Au) control surface within 24 hours of incubation in serum-free media (SFM) at 37oC. After 5days of incubation in SFM at 37oC, the contact angles for the –COOH terminated SAMsurfaces increased markedly. AFM analysis confirmed the desorption of –CH3 terminated SAM molecules from the surface with increased roughness and marked appearance of peaks andvalleys within 2 hours. A decrease in the thickness of the –COOH terminated SAM surface also suggests molecular desorption over time. No significant changes in contact angle or AFM analyses were observed on the –OH terminated SAM surfaces. Cellular adhesion decreased morerapidly on the Au control and –CH3 terminated SAM surfaces in comparison to the other surfaces. However by day 10, cellular adhesion, fusion, and apoptosis were comparable on all SAM surfaces and the Au control . These studies suggest that SAM surfaces may not be suitable for long-term studies where material dependent properties are investigated.
Keywords: self-assembled monolayers, instability, macrophage, foreign body giant cell, contact angles
Introduction
It is well understood that inflammation, wound healing, and the foreign body reaction occurfollowing implantation of a biomaterial. Significant research has been conducted to gain insight into the cellular/biomaterial interactions involved in and directing these responses. Theoretically, an in-depth understanding of the relationships between surface chemistry and the behavior of key inflammatory and wound healing cells (i.e. monocytes, macrophages, foreign body giant cells, and fibroblasts) would provide a basis for establishing criteria for designing biomaterials for use in specific applications. This study utilizes homogeneous SAM surfaces to investigate the effects of particular surface chemistries (hydrophobic and hydrophilic) and functional groups (– CH3, –COOH, and –OH) on the behavior of macrophages andforeign body giant cells (FBGCs), two key cells involved in inflammation, wound healing, and the foreign body reaction.
Self-assembled monolayer (SAM) surfaces are a unique type of surface that can present a variety of distinct surface chemistries offering a novel means for studying cellular/biomaterial interactions. SAMs consist of long-chained alkanethiols (HS-(CH2)n- R, where n=10-18 and R is a terminal group) that spontaneously adsorb onto a gold surface from a solution and self-assemble to form a homogeneous monolayer. These surfaces are very advantageous in that they can be quickly synthesized and easily modified to present a variety of homogenous, mixed, or pattern surface chemistries. In addition, the composition and properties of the surface are easily controlled by the synthesis parameters, distinct alkanethiol units and terminal groups (–CH3, –COOH,– OH, –NH2) used, post-synthesis modifications adding specific ligands or additional chemistries, and micropatterning techniques that pattern the functional groups within the monolayer.1-6 Using these techniques, tailored SAM surfaces have been created to study the effects of specific surface chemistries on protein adsorption within 24 hours and numerous cellular behaviors over time periods ranging from hours to days including cellular adhesion, recruitment, orientation, migration, and cytokine release.2,7-18 In addition, thesesurfaces are being utilized in other applications to examine ligand interactions, improve the reproduction of microarrays, and alter nanostructures on the surfaces of thin metal films.1,6,19,20 Significant research has been conducted with these surfaces since their inceptionin the 1980s; however more recently, the stability of these surfaces has come into question.6,21-30
Recent studies in our laboratory have shown that hydrophobic, hydrophilic/neutral, and hydrophilic/ionic surface chemistries affect macrophage adhesion, fusion, apoptosis, and activation in opposing manners.31-37 Particularly, hydrophobic and hydrophilic/ionic surfaces promote macrophage adhesion and fusion and inhibit macrophage apoptosis, while hydrophilic/neutral surfaces inhibit macrophage adhesion and fusion and promote macrophage apoptosis and activation.31,36,37 These findings prompted a more intensive investigation into the effects of particular surface chemistries and functional groups on macrophage behavior.
In this study, we employ these unique SAM surfaces to evaluate the effects of the commonly utilized functional groups (–CH3, –COOH, –OH) and hydrophobic/hydrophilic surface chemistries on macrophage adhesion, fusion, and apoptosis. As the study progressed, the SAM material stability became uncertain; therefore, the focus of this research was revisedto include an evaluation of these surfaces as a stable material for use in our 10 day, in vitro human monocyte/macrophage cell culture system.
Materials and Methods
Self-Assembled Monolayer Synthesis
The SAM surfaces investigated within this study have a chemistry of HS(CH2)11X with either a –CH3 (hydrophobic), –COOH (hydrophilic), or –OH (hydrophilic) functional group. Unmodified gold surfaces were utilized as a control within these studies. Recent studies suggest that the stability of the SAM surface is questionable over time dueto various factors explained later; therefore a SAM synthesis protocol was developed based upon commonly utilized techniques in order to synthesize fresh SAM surfaces for each experiment.2,7,13,14,38-41 Prior to alkanethiol assembly, glass coverslips (no. 2, d=18mm, Scientific, Pittsburgh, PA) were primed with a 100Å thick layer of titanium to improve the adhesion of the gold coating, and then were coated with a layer of gold (1000Å thick) using asputter coating technique (2M/torr deposition rate, 3×10-6 torr power). The pre-coated glass substrates were cleaned to remove any debris or contaminants using a series of acetone, absolute ethanol, and deionized, distilled water washes. Clean substrates were immersed in a 1.0 mM ethanolic, alkanethiol solution under a nitrogen environment for 18-20 hours at room temperature to allow the monolayers to spontaneously form and assemble. Subsequently, the alkanethiol surfaces were rinsed in absolute ethanol (3x) to remove any non-adsorbed alkanethiol molecules. Surfaces were then dried ina nitrogen stream and equilibrated in Dulbecco’s Phosphate Buffer Saline with Mg+2 and Ca+2 (PBS++) for 15 minutes at room temperature.
Surface Characterization
SAM surfaces were characterized using water contact angle analysis via the sessile drop method and a goniometer (Edmund Scientific, Barrington, NJ) at 22oC room temperature. Contact angles were measured immediately upon synthesis at day 0 and subsequently on untreated samples at day 40 to determine modification long-term stability. In addition, select samples weretreated via incubation in serum-free media (SFM) (Gibco, Grand Island, NY) at 37oC to determine modification stability under aqueous culture conditions. Treated samples were measured for contact angles after 2 hours, 6 hours, 24 hours, 5 days, 10 days, and 18 days of treatment. Contact angles were taken over two areas on each of the surfaces to elucidate surface homogeneity. Both advancing and receding water contact angles were measured and are reported as the mean ± standard deviation to further detect surface inhomogeneities.
Atomic force microscopy was conducted using a Nanoscope IIIa (Digital Instruments, Santa Barbara, CA). Measurements were taken using a silicon nitride cantilever tip (spring constant = 0.58 N/m) in air. Dry, freshly synthesized surfaces were imaged at t=0 and after 2 hours of treatment in serum-free media at 37oC to determine changes in the topography of the surfaces within 2 hours in an aqueous, 37oC environment. A total of three samples were analyzed over two areas on each sample. AFM 3-D images and roughness measurements are presented.
Macrophage/FBGC In Vitro Cell Culture System
Equilibrated SAM surfaces were sterilized with 100% ethanol, placed into 12-well tissue culture polystyrene (TCPS) plates (Fisher Scientific, Pittsburgh, PA) and secured with silicone rings (ID=1.59cm, OD=2.22cm, h≈8mm) for use in culture within two hours of synthesis. Silicone rings were cut from silicone tubing (Cole-Parmer, Vernon Hills, IL), sonicated for 5 minutes in 100% ethanol, rinsed with distilled water, air-dried, and sterilized using ethyleneoxide prior to insertion. The resultant surface area was 198mm2.
Human blood monocytes and serum were isolated using a Ficoll and density gradient centrifugation technique as described previously.42 Freshly isolated monocytes were plated at a concentration of 7.5 × 105 cells in 0.5 mL of serum-free media (SFM) with 20% autologous serum and were allowed to adhere for 2 hours. After 2 hours, supernatants containing non-adherent cells were collected via pipetting for non-adherent cell analysis, adherent cells were rinsed with warmed PBS++ and refed fresh media containing SFM with 20% heat-treated (56oC for 1 hour) autologous serum. Cell cultures were incubated at 37oC in a 5% CO2 environment for 3, 7 and 10 days. At days 3 and 7, IL-4, a fusion inducing cytokine, was added to the fresh media (15ng/mL) for select samples. Adherent and non-adherent cell cultures were examined using the following procedures.
Adherent Cell Analysis
Late stage apoptosis was identified in adherent cell cultures obtained at day 3, day 7, andday 10 using TDT-mediated dUTP-flourescein nick end-labeling (TUNEL) (Roche, Indianapolis, IN)in accordance with the manufacturer’s instructions. Subsequently, the adherent cell cultures were labeled with DAPI (Roche, Indianapolis, IN), a nuclear label, and Alexa Flour 594 (Invitrogen, Carlsbad, CA), a phalloidin that binds to filamentous actin in the cytoskeleton, in accordance with manufacturer’s instructions. At day 0 (2 hours), adherent cells were visualized using only Alexa Flour 594 and DAPI.
A total of 10 fields (40x) were analyzed for the total number of adherent cells, the numberof nuclei in FBGCs, the number of FBGCs, and the number of apoptotic cells. The adherent cell density is reported in cells/mm2, while percent fusion was calculated to be the number of nuclei in FBGCs divided by the total number of nuclei. Apoptosis results are presented as the percentage of apoptotic cells of the total number of adherent cells. All data is reported as the mean ± the standard error of the mean (SEM).
Results
Surface Characterization
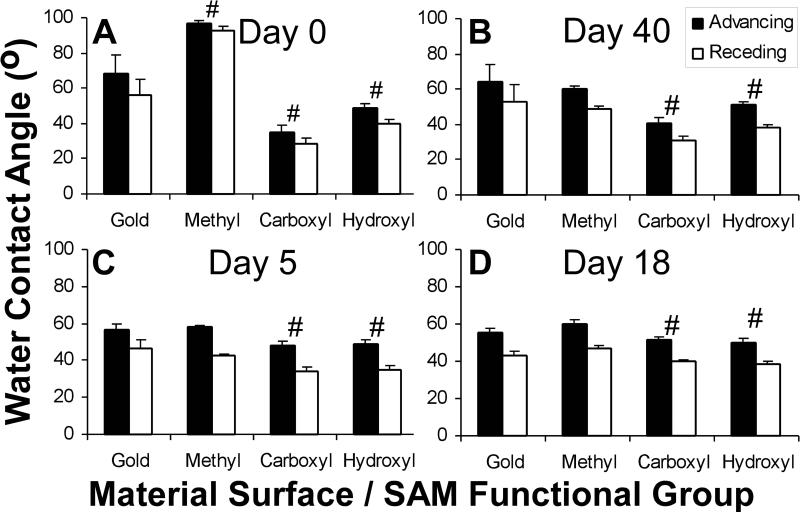
Contact angle analysis was measured to determine the presence of the SAM modifications overtime. At day 0, the advancing water contact angles for the Au control and the –CH3, –COOH, and –OH terminated SAM surfaces were 68o ± 11o, 97o ± 2o, 35o ± 4o, and 49o ± 3o, respectively (Figure 1A). The –CH3 terminated SAM surface contact angle decreased significantly to 58o ± 1o after incubation in an aqueous environment of serum-free media at 37oC for 5 days (Figure 1B). The contact angle for the –COOH terminated SAM surfaces increased slightly to 49o ± 2o, while the –OH terminated SAM surface contact angles remained unchanged. After 18 days of treatment in serum-free media at 37oC (Figure 1C), the contact angles for the Au control (55o ± 3o) and the –CH3(60o ± 2o), –COOH (50o ± 2o), and –OH (52o ± 2o) terminated SAM surfaces were comparable to day 5 values. Forty days after synthesis, the contact angles for dry, untreated samples at room temperature in the dark (Figure 1D) decreased for the –CH3 terminated SAM surfaces to levels (60 o ± 2o) equivalent to those measured on the treated samples at days 5 and 18. In contrast, the –COOH and –OH terminated SAM surfaces remained relatively comparable to the values seen at day 0 (40o ± 4o and 51o ± 2o).
Figure 1.
Advancing and receding contact angles on SAM surfaces. Untreated, dry samples were examined at days 0 (A) and 40 (D), after synthesis. Treated samples were incubated in SFM at 378C for 5 (B) and 18 (C) days post synthesis. Mean 6 standard deviation (n 5 6, 3). ‘‘#’’ indicates statistical difference between advancing and receding values for this material in comparison to the advancing and receding values for gold (p < 0.05).
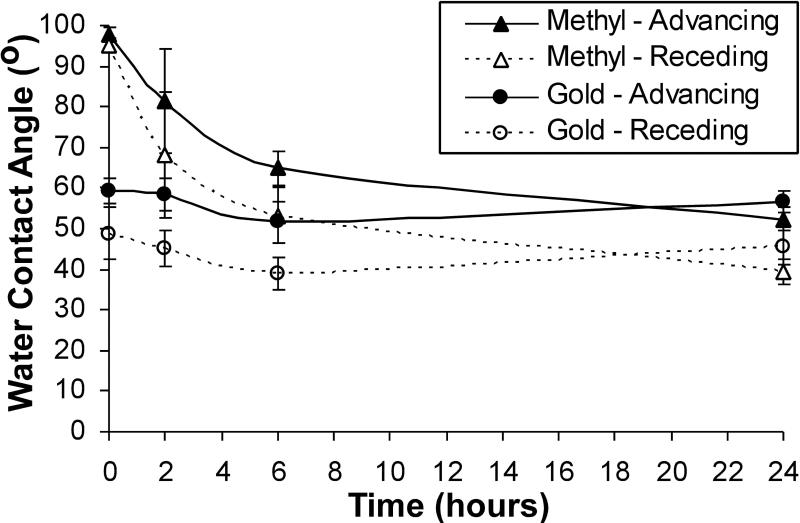
A time course experiment on the –CH3 terminated SAM and Au control surfaces wasconducted to determine changes in contact angle values within the first 24 hours of treatment in serum-free media at 37oC (Figure 2). The advancing and receding contact angles for the –CH terminated SAM surface decreased from t=0 (98o ± 2o) to 2 hoursof treatment (81o ± 13o) and decreased significantly (p<0.05) 6 hours (65o ± 4o) and 24 hours (52o ± 2o). The contact angles for the Au control surfaces were comparable to one another over time and ultimately comparable to the –CH3 terminated SAM surface by 24 hours of treatment (56o ± 3o).
Figure 2.
Early changes in water contact angles on methyl SAM surfaces in an aqueous environment. Samples were incubated in SFM at 378C. Mean 6 Standard deviation (n 5 3). ‘‘*’’ Indicates a statistical difference between the contact angles for the _CH3 terminated surface and angles for the Au surface at this time-point (p < 0.05).
AFM Analysis
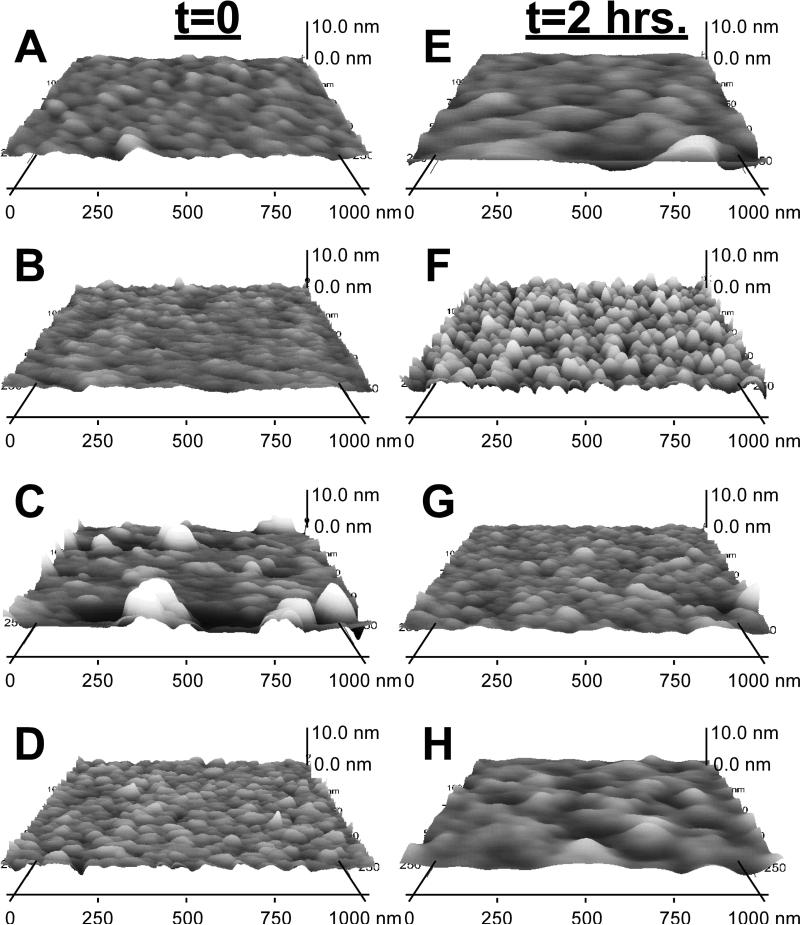
AFM analysis was conducted on the SAM surfaces immediately (<2hours) following synthesis and after 2 hours of treatment in serum-free media at 37oC in order to further investigate the stability of the SAM alkanethiols. AFM images are shown in Figure 3 and the roughness and thickness values are shown in Table 1. The Au control surface remained unchanged between t=0 and t=2 hours with roughness values of 0.40 ± 0.06 and 0.44 ± 0.03 respectively (Figures 3A & 3E). The topography for the –CH3 terminated SAM surface was relatively smooth at t=0 with a roughness of 0.25 ± 0.10 and a thickness of 0.58 ± 0.39 nm (Figure 3B). As suspected, this topography changed after 2 hours of incubation in serum-free media at 37oC increasing significantly in roughness (0.76 ± 0.34) and thickness (1.89 ± 0.58 nm) (Figure 3F). This change in topography supports the hypothesis that the alkanethiols desorbed from the surface leaving pits in place of the desorbed alkanethiols. In Figures 3C & 3G, strong peaks with average heights of 5.00 ± 2.91 nm were present on the –COOH terminated SAM surfaces following synthesis at t=0 and were reduced after 2 hours of treatment to 3.49 ± 1.72 nm. Lastly, the –OH terminated SAM surfaces remained relatively comparable before and after treatment in serum-free media at 37oC.
Figure 3.
AFM images of the gold control (A, E) and the methyl (B, F), carboxyl (C,G), and hydroxyl (D, H) SAM surfaces immediately after synthesis (A–D) and after 2 h of treatment (E–H). Samples treated were incubated in SFM at 378C for 2 h.
Table 1.
AFM Results for the SAM Surfaces Prior to and After Treatment^
| Parameter | Surface | At t=0 | After 2 hours of Treatment* |
|---|---|---|---|
| Roughness | Gold | 0.40 ± 0.06 | 0.44 ± 0.03 |
| Methyl | 0.25 ± 0.10 | 0.76 ± 0.34 | |
| Carboxyl | 1.97 ± 1.15 | 1.23 ± 0.52 | |
| Hydroxyl | 0.61 ± 0.03 | 0.65 ± 0.30 | |
| Thickness/Peak Height (nm) | Gold | - | - |
| Methyl | 0.58 ± 0.39 | 1.89 ± 0.58 | |
| Carboxyl | 5.00 ± 2.91 | 3.49 ± 1.72 | |
| Hydroxyl | 1.67 ± 0.12 | 1.77 ± 1.16 |
Treated samples were incubated in serum-free media at 37CC for 2 hours
Results are the mean ± standard deviation (n=3).
Cellular Adhesion
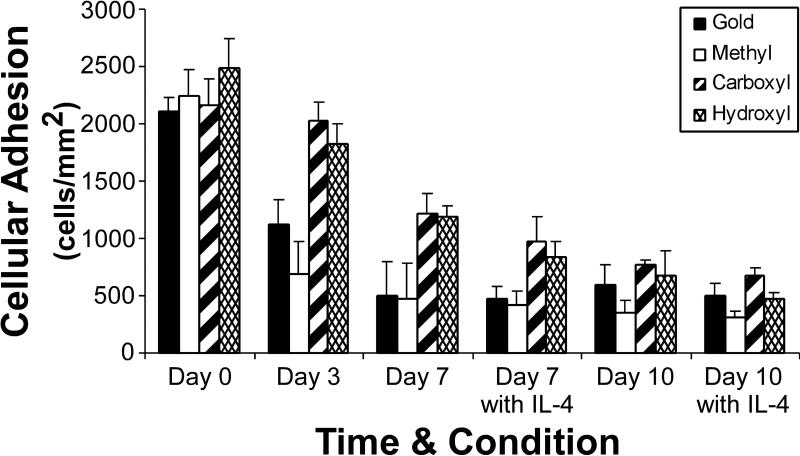
Cellular adhesion was comparable on all surfaces at day 0 averaging 2,250±90 cells/mm2 (Figure 4). The adherent cell density decreased significantly on the –CH3 terminated SAM (691±279 cells/mm2) and Au control (1,120±240 cells/mm2) surfaces by day 3 to values statistically less than the –COOH (2,030±160 cells/mm2) and –OH (1830±170 cells/mm2) terminated SAM surfaces (p<0.05). A decrease in adherent cell densities was observed on all surfaces by days 7 and 10 in the presence and absence of IL-4; however there were no significant differences between surfaces at these later timepoints.
Figure 4.
Cellular adhesion on SAM surfaces over time. Mean 6 SEM, n 5 3. ‘‘^’’ indicates a significant decrease in adhesion occurs after this time-point (p 5 0.002). ‘‘*’’ Indicates that the values for these materials are statistically different from the methyl SAM surface (p 5 0.03).
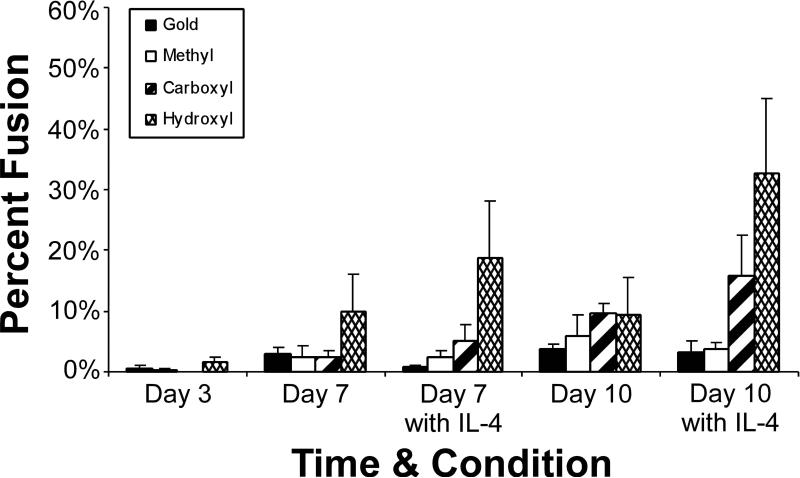
Macrophage Fusion into FBGCs
Macrophage fusion into FBGCs was equivalently minimal on all materials at day 3 (Figure 5).Percent fusion increased over time and remained comparable on the majority of the surfaces. Macrophage fusion was significantly greater on the –OH terminated SAM surface at day 7 with (19%±9%) or without (10%±6%) IL-4 and at day 10 with IL-4 (33%±12%). Also, fusion was greater on the –COOH terminated SAM surface than on the Au control or –CH3 terminated SAM surface at day 10 with IL-4 (16%±6%).
Figure 5.
Macrophage fusion into FBGCs on SAM surfaces over time. Mean 6 SEM, n 5 3. ‘‘^’’ indicates that the values for this material are statistically greater than gold (p 5 0.04).
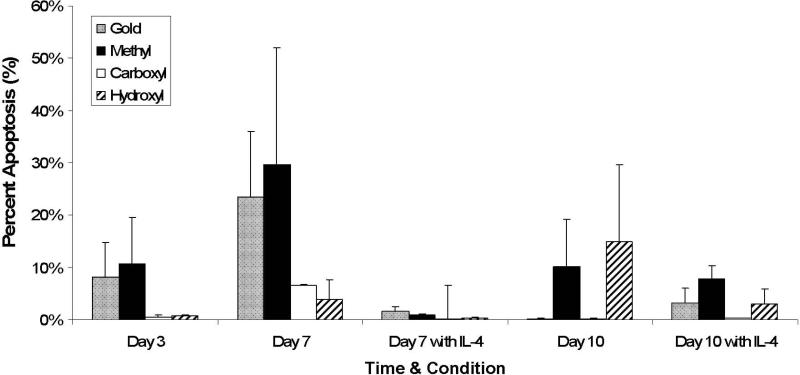
Monocyte/Macrophage Apoptosis
The percentage of apoptotic cells on the Au control and –CH3 terminated SAM surfaces were comparable (8%±7% and 11%±9%) and slightly greater than the percentages on the –COOH (1%±0%) and –OH terminated SAM (1%±0%) surfaces at day 3 correlating with the significant decrease in adhesion seen on these surfaces at this time (Figure 6). This trend remained at day 7 without IL-4, however at day 7 in the presence of IL-4 and by day 10, percent apoptosis was relatively comparable on all materials.
Figure 6.
Cellular apoptosis on SAM surfaces over time. Percent apoptosis is the percentage of total number of adherent nuclei that are apoptotic. Mean 6 SEM, n 5 3.
Discussion
Immediately following synthesis, the presence of the alkanethiol on the material surface was confirmed by contact angle analysis of each of the SAM surfaces. Advancing contact angles onthe –CH3 terminated SAM surfaces were comparable to the expected, previously reported values of 101o, 107o, and 117o.12,43 In contrast, contact angles for both the –COOH and –OH terminated SAM surfaces were higher than the expected values, 25o and 20o, respectively, but were within range of other previously reported values.12,22,43,44 Prior research conducted in numerous laboratories have reported a wide range of contact angle values for the –COOH (28o, 42o, and 72o) and –OH (25o and 82o) terminated SAM surfaces.12,22,43,44 This observed range may be due to defects in the SAM surface that resulted during synthesis and/or subsequent alkanethiol degradation that altered the chemical groups exposed at the material surface varying the contact angles measured.
The SAM synthesis parameters utilized in this study were chosen based upon commonly utilized SAM synthesis techniques to minimize inhibitory factors that result in surface defects such as debris or defects on the gold substrate, low concentrations or impure alkanethiol solutions, insufficient time allowed for assembly (typically 2-12 hours for long chain thiols and 24 hours for short chain thiols), and high oxygen content in the alkanethiol solution and environment.2,6,7,13,14,38-41,45,46 In addition, studies have shown that longer alkanethiol chains (C18) form SAM surfaces with fewer defects than short chain alkanethiols (n<9) and that fewer defects occur with higher incubation temperatures; therefore, the alkanethiols utilized in this study were of moderate to long chain length (C12) and were incubated at room temperature in a nitrogen atmosphere.28,46
Variations in the contact angles over time revealed that the –CH3 terminated SAM surfaces were unstable in both serum-free media at 37oC (treated condition) within days and in a dry, room temperature environment (untreated condition) over longer periods of time. This instability began as quickly as 2 hours when incubated in serum-free media, in agreement with similar findings by Scotchford et.al.13 Significant research has demonstrated that SAM surfaces become unstable over time as indicated by changes in contact angles, AFM, IR,XPS, SPR, and Raman spectroscopy results.13,23,24,26,29 AFM analysis of these surfaces in our study confirmed the partial desorption of the –CH3 terminated SAM moleculesbefore and after 2 hours of treatment in serum-free media at 37oC by changes in the topography of the surface from a relatively smooth topography to a more rough, thicker topographycomposed of taller peaks and valleys.
Alkanethiols can easily be removed from the material surface as a result of direct desorption as disulfides upon exposure to liquid media or due to oxidation into sulfinates and sulfonates upon exposure to air followed by desorption when exposed to solvents, both of which are promoted with increasing temperatures.21,23,25,29,47 Studies have shown that this degradation occurs rapidly within hours of exposure to air and that subsequent immersion in liquids furthers this degradation.25,29 In addition, degradation of SAMs has also been shown to begin at boundaries of defects; therefore, any potential defects resulting during synthesis, discussed above, provide additional locations for degradation.21,27 A combination of these mechanisms: direct-desorption, oxidation, and synthesis defects; may explain the instability of these SAM surfaces over time.
Instability in the –COOH terminated SAM surfaces was observed with contact angle analysis after 5 days of incubation in serum-free media at 37oC and within 40 days when leftuntreated in a dry, room temperature environment. Previous research has shown that unbound alkanethiols can remain at the material surface, and it has been suggested that these alkanethiols in the solution form interplanar hydrogen bonds with the –COOH terminal groups of alkanethiols within the monolayer potentially forming a partial bi-layer. 45,46 AFM analysisrevealed the potential presence of this bi-layer effect on the –COOH terminated SAM surfaces with peak heights over twice the size of the alkanethiol chains. These peaks diminish after 2 hours of treatment in serum-free media at 37oC suggesting that the bi-layer desorbed within the aqueous environment. This bi-layer effect may also account for the slightly greater contact angle initially seen following synthesis of these materials. Contact angle and AFM analysis of the –OH terminated SAM surfaces did not indicate any significant instabilities in the surface over time in either condition suggesting that these surfaces are more stable than the –CH3 and –COOH terminated SAM surfaces.
SAM stability may also have a cellular dependent component. Previous research demonstrated that endothelial cell attachment could be controlled using micropatterned SAM surfaces and that this attachment could remain localized to the microprinted patterns over time indicating that the integrity of mixed micropatterned SAM surfaces was maintained up to 24 hours.7,16,48 Later, Chen et.al. investigated the cell-dependent nature of SAM stability by seeded 3T3-L1 pre-adipocytes or bovine pulmonary artery endothelial cells to non-adhesive hexa(ethylene glycol)-terminated alkanethiols (EG6 SAM) SAM surfaces that were patterned with adhesion-promoting hexadecanethiol. The time until whichthe patterned adhesion failed was measured as an indication of rate of degradation of the non-adhesive EG6 SAM surface over time.26 The findings from this study demonstrated that SAM instability was further promoted in the presence of the adipocytes in comparison to the endothelial cells as a result of the adipocyte secretion of alcohol dehydrogenase (ADH) and/or aldehyde hydrogenase, two enzymes that are associated with fatty acid metabolism and have been shown to oxidize and degrade poly(ethylene glycol) chains. Addition of inhibitors of ADH activity minimized these degratory effects confirming the hypothesis that the instability was enhanced by products of the adipocytes. Macrophages and FBGCs are known to have a low pH (3.6-3.7) inthe pericellular space and to secrete enzymes, oxygen free radicals, and acids that can oxidize, degrade, and/or desorb SAM molecules providing a mechanism by which adherent macrophages and FBGCs in our study can reduce SAM stability over time in culture.21,26,49
Ultimately, the SAM surfaces in our study were sufficiently similar to result in relativelysimilar amounts of adhesion, fusion and apoptosis over time. Interestingly, the higher levels of adhesion on the –COOH terminated SAM surfaces observed in comparison to the –CH3 terminated SAM surfaces at early timepoints and the instability of the surfaces within a short period of time mirrors a previous study conducted analyzing osteoblast-like cells and the stability of a HS(CH2)2COOH and HS(CH2)7CH3 containing SAM surfaces within 24 hours.13 This study found that a greater number of osteoblast-like cells adhered to –COOH terminated SAM surfaces at 24 hours and that the contact angles decreased significantly for both the –CH3 and –COOH terminated SAM surfaces incubated in serum-free media or DMEM containing fetal bovine serum.13 Although it is a different cell type, the studies were conducted using similar synthesistechniques and conditions suggesting that the cellular/biomaterial interactions seen in this study are valid with respect to these particular SAM surfaces. Conclusions regarding the effects of specific functional groups can not be made due to the unknown nature of the SAM surface over time.
In conclusion, self-assembled monolayer surfaces display significant instability making them a questionable model surface system for utilization in studies of cellular/biomaterial interactions. Nevertheless, numerous studies have been conducted with these seemingly simplistic materials over both short (minutes to <24 hours) and long (days) periods of time oftentimeswithout a full analysis of the SAM stability over the timeframe of the experiments. This research demonstrates the instability of the SAM surfaces over the experimental timeframe occurringas early as within 2 hours of incubation in serum-free media at the culture temperature of 37oC suggesting that SAM surfaces may not be suitable for long-term studies where materialdependent properties are investigated. In future studies, further analysis of the material stability post synthesis, prior to use, and over the time period of the study is crucial to permit sound conclusions regarding material-dependent protein adsorption or cellular behavior. In addition, this research prompts further investigation into the cell-dependent nature of SAM surface stability.
Acknowledgments
Contract Grant Sponsor: National Institute of Health National Institute of Biomedical Imaging and Bioengineering
Contract Grant Number: EB-000275
References
- 1.Lahiri J, Isaacs L, Tien J, Whitesides GM. A strategy for the generation of surfaces presenting ligands for studies of binding based on an active ester as a common reactive intermediate: a surface plasmon resonance study. Analytical Chemistry. 1999;71(4):777–790. doi: 10.1021/ac980959t. [DOI] [PubMed] [Google Scholar]
- 2.Mrksich M, Dike L, Tien J, Ingber D, Whitesides G. Using microcontact printing to pattern the attachment of mammalian cells to self-assembled monolayers of alkanethiolates on transparent films of gold and silver. Experimental Cell Research. 1997;235:305–313. doi: 10.1006/excr.1997.3668. [DOI] [PubMed] [Google Scholar]
- 3.Ito Y. Surface micropatterning to regulate cell functions. Biomaterials. 1999;20:2333–2342. doi: 10.1016/s0142-9612(99)00162-3. [DOI] [PubMed] [Google Scholar]
- 4.Kane RS, Takayama S, Ostuni E, Ingber DE, Whitesides GM. Patterning proteins and cells using soft lithography. Biomaterials. 1999;20:2363–2376. doi: 10.1016/s0142-9612(99)00165-9. [DOI] [PubMed] [Google Scholar]
- 5.Whitesides GM, Ostuni E, Takayama S, Jiang X, Ingber DE. Soft lithography in biology and biochemistry. Annual Reviews of Biomedical Engineering. 2001;3:335–373. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- 6.Love JC, Estroff LA, Kreibel JK, Nuzzo RG, Whitesides GM. Self-assembled monolayers as thiolates on metals as a form of nanotechnology. Chemical Reviews. 2005;105:1103–1169. doi: 10.1021/cr0300789. [DOI] [PubMed] [Google Scholar]
- 7.Mrksich M, Chen CS, Xia Y, Dike LE, Ingber DE, Whitesides GM. Controlling cell attachment on contoured surfaces with self-assembled monolayers of alkanethiolates on gold. Proceedings of the National Academy of Science. 1996;93:10775–10778. doi: 10.1073/pnas.93.20.10775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang F, Kang ET, Neoh KG, Huang W. Modification of gold surface by grafting of poly(ethylene glycol) for reduction in protein adsorption and platelet adhesion. Journal of Biomaterial Science Polymer Edition. 2001;12(5):515–531. doi: 10.1163/156856201300194252. [DOI] [PubMed] [Google Scholar]
- 9.Latour RA, Rini CJ. Theoretical analysis of adsorption thermodynamics for hydrophobic peptide residues on SAM surfaces of varying functionality. Journal of Biomedical Materials Research. 2002;60:564–577. doi: 10.1002/jbm.10052. [DOI] [PubMed] [Google Scholar]
- 10.Martins C, Naeemi E, Ratner B, Barbosa M. Albumin adsorption on Cibaron Blue F3G-A immobilized onto oligo(ethylene glycol)-terminated self-assembled monolayers. Journal of Material Science: Materials in Medicine. 2003;14:945–954. doi: 10.1023/a:1026394431100. [DOI] [PubMed] [Google Scholar]
- 11.Martins MCL, Fonseca C, Barbosa MA, Ratner BD. Albumin adsorption on alkanethiols self-assembled monolayers on gold electrodes studied by chronopotentiometry. Biomaterials. 2003;24:3697–3706. doi: 10.1016/s0142-9612(03)00244-8. [DOI] [PubMed] [Google Scholar]
- 12.Michael KE, Vernekar VN, Keselowsky BG. Adsorption-Induced conformational changes in fibronectin due to interactions with well-defined surface chemistries. Langmuir. 2003;19:8033–8040. [Google Scholar]
- 13.Scotchford CA, Cooper E, Leggett GJ, Downes S. Growth of human osteoblast-like cells on alkanethiol on gold self-assembled monolayers: The effect of surface chemistry. Journal of Biomedical Materials Research. 1998;41:431–442. doi: 10.1002/(sici)1097-4636(19980905)41:3<431::aid-jbm13>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 14.Kalltorp M, Oblogina S, Jacobsson S, Karlsson A, Tengvall P, Thomsen P. In vivo cell recruitment, cytokine release, and chemiluminescence response at gold, and thiol functionalized surfaces. Biomaterials. 1999;20:2123–2137. doi: 10.1016/s0142-9612(99)00115-5. [DOI] [PubMed] [Google Scholar]
- 15.Lindblad M, Lestelius M, Johansson A, Tengvall P, Thomsen P. Cell and soft tissue interactions with methyl- and hydroxyl-terminated alkanethiols on gold surfaces. Biomaterials. 1997;18:1059–1068. doi: 10.1016/s0142-9612(97)00029-x. [DOI] [PubMed] [Google Scholar]
- 16.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Micropatterned surfaces for control of cell shape, position, and function. Biotechnology Progress. 1998;14:356–363. doi: 10.1021/bp980031m. [DOI] [PubMed] [Google Scholar]
- 17.Jiang X, Bruzewicz DA, Wong AP, Piel M, Whitesides GM. Directing cell migration with asymmetric micropatterns. Proceedings of the National Academy of Science. 2005;102(4):975–978. doi: 10.1073/pnas.0408954102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang A, Liang X, McAllister JP, Li J, Brabant K, Black C, Finlayson P, Cao T, Tang H, Salley SO. Stability of and inflammatory response to silicon coated with fluoroalkyl self-assembled monolayer in the central nervous system. Journal of Biomedical Materials Research. 2007;81A:363–372. doi: 10.1002/jbm.a.31034. others. [DOI] [PubMed] [Google Scholar]
- 19.Datwani SS, Vijayendran RA, Johnson E, Biondi SA. Mixed alkanethiol self-assembled monolayers as substrates for microarraying applications. Langmuir. 2004;20:4970–4976. doi: 10.1021/la030140x. [DOI] [PubMed] [Google Scholar]
- 20.Slaughter GE, Bieberich E, Wnek GE, Wynne KJ, Guiseppi-Elie A. Improving neuron-to-electrode surface attachment via alkanethiol self-assembly: an alternating current impedence study. Langmuir. 2004;20:7189–7200. doi: 10.1021/la049192s. [DOI] [PubMed] [Google Scholar]
- 21.Yang G, Amro NA, Starkwolfe ZB, Liu G. Molecular-level approach to inhibit degradations of alkanethiol self-assembled monolayers in aqueous media. Langmuir. 2004;20:3995–4003. doi: 10.1021/la0499160. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Chen S, Li L, Jiang S. Improved method for the preparation of carboxylic acid and amine terminated self-assembled monolayers of alkanethiolates. Langmuir. 2005;21(7):2634–2636. doi: 10.1021/la046810w. [DOI] [PubMed] [Google Scholar]
- 23.Flynn NT, Tran TNT, Cima MJ, Langer R. Long-term stability of self-assembled monolayers in biological media. Langmuir. 2003;19:10909–10915. [Google Scholar]
- 24.Jiang X, Bruzewicz DA, Thant MM, Whitesides GM. Palladium as a substrate for self-assembled monolayers used in biotechnology. Analytical Chemistry. 2004;76:6116–6121. doi: 10.1021/ac049152t. [DOI] [PubMed] [Google Scholar]
- 25.Kondoh H, Kodama C, Sumida H, Nozoye H. Molecular processes of adsorption and desorption of alkanethiol monolayers on Au(111). Journal of Chemical Physics. 1999;111(3):1175–1184. [Google Scholar]
- 26.Nelson CM, Raghavan S, Tan JL, Chen CS. Degradation of micropatterened surfaces by cell-dependent and -independent processes. Langmuir. 2003;19:1493–1499. [Google Scholar]
- 27.Noh J, Hara M. Molecular-scale desorption processes and the alternating missing-row phase of alkanethiol self-assembled monolayers on Au(111). Langmuir. 2001;17:7280–7285. [Google Scholar]
- 28.Prathima N, Harini M, Raj N, Chandrashekara RH, Ayappa KGS,S, Biswas SK. Thermal study of accumulation of conformational disorders in the self-assembled monolayers of C8 and C18 alkanethiols on the Au(111) surface. Langmuir. 2005;21:2364–2374. doi: 10.1021/la048654z. [DOI] [PubMed] [Google Scholar]
- 29.Schoenfisch MH, Pemberton JE. Air stability of alkanethiol self-assembled monolayers on silver and gold surfaces. Journal of American Chemical Society. 1998;120:4502–4513. [Google Scholar]
- 30.Zhang Y, Terrill RH, Tanzer TA, Bohn PW. Ozonolysis is the primary cause of UV photooxidation of alkanethiolate monolayers at low irradiance. Journal of Americal Chemical Society. 1998;120:2654–2655. [Google Scholar]
- 31.Brodbeck W, Shive M, Colton E, Nakayama Y, Matsuda T, Anderson JM. Influence of biomaterial surface chemistry on the apoptosis of adherent cells. Journal of Biomedical Materials Research. 2001;55:661–668. doi: 10.1002/1097-4636(20010615)55:4<661::aid-jbm1061>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 32.Brodbeck W, Nakayma Y, Matsuda T, Colton E, Ziats N, Anderson JM. Biomaterial surface chemistry dictates adherent monocyte/macrophage cytokine expression in vitro. Cytokine. 2002;18(6):311–319. doi: 10.1006/cyto.2002.1048. [DOI] [PubMed] [Google Scholar]
- 33.Brodbeck W, Patel J, Voskerician G, Christenson E, Shive M, Nakayama Y, Matsuda T, Ziats N, Anderson JM. Biomaterial adherent macrophage apoptosis is increased by hydrophilic and anionic substrates in vivo. Proceedings of the National Academy of Science. 2002;99(16):10287–10292. doi: 10.1073/pnas.162124199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brodbeck W, Voskerician G, Ziats N, Nakayama Y, Matsuda T, Anderson JM. In vivo leukocyte cytokine mRNA responses to biomaterials are dependent on surface chemistry. Journal of Biomedical Materials Research. 2003;64A:320–329. doi: 10.1002/jbm.a.10425. [DOI] [PubMed] [Google Scholar]
- 35.Jones J, Dadsetan M, Collier T, Ebert M, Stokes K, Ward R, Hiltner A, Anderson JM. Macrophage behavior on surface modified biomaterials. Journal of Biomaterial Science Polymer Edition. 2004;15(5):567–564. doi: 10.1163/156856204323046843. [DOI] [PubMed] [Google Scholar]
- 36.Jones J, Chang D, Colton E, Kwon I, Matsuda T, Anderson J. Proteomic analysis and quantification of cytokines and chemokines from biomaterial surface-adherent macrophages and foreign body giant cells. Journal of Biomedical Materials Research. 2006 doi: 10.1002/jbm.a.31221. In Press. [DOI] [PubMed] [Google Scholar]
- 37.Jones JA, McNally AK, Chang DT, Qin LA, Meyerson H, Colton E, Kwon IK, Matsuda T, Anderson JM. Matrix metalloproteinases and their Inhibitors in the foreign body reacion. Journal of Biomedical Materials Research. 2006 doi: 10.1002/jbm.a.31220. In Press. [DOI] [PubMed] [Google Scholar]
- 38.Keselowsky B, Collard D, Garcia A. Surface chemistry modulates focal adhesion composition and signaling through changes in integrin binding. Biomaterials. 2004;25:5947–5954. doi: 10.1016/j.biomaterials.2004.01.062. [DOI] [PubMed] [Google Scholar]
- 39.Keselowsky B, Collard D, Garcia A. Integrin binding specificity regulates biomaterial surface chemistry effects on cell differentiation. Proceedings of the National Academy of Science. 2005;102(17):5953–5957. doi: 10.1073/pnas.0407356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanahaski M, Matsuda T. Surface functional group dependence on apatite formation on self-assembled monolayers in a simulated body fluid. Journal of Biomedical Materials Research. 1997;34:314–315. doi: 10.1002/(sici)1097-4636(19970305)34:3<305::aid-jbm5>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 41.Sayre C, Collard D. Electrooxidative deposition of polypyrirole and polyaniline on self-assemled monolayer modified electrodes. Langmuir. 1997;13:714–722. [Google Scholar]
- 42.McNally AK, Anderson JM. Complement C3 participation in monocyte adhesion to different surfaces. Proceedings of the National Academy of Science. 1994;91:10119–10123. doi: 10.1073/pnas.91.21.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang MS, Palmer LB, Schwartz JD, Razatos A. Evaluating protein attraction and adhesion to biomaterials with the atomic force microscope. Langmuir. 2004;20:7753–7759. doi: 10.1021/la049849+. [DOI] [PubMed] [Google Scholar]
- 44.Shyue JJ, de Guire MR. Acid-base properties and zeta potentials of self-assembled monolayers obtained via in situ transformations. Langmuir. 2004;20:8693–8698. doi: 10.1021/la049247q. [DOI] [PubMed] [Google Scholar]
- 45.Li L, Chen S, Jiang S. Protein adsorption on alkanethiolate self-assembled monolayers: nanoscale surface structural and chemical effects. Langmuir. 2003;19:2974–2982. [Google Scholar]
- 46.Li L, Chen S, Jiang S. Molecular-scale mixed alkanethiol monolayers of different terminal groups on Au(111) by low-current scanning tunneling microscopy. Langmuir. 2003;19:3266–3271. [Google Scholar]
- 47.Garg N, Carrasquillo-Molina E, Lee TR. Self-assembled monolayers composed of aromatic thiols on gold: structural characterization and thermal stability in solution. Langmuir. 2002;18:2717–2726. [Google Scholar]
- 48.Mrksich M, Dike LE, Tien J, Ingber DE, Whitesides GM. Using microcontact printing to pattern the attachment of mammalian cells to self-assembled monolayers of alkanethiolates on transparent films of gold and silver. Experimental Cell Research. 1997;235:305–313. doi: 10.1006/excr.1997.3668. [DOI] [PubMed] [Google Scholar]
- 49.Silver IA, Murrills RJ, Etherington DJ. Microelectrode studies on the acid microenvironments beneath adherent macrophages and osteoclasts. Experimental Cell Research. 1988;175:266–276. doi: 10.1016/0014-4827(88)90191-7. [DOI] [PubMed] [Google Scholar]