Abstract
BACKGROUND
Inflammatory bowel diseases (IBD), which include ulcerative colitis (UC) and Crohn’s disease (CD), are debilitating and chronic disorders with unpredictable courses and complicated treatment measures. Therefore, an efficient treatment protocol seems necessary as therapeutic prophylaxis for these disorders. This study aims to determine the healing effect of Teucrium polium (T. polium) in acetic acid-induced UC in an experimental dog model.
METHODS
From September to December 2010, eight male (20-25 kg) crossbred dogs were used for induction of UC by 6% acetic acid, transrectally. After one week, three biopsies (10, 20 and 30 cm proximal to the anal verge) were taken from the colon of each animal for histological studies. In the presence of UC, 400 mg/kg/day of T. polium extract was administered orally and transrectally (via enema) for 30 days in six of the dogs. The remaining two dogs were used as controls and did not receive T. polium. Multiple biopsies were taken 7, 14, and 30 days after discontinuation of T. polium in the same manner as before treatment.
RESULTS
After administration of acetic acid, we noted the presence of multiple ulcers, diffuse inflammation, PMN infiltration in the lamina propria, glandular destruction and goblet cell depletion. Treatment with T. polium restored the colonic architecture with an increased number of healthy cells and a reduction in inflammatory cells. Damage of the surface epithelial cells and mucosal layer of the lumen were reversed, which lead to faster ulcer healing.
CONCLUSION
T. polium may be a treatment choice for UC and can broaden the current therapy options for UC.
Keywords: Ulcerative colitis, Acetic acid, Teucrium polium, Treatment, Dog
INTRODUCTION
Inflammatory bowel diseases (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD), are debilitating and chronic disorders that have unpredictable courses and complicated treatment measures. Similar to other chronic diseases, IBD negatively affects quality of life (HRQOL).1-4
The clinical spectrum ranges from an inactive or quiescent phase to low-grade active disease to fulminant disease.5 Common symptoms include diarrhea, rectal bleeding, passage of mucus, tenesmus, urgency and abdominal pain. In more severe cases, fever and weight loss may be visible. The symptom complex tends to differ according to the extent of disease.6,7 The etiology of UC is presently unknown, but likely multifactorial. The currently held paradigm involves a complex interaction of three elements including genetic susceptibility, host immunity and environmental factors.6 UC is most commonly diagnosed between the third and fourth decades of life, with no difference between males and females.8 Anti-inflammatory medications, corticosteroids, immunomodulators and biological agents against tumor necrosis factor-α (TNF-α) have been recommended as treatments for UC.9-13
Colonocytes express class II antigens. They can serve as antigen-presenting cells,14 express cytokine receptors and leukocyte adhesion molecules, and secrete various cytokines and chemokines.15-18 Patients with UC have an increased turnover rate of colonic epithelium19 and other abnormalities of epithelial cells such as a decrease in metabolism of short-chain fatty acids, particularly butyrate, abnormal membrane permeability,20 and altered composition of glycoprotein mucus produced by the colonic epithelium.21 The role of epithelial cells in the pathogenesis of IBD is supported further by animal models of colitis in which the colonic epithelium has been disrupted.22
In patients with IBD, especially UC, the repeated cycle of injury and repair of intestinal mucosa has been shown to increase the risk of colon cancer.6
The extent and severity of UC and its anatomic location affects agents used to induce remission in UC patients, including both oral and topical regimens.21 Sulfasalazine and its aminosalicylate analogues, corticosteroids, immunomodulators, suppressive antimetabolites, anti-tumor necrosis factor-biologics including infliximab, and in some cases antibiotics have been reported as treatments of choice.12,13
Identical to the initial treatment measures, maintenance therapy is usually individualized in coordination with the patient’s medical team. Patients with mild to moderate disease may be maintained on similar medications and dosages, with the exception of high-dose sulfasalazine.23 In some cases, oral and topical combinations provide the best results for long-term maintenance. As corticosteroids are generally ineffective for maintenance therapy, patients can be tapered and switched to 5-ASA, azathioprine, or 6-MP. A small number of patients are refractory to induction or relapse soon after the start of maintenance therapy. Total colectomy remains the last choice in these patients.23 So, a safer therapy that is more efficient seems necessary in treatment and prophylaxis of IBD.
Herbal extracts have been used to treat IBD in many studies.24-26 Medhi et al. in an experimentally induced UC model in rats demonstrated that Manuka honey and sulfasalazine showed synergic effects and enhancement of the antioxidant defense system.24 Souza et al. noticed that enemas of budesonide and probiotics enhanced the mucosal trophism in experimental colitis in rats.26
Ansari et al. reported that Teucrium polium(T. Polium) honey could accelerate burn wound healing.27 T. polium (Lamiaceae) is a wild-flowering plant, found abundantly in South-western Asia, Europe and North Africa. T. polium has about 220 genera and almost 4000 species worldwide.28,29 Teucrium species have been used as medicinal herbs for more than 2000 years. Different properties are reported for various Teucrium species.30 T. poliumis well-known for its diuretic, antipyretic, diaphoretic, antispasmodic, tonic, anti-inflammatory, antihypertensive, anorexic, analgesic antibacterial34 and antidiabetic properties.35
The therapeutic benefits of these medicinal plants are often attributed to their antioxidant properties.36-39 It has been shown that an aqueous extract of the leaves and stems of T. poliumcould inhibit iron-induced lipid peroxidation in a rat liver homogenate at concentrations not toxic to the cultured hepatic cells.40,41 T. poliumhad effective protection against ethanol-induced gastric mucosal damage42 and was reported to reduce NADPH-initated lipid peroxidation in rat liver microsomes.43 The in vitro antioxidant activity of T. polium extract was shown in another study.44 Herein, we evaluated the healing effect of T. polium in acetic acid induced UC in an experimental dog model.
MATERIALS AND METHODS
From September to December 2010, eight male (20-25 kg) cross-bred dogs were obtained from the Laboratory Animal Center affiliated with Shiraz University of Medical Sciences, Shiraz, Iran. The animals underwent enemas that consisted of 6% acetic acid (10 mg/kg body weight, in distilled water) administered with a no.18 feeding tube to induce UC. Bowel preparation was performed for all animals on the day of induction with senagraph syrup (30 ml; Sina Daru, Tehran, Iran) through a nasogastric tube.
One week following post-induction of UC, a rigid rectosigmoidoscope was used to evaluate the colonic mucosa. Three biopsies (10, 20 and 30 cm proximal to the anal verge) were taken from the colon of each animal and transferred into formalin for histological analyses as described by Mehrabani et al. (2009).44 Fromeach colon sample, we took 5 μm tissue sections and stained them with hematoxylin and eosin (H&E). All cases were evaluated for the presence of any macroscopic and microscopic mucosal ulcers as described by Morris et al. (1994).45
One week after transrectal acetic acid administration and histological confirmation of UC in all animals, 400 mg/kg/day of T. poliumextract (Barieej Essence Company, Tehran, Iran) was administered transrectally (via enema) for a one month period in six of the dogs. To prepare T. polium, 100 g of its dehydrated form was ground into a fi ne texture and extracted repeatedly with 80% ethanol. The extract was completely vacuum dried and then dissolved in distilled water. The control group consisted of two dogs that did not receive any therapy but were similarly treated for biopsies.
We took multiple biopsies 7, 14, and 30 days after discontinuation of T. polium in the same manner as before treatment. Histologically, samples were examined for the presence or absence of ulcers, mucosal cell depletion, inflammatory cysts, congestion, mucosal atrophy, submucosal edema, inflammatory cells, and vascular dilatation. For histological scoring, we choose 20 random fields per section from each specimen. We scored samples from all dogs according to the criteria described by Dundar et al. (2008)46 for colitis severity. To eliminate observer bias, the pathologists were blinded to the treatment.
The study was approved by the Ethics Committee of Shiraz University of Medical Sciences (registration number: 90-5077). All experiments were carried out under aseptic conditions in the Laboratory Animal Center at Shiraz University of Medical Sciences. The protocols for anesthesia, surgical procedures and postoperative care were identical for all animals. Dogs were initially evaluated for any illnesses by physical examination and laboratory screening. Before ulcer induction, all animals were deprived of food for 24 h but had free access to water to prevent excessive dehydration during starvation. To check for the presence of ulcers in the colon, 0.1 mg/kg of acepromazine was administered.
SPSS software (version 15.0, Chicago, IL, USA) was used for statistical analyses. Histological ulcer index in the stomach was evaluated by the independent sample test. Differences were considered significant at p < 0.05.
RESULTS
After seven days, we observed inflammatory cells [polymorphonuclear (PMN) leukocytes and lymphocytes] in the mucosa and around the crypts. Multiple ulcerations were visible that denoted the formation of a crypt abscess. In the submucosa, multifocal loci of ulceration and inflammation were present, which were diffusely edematous.
Table 1 shows the healing effects of T. polium in the dogs. On day 7 the scoring was 3-8 (p >0.05), on day 14 it was 2-5 (p=0.04), and on day 30 it was 0-1(p=0.005). Mucosal healing on days 14 (p=0.04) and 30 (p=0.005) were statistically significant compared to the control group.
Table 1. Treatment of UC by T. polium in dogs.
| Variables | Without treatment |
T. polium
After 7 days ( p>0.05) |
T. polium
After 14 days ( p =0.04) |
T. polium
After 30 days
( p =0.004) |
| 1 2 3 4 5 | 1 2 3 4 5 | 1 2 3 4 5 | 1 2 3 4 5 | |
| Ulceration | 1 1 1 0 0 | 0 1 1 0 0 | 0 1 0 0 0 | 0 0 0 0 0 |
| Mucosal cell depletion | 1 1 1 2 2 | 1 1 0 1 1 | 0 1 0 0 0 | 0 0 0 0 0 |
| Crypt abscess | 1 1 1 1 1 | 0 0 1 1 1 | 0 0 0 0 1 | 0 0 0 0 0 |
| Inflammatory cyst | 0 1 0 0 0 | 0 1 0 0 0 | 0 0 0 0 0 | 0 0 0 0 0 |
| Mucosal atrophy | 1 1 1 1 1 | 0 0 1 1 1 | 0 0 0 0 1 | 0 0 0 0 0 |
| Submucosal edema | 1 1 1 1 1 | 0 0 1 1 1 | 0 0 0 0 1 | 0 0 0 0 0 |
| Inflammatory cells | 2 2 3 2 2 | 1 1 2 1 2 | 1 1 1 11 | 0 0 0 1 1 |
| Vascular dilatation | 2 2 2 2 2 | 1 1 2 2 2 | 1 2 2 1 1 | 0 0 0 0 0 |
| Total | 9 10 10 9 9 | 3 5 8 7 8 | 2 5 3 2 5 | 0 0 0 1 1 |
Biopsies taken before (Figure 1) and after administration of acetic acid showed typical changes related to UC, e.g., multiple ulcers and diffuse inflammation (Figure 2). Figure 3 shows the presence of severe inflammation, PMN infiltration in the lamina propria, glandular destruction and goblet cell depletion that denoted UC after seven days (400x, H&E).As Figures 4 and 5 demonstrate, after treatment with T. polium an improvement in colonic ulcers as evidenced by increased numbers of healthy epithelial cells was noted. T. polium minimized the inflammatory burden and helped in reversing the damage to the surface of the epithelial cells and mucosal layer that was caused by acetic acid administration.
Fig. 1 .
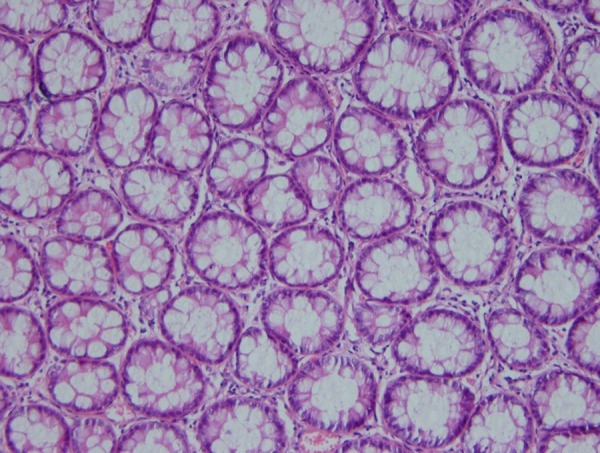
Normal colonic mucosa (H&E, 250x).
Fig. 2 .
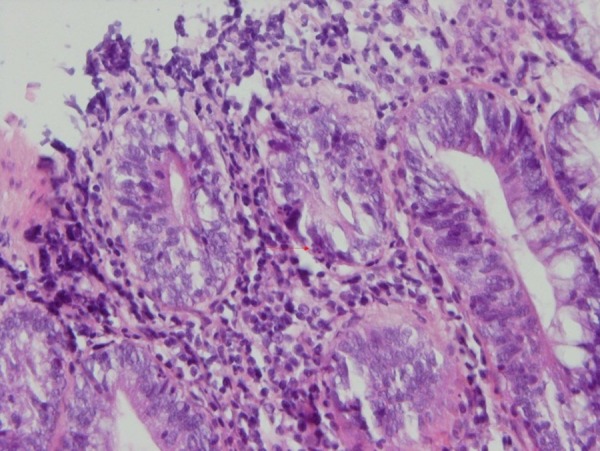
PMN permeation into the colonic gland (H&E, 400x).
Fig. 3 .
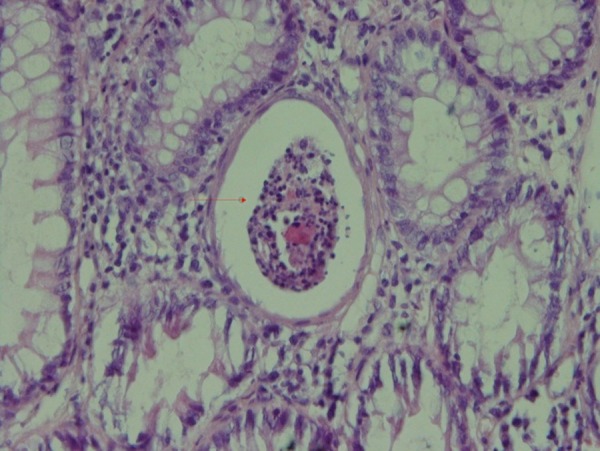
Inflammation in the lamina propria and crypt abscess
(H&E, 400x).
Fig. 4 .
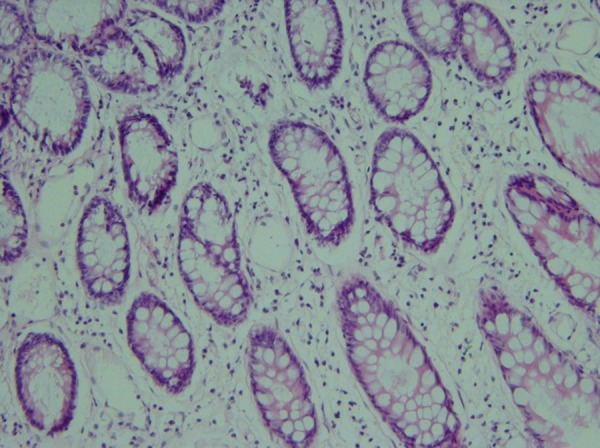
Mild chronic inflammation in the lamina propria With mild atrophy after T. polium administration (H&E, 250x).
Figure 5 .
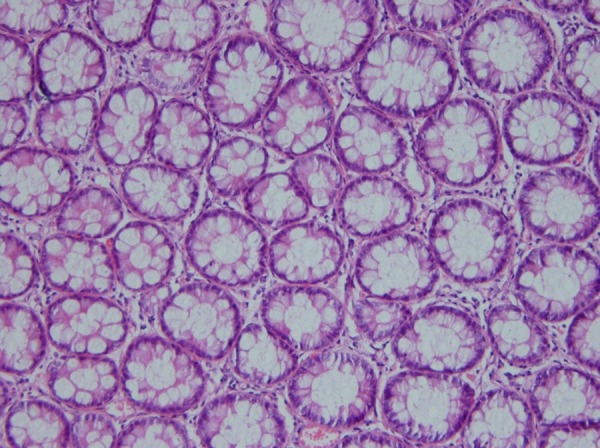
Complete healing 30 days after treatment (H&E, 400x).
T. polium consumption resulted into a faster healing of the ulcers. In the two dogs from the control group, severe loss of body weight was noticed along with crypt damage, loss of epithelium, infiltration of inflammatory cells and depletion of goblet cells, these changes were severe, with no mucosal healing.
DISCUSSION
The mainstay of medical therapy for UC focuses on medications that change the host response to decrease mucosal inflammation. Treatment regimens targeting other aspects of the systemic inflammatory process or manipulating the enteric flora have also been developed.6 To determine the etiology of IBD, experimental colitis has been induced using animal models. Anti-inflammatory treatments were investigated for IBD in animal models.47 Some authors have shown that dogs can be used as an experimental model for UC.48,49
Various chemicals, including dextran sodium sulfate, 2,4,6-trinitrobenzene sulfonic acid, oxazolone, indomethacin, and acetic acid, have been used to induce experimental colitis.47
T. polium has strong in vitro antioxidant properties, therefore this study investigated the healing effects of T. polium in the repair of acetic acid-induced UC in an experimental dog model. In this study we used 6% acetic acid, a readily available, inexpensive chemical agent, as an enema to induce UC in dogs. This resulted in the rapid development of signs of UC that included severe ulceration and inflammation of the colon, weight loss, diarrhea and hematochezia. Rigid rectosigmoidoscopy revealed frank ulceration and hemorrhage. Histologically, the typical early lesions consisted of an infiltration of inflammatory cells, primarily polymorphonuclear leukocytes, into the crypts at the base of the mucosa, forming crypt abscesses.
In this study, after the administration of 400 mg/kg/day T. polium for one month (transrectally) in dogs with induced UC, a significant improvement was visible both clinically and histologically. T. polium has long been recognized in folk medicine as a treatment for many pathophysiological conditions such as gastrointestinal disorders, inflammations, diabetes and rheumatism.44 Most of these effects were related to the antioxidant and free radical scavenging properties of T. polium.50
Kadifkova-Panovska et al. reported that an aqueous extract of T. polium was not toxic to cultured hepatic cells since mitochondrial respiration was fully preserved and the cell membrane integrity remained intact. In their study, cells were exposed for 24 h to the plant powder at concentrations of up to 1 mg/ml.40
Mehrabani et al. (unpublished data) investigated the effects of Calendula officinalis as treatment for acetic acid-induced UC in dogs, as an animal model. They noted significant mucosal healing after administration of Calendulaofficinalis after 30 and 45 days.49
The effects of corticosteroids as treatment for acetic acid-induced UC in dogs have been investigated.48,49 Two methods of steroid therapy were compared for UC via appendicostomy (antegrade) and enema (retrograde). Better results were visible in the antegrade method in comparison to the retrograde route. The difference may be due to peristaltic movement of the colon pushing materials from the proximal to the distal part of the colon.48 Our results are in agreement with those reports that have investigated the healing effects of herbal extracts such as T. polium in peptic ulcers and UC.44,48,49
However, many factors may be considered in determining the optimal therapy for UC patients. The current therapeutic measures can be classified upon disease activity into those that treat active disease (induction therapy) and those that prevent recurrence of disease once remission is achieved (maintenance therapy).6 The concept of induction and maintenance of remission is a basis of evaluating the efficacy of a specific therapy. The extent of disease in any given patient has an important role in determining the route of medication administration. Enema preparations may be used alone or in combination with systemic therapy in patients with left-sided disease. Other important factors are previous responses to or side effects from a specific medication and patient compliance. These factors can favor or preclude the use of a specific agent.6
The strength of our study was that we used the dog as an animal model for UC, which has been shown to be the most identical model for the human digestive system. The limitations of our study were vaccination of the animals for rabies and therapy for helminthic parasites, both of which were time consuming. Dogs are more expensive to maintain and keep when compared with mice and rats.
Our findings suggest that T. polium could accelerate UC remission in dogs. We did not attempt to identify the chemical constituents that could account for the anti-inflamatory action of the extract. Therefore, T. polium may be considered as a treatment choice for UC and can broaden the current therapy options of the disease.
ACKNOWLEDGEMENTS
The authors would like to thank the Office of Vice Chancellor for Research at Shiraz University of Medical Sciences for financial support, ST Heydari for statistical consult and the personnel of the Laboratory Animal Center at Shiraz University of Medical Sciences for their laboratory assistance.
CONFLICT OF INTEREST
The authors declare no conflict of interest related to this work.
Please cite this paper as :
D Mehrabani, F Bahrami, SV Hosseini, MJ Ashraf, N Tanideh, A Rezaianzadeh, M Amini, A Amini. The Healing Effect of Teucrium polium in Acetic Acid-induced Ulcerative Colitis in the Dog as an Animal Model. Middle East J Dig Dis 2012;4:40-47.
References
- 1.Karwowski CA, Keljo D, Szigethy E. Strategies to improve quality of life in adolescents with inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1755–64. doi: 10.1002/ibd.20919. [DOI] [PubMed] [Google Scholar]
- 2.Hendriksen C, Binder V. Social prognosis in patients with ulcerative colitis. BMJ. 1980;281:581–3. doi: 10.1136/bmj.281.6240.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burger D, Travis S. Conventional medical management of inflammatory bowel disease. Gastroenterology. 2011;140:1827–37. doi: 10.1053/j.gastro.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 4.Triantafi llidis JK, Merikas E, Georgopoulos F. Current and emerging drugs for the treatment of inflammatory bowel disease. Drug Des Devel Ther. 2011;5:185–210. doi: 10.2147/DDDT.S11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brunicardi FC, Schwartz SI. Schwartz’s Principles of Surgery, 9th ed, New York, McGraw-Hill 2010; pp. 1033-6.
- 6. Feldman, Sleisenger & Fortran`s Gastrointestinal and Liver Disease. 8th ed, New York, Elsevier2007; pp. 2498-548.
- 7.Both H, Torp-Pedersen K, Kreiner S, Hendriksen C, Binder V. Clinical appearance at diagnosis of ulcerative colitis and Crohn’s disease in a regional patient group. Scand J Gastroenterol. 1983;18:987–91. doi: 10.3109/00365528309182127. [DOI] [PubMed] [Google Scholar]
- 8.Ho GT, Chiam P, Drummond H, Loane J, Arnott ID, Satsangi J. The effi cacy of corticosteroid therapy in infl amatory bowel disease: Analysis of a 5 year UK inception cohorot. Aliment Pharmacol Ther. 2006;2:319–30. doi: 10.1111/j.1365-2036.2006.02974.x. [DOI] [PubMed] [Google Scholar]
- 9.Menassa R, Du C, Yin ZQ, Ma S, Poussier P, Brandle J. et al. Therapeutic effectiveness of orally administered transgenic low-alkaloid tobacco expressing human interleukin-10 in a mouse model of colitis. Plant Biotechnol J. 2007;5:50–9. doi: 10.1111/j.1467-7652.2006.00214.x. [DOI] [PubMed] [Google Scholar]
- 10.Bossa F, Colombo E, Andriulli A, Annese V. Treatment of steroid-naive ulcerative clitis. Exp Opin Pharmacother. 2009;10:1449–1460. doi: 10.1517/14656560902973728. [DOI] [PubMed] [Google Scholar]
- 11.Faure M, Moennoz D, Montigon F, Mettraux C, Mercier S, Schiffrin EJ. et al. Mucin production and composition is altered in dextran sulfate sodium-induced colitis in rats. Dig Dis Sci. 2003;48:1366–73. doi: 10.1023/a:1024175629909. [DOI] [PubMed] [Google Scholar]
- 12.Hendriksen C, Binder V. Social prognosis in patients with ulcerative colitis. BMJ. 1980;281:581–3. doi: 10.1136/bmj.281.6240.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burger D, Travis S. Conventional medical management of inflammatory bowel disease. Gastroenterology. 2011;140:1827–37. doi: 10.1053/j.gastro.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 14.Mayer L, Shlien R. Evidence for function of Ia molecules on gut epithelial cells in man. J Exp Med. 1987;166:1471. doi: 10.1084/jem.166.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reinecker HC, Podolsky DK. Human intestinal epithelial cells express functional cytokine receptors sharing the common gamma c chain of the interleukin-2 receptor. Proc Natl Acad Sci USA. 1995;92:8353. doi: 10.1073/pnas.92.18.8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe M, Ueno Y, Yajima T, Iwao Y, Tsuchiya M, Ishikawa H. et al. Interleukin-7 is produced by human intestinal epithelial cells and regulates the proliferation of intestinal mucosal lymphocytes. J Clin Invest. 1995;95:2945–53. doi: 10.1172/JCI118002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung HC, Eckmann L, Yang SK, Panja A, Fierer J, Morzycka-Wroblewska E. et al. A distinct array of pro inflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang GT, Eckmann L, Savidge TC, Kagnoff MF. Infection of human intestinal epithelial cells with invasive bacteria upregulates apical intercellular adhesion molecule-1 (ICAM)-1) expression and neutrophil adhesion. J Clin Invest. 1996;98:572–83. doi: 10.1172/JCI118825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allan A, Bristol JB, Williamson RC. Crypt cell production rate in ulcerative proctocolitis: Differential increments in remission and relapse. Gut. 1985;26:999–1003. doi: 10.1136/gut.26.10.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC. et al. Four-week open-label trial of metronidazole and ciprofloxacin for the treatment of recurrent or refractory pouchitis. Aliment Pharmacol Ther. 2002;16:909–17. doi: 10.1046/j.1365-2036.2002.01203.x. [DOI] [PubMed] [Google Scholar]
- 21.Gionchetti P, Amadini C, Rizzello F, Venturi A, Campieri M. Review article: Treatment of mild to moderate ulcerative colitis and pouchitis. Aliment Pharmacol Ther. 2002;16:13–9. doi: 10.1046/j.1365-2036.16.s4.3.x. [DOI] [PubMed] [Google Scholar]
- 22.Sambuelli A, Boerr L, Negreira S, Gil A, Camartino G, Huernos S. et al. Budesonide enema in pouchitis: A doubleblind, double-dummy, controlled trial. Aliment Pharmacol Ther. 2002;16:27–34. doi: 10.1046/j.1365-2036.2002.01139.x. [DOI] [PubMed] [Google Scholar]
- 23. Zinner M J, Ashley SW. Maingot`s Abdominal Operations, 11th ed, New York, Mc Graw Hill 2007; pp. 551-87.
- 24.Parente LM, Andrade MA, Brito LA, Moura VM, Miguel MP, Lino-Júnior Rde S. et al. Angiogenic activity of Calendula offi cinalis fl owers Lin rats. Acta Cir Bras. 2011;26:19–24. doi: 10.1590/s0102-86502011000100005. [DOI] [PubMed] [Google Scholar]
- 25.Medhi B, Prakash A, Avti PK, Saikia UN, Pandhi P, Khanduja KL. Effect of Manuka honey and sulfasalazine in combination to promote antioxidant defense system in experimentally induced ulcerative colitis model in rats. Indian J Exp Biol. 2008;46:583–90. [PubMed] [Google Scholar]
- 26.Souza MM, Aguilar-Nascimento JE, Gomes-da-Silva MH, Carlos Junior R. Effects of budesonide and probiotics enemas on the colonic mucosa of rats with experimental colitis. Acta Cir Bras. 2007;22:34–8. doi: 10.1590/s0102-86502007000100006. [DOI] [PubMed] [Google Scholar]
- 27.Ansari M, Alizadeh AM, Paknejad M, Khaniki M, Naeimi SM. Effect of Teucrium Polium Honey on Burn Wound Healing Process. J Babol Univ Med Sci. 2009;11:5–7. [Google Scholar]
- 28.Naghibi F, Mosaddegh M, Mohammadi-Motamed S, Ghorbani A. Labiatae Family in folk Medicine in Iran: from Ethnobotany to Pharmacology. Iran J Pharmaceu Res. 2005;2:63–79. [Google Scholar]
- 29.Jamzad Z, Ingrouille M, Simmonds MSJ. Three new species of Nepeta (Lamiaceae) from Iran. Toxon. 2003;52:93–8. [Google Scholar]
- 30.Agel MB, Garaibeh MN, Salhab AS. The calcium antagonistic effect of the volatile oil of Teucrium polium. Int J Crude Drug Res. 1990;28:201–10. [Google Scholar]
- 31.Tariq M, Ageel AM, Al-Yahya MA, Mossa JS, Al-Said MS. Anti-inflammatory activity of Teucrium polium. Int J Tiss React. 1989;11:185–8. [PubMed] [Google Scholar]
- 32.Mansouri S. Inhibition of staphylococcus areus mediated by extracts of Iranian plants. Pharmacbiol. 1999;37:375–7. [Google Scholar]
- 33.Esmaeili MA, Yazdanparast R. Hypoglycaemic effect of Teucrium polium; studies with rat pancreatic islets. J Ethnopharmacol. 2004;9:527–30. doi: 10.1016/j.jep.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 34.Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant fl avonoids and risk of coronary heart disease: the Zutphen elderly study. Lancet. 1993;342:1007–11. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z, Chang Q, Zhu M, Huang Y, Ho WKK, Chen ZY. Characterization of antioxidants present in hawthorn fruits. J Nutr Biochem. 2001;12:144–52. doi: 10.1016/s0955-2863(00)00137-6. [DOI] [PubMed] [Google Scholar]
- 36.Rice-Evans C. Flavonoids and isofl avones: absorption, metabolism and bioactivity. Free Radic Biol Med. 2004;36:827–8. doi: 10.1016/j.freeradbiomed.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 37.Dixon RA, Xie DY, Sharma SB. Proanthocyanidins-a final frontier in flavonoid research? New Phytol. 2005;165:9–28. doi: 10.1111/j.1469-8137.2004.01217.x. [DOI] [PubMed] [Google Scholar]
- 38.Suboh SM, Bilto YY, Aburjai TA. Protective effects of selected medicinal plants against protein degradation, lipid peroxidation and deformability loss of oxidatively stressed human erythrocytes. Phytother Res. 2004;18:280–4. doi: 10.1002/ptr.1380. [DOI] [PubMed] [Google Scholar]
- 39.Kadifkova-Panovska T, Kulevanova S, Stefova M. In vitro antioxidant activity of some Teucrium species (Lamiaceae) Acta Pharm. 2005;55:207–14. [PubMed] [Google Scholar]
- 40.Alkofahi A, Atta AH. Pharmacological screening of the anti-ulcerogenic effect of some Jordanian medical plants in rats. J Ethnopharmacol. 1999;67:341–5. doi: 10.1016/s0378-8741(98)00126-3. [DOI] [PubMed] [Google Scholar]
- 41.Hasani P, Yasa N, Vosough-Ghanbari S, Mohammadirad A, Dehghan G. In vivo antioxidant potential of Teucrium polium, as compared to a-tocopherol. Acta Pharma. 2007;57:1–12. doi: 10.2478/v10007-007-0010-z. [DOI] [PubMed] [Google Scholar]
- 42.Ljubuncic P, Azaizeh H, Portnaya I, Cogan U, Said O, Saleh KA. et al. Antioxidant activity and cytotoxicity of eight plants used in traditional Arab medicine in Israel. J Ethnopharmacol. 2005;99:43–7. doi: 10.1016/j.jep.2005.01.060. [DOI] [PubMed] [Google Scholar]
- 43.Mehrabani D, Rezaee A, Azarpira N, Fattahi MR, Amini M, Tanideh N. et al. The healing effects of Teucrium polium in the repair of indomethacin-induced gastric ulcer in rats. Saudi Med J. 2009;30:494–9. [PubMed] [Google Scholar]
- 44.Morris DL, Montgomery SM, Galloway ML, Pounder RE, Wakefi eld AJ. Inflammatory bowel disease and laterality: is left handedness a risk? Gut. 2001;49:199–202. doi: 10.1136/gut.49.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dundar E, Olgun EG, Isiksoy S, Kurkcuoglu M, Baser KH, Bal C. The effects of intra-rectal and intra-peritoneal application of Origanum onites Lessential oil on 2,4,6-trinitrobenzenesulfonic acid-induced colitis in the rat. Exp Toxicol Pathol. 2008;59:399–408. doi: 10.1016/j.etp.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 46.Kawada M, Arihiro A, Mizoguchi E. Insights from advances in research of chemically induced experimental models of human inflammatory bowel disease. World J Gastroenterol. 2007;13:5581–93. doi: 10.3748/wjg.v13.i42.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mehrabani D, Nasibi M, Izadpanah A, Rezaianzadeh A, Ashraf MJ, Tanideh N, et al. The effect of corticosteroids in therapy of acetic acid induced ulcerative colitis in dog as an animal model. Submitted to Arch Iran Med.
- 48.Mehrabani D, Ziaei M, Hosseini SV, Ghahramani L, Bananzadeh AM, Tanideh N. The Effect of Calendula officinalis in Therapy of Acetic Acid Induced Ulcerative Colitis in Dog as an Animal Model. Iran Red Crescent Med J. 2012 [In press] [PMC free article] [PubMed] [Google Scholar]
- 49.Cholbi MR, Paya M, Alcaraz MJ. Inhibitory effects of phenolic compounds on CCl4-induced microsomal lipid peroxidation. Experientia. 1991;7:195–9. doi: 10.1007/BF01945426. [DOI] [PubMed] [Google Scholar]


